This article was downloaded by: [National Chiao Tung University 國立交通大學] On: 28 April 2014, At: 05:34
Publisher: Taylor & Francis
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK
Ozone: Science & Engineering: The
Journal of the International Ozone
Association
Publication details, including instructions for authors and subscription information:
http://www.tandfonline.com/loi/bose20
A refined model for ozone mass transfer
in a semibatch stirred vessel
C.Y. Chiu a , C.Y. Chang a , W.H. Huang a , S.J. Lee a , Y.H. Yu a , H.T. Liou b , Y. Ku c & J.N. Chen d
a
Graduate Institute of Environmental Engineering , National Taiwan University , Taipei, 106, Taiwan
b
Institute of Atomic and Molecular Sciences , Academia Sinica , Taipei, 106, Taiwan
c
Department of Chemical Engineering , National Taiwan Institute of Technology , Taipei, 106, Taiwan
d
Graduate Institute of Environmental Engineering , National Chiao‐Tung University , Hsin‐Chu, 300, Taiwan
Published online: 29 Nov 2010.
To cite this article: C.Y. Chiu , C.Y. Chang , W.H. Huang , S.J. Lee , Y.H. Yu , H.T. Liou , Y. Ku & J.N. Chen (1997) A refined model for ozone mass transfer in a semibatch stirred vessel, Ozone: Science & Engineering: The Journal of the International Ozone Association, 19:5, 439-456, DOI: 10.1080/01919512.1997.10382870
To link to this article: http://dx.doi.org/10.1080/01919512.1997.10382870
PLEASE SCROLL DOWN FOR ARTICLE
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://www.tandfonline.com/page/terms-and-conditions
Vol. 19, pp. 439-456 Printed in the U.S.A.
International Ozone Association Copyright © 1997
A Refined Model for Ozone Mass Transfer
in a Semibatch Stirred Vessel
C.Y. Chiu1, C.Y. Chang1+, W.H. Huang1, S.J. Lee1,
Y.H. Yu1, H.T. Liou2, Y. Ku3, and J.N. Chen4 1 Graduate Institute of Environmental Engineering,
National Taiwan University, Taipei 106, TAIWAN
2 Institute of Atomic and Molecular Sciences,
Academia Sinica, Taipei 106, TAIWAN
3 Department of Chemical Engineering,
National Taiwan Institute of Technology, Taipei 106, TAIWAN
4 Graduate Institute of Environmental Engineering,
National Chiao-Tung University, Hsin-Chu 300, TAIWAN
+ Author to whom all correspondence should be addressed.
Received for Review : 6 March 1996 Accepted for Publication : 10 June 1997
Abstract
The mathematical model proposed by Anselmi et al. (1984) for a semibatch stirred gas-liquid contactor is refined to describe the mass transfer of ozone absorption and decomposition in aqueous solution with the decomposition rate expression of general reaction orders (not necessarily integers). Three system equations are employed to describe the ozone concentrations in the bulk liquid (CALb), the hold-up gas (CAGi ), and the outlet gas in the free
volume above the liquid surface (CAGe), respectively. The effect of ozone
decomposition on the mass transfer, which is reflected by the enhancement factor (Er) defined as the ratio of mass absorbed per unit area in time t with
chemical reaction (r) to that without chemical reaction or of the purely physical absorption, is considered in the refined model. Furthermore, the refined model also takes into account the variation of Er with CALb, which
changes with time during the course of gas-liquid contacting. Thus this analysis extends the applicability of the model of Anselmi et al. (1984) and is of special importance for ozone mass transfer in the cases of basic solutions and of low mass transfer coefficients, in which the effect of decomposition on absorption is significant, and in the system with variable liquid phase ozone concentration.
439
440 C.Y. Chiu et al.
Introduction
Ozone mass transfer is of importance for ozone utilization in a semibatch stirred gas-liquid contactor (Yocum, 1979; Anselmi et al., 1984). For such a semibatch stirred vessel, Anselmi et al. (1984) have developed a three-equation model describing the concentrations of ozone in the bulk liquid (Cyj,), the hold-up gas (CAffl), and the free volume above the
liquid surface (CAG<). Their model is better than the usual model describing C^j, with the
assumption of constant CACS such as CAGi = CAOi° (the ozone concentration of the inlet gas).
The second order kinetics of ozone decomposition at pH = 5.4 was initially introduced in the system equations, but finally disregarded in obtaining the solution. Thus, their model cannot be applicable for other kinetic expressions of ozone decomposition reported by many investigators (for example, Weiss, 1935; Sheffer and Esterson, 1982; Sotelo et al., 1987; Nadezhdin, 1988; Farooq and Ahmed, 1989; Mehta et al., 1989; Chang et al., 1995). Moreover, in the study of Anselmi et al. (1984), neither the effects of the decomposition reaction (r) on the enhancement factor E, nor the differentiation between the purely physical (kL°) and the chemical ( k j mass transfer coefficients has been provided.
The term E,. is defined as the ratio of mass absorbed per unit area in time t with chemical reaction to that of purely physical absorption (Danckwerts, 1970). This lack of infor-mation seriously compEcates the use of the data for the purposes of : I) performing mass transfer computations, and 2) computing the mass transfer in combination with different reactions (Bollyky, 1981). Further, the value of E, varies with C ^ , which changes with time during the course of gas-liquid contacting. All these factors would have to be taken into consideration for properly modeling the ozone mass transfer in a semibatch stirred vessel.
Ozone decomposition reactions have been studied by numerous investigators as can be seen from the tables compiled by Peleg (1976), Teramoto et al. (1981), Gurol and Singer (1982), Sotelo et al. (1987), Nadezhdin (1988), Farooq and Ahmed (1989), and Miyahara and Hirokawa (1994). The overall reaction kinetic orders varied from 0 to 2 for ozone while from 0 to 1 for hydroxide ion (Sheffer and Esterson, 1982; Anselmi et al., 1984; Sotelo et al., 1987; Nadezhdin, 1988; Farooq and Ahmed, 1989). The only agreement reached by these workers is the radical-chain nature of ozone decomposition, which is catalyzed by the hydroxide ion (Sotelo et al., 1987; Nadezhdin, 1988). Among those kinetic schemes based on radical-chain reaction mechanisms, the pioneering work of Weiss (1935) led to a kinetic expression of the form: for pH 2 to 8:
[1] Sotelo et at (1987) added a second initiation reaction between ozone and water to the scheme of Weiss (1935) to explain an apparent independence of the rate on pH in the acidic region. They suggested that for pH = 2.5 to 9 and temperature = 283 to 313 K,
[2] Kuo (1982) conducted theoretical studies on the mass transfer during ozone absorption. The decomposition reaction was considered first order with respect to ozone. Mehta et al. (1989) modified the model of Kuo (1982) taking three-halves order with respect to ozone concentration for the decomposition reaction of ozone. The effects of pH value and
temperature were not included in their models. Miyahara and Hirokawa (1994) also suggested a rate expression of ozone decomposition in the form:
[3] Recently, Chang et al. (1995) gave the following reaction rate expression which is applicable to ozone decomposition of the general form:
[4] In Equation [4], k ^ and !%„ represent the acidic and basic decomposition reaction rate constants, respectively. Equation [4] reduces to Equation [1] with k ^ = k,JHO], m = 1. k&, = MHO"]", and n = 1.5, to Equation [2] with k ^ = k^, m = 1, kBn = kBS[HO]05,
and n = 1.5, to Equation [3] with:
and n = 1.5, and to the rate expressions proposed by other investigators with k^, = 0, and n = 0-2 (not necessarily an integer). Employing the general reaction rate expression of Equation [4] and applying the film model of gas liquid absorption, Chang et al. (1995) examined the role of the ozone decomposition reaction on Er and the efficient use of
ozone. Further, according to Sotelo et al. (1989 a,b), the kinetic constant of ozone decomposition in solution also is affected by the type of salts, the ionic strength (I), and the hydroxyl radical scavenger concentration (Cais). For example, at pH = 7, and salt type
= sodium phosphate (buffered solution), Sotelo et al. (1989a) reported -ro3 as follows:
[5] The units of kcS, T, I, Co,IS and [O3 ] in Equation [5] are M'!s"\ K, M, M and M,
respectively. Equation [5] also may be regarded as a special form of Equation [4]. Noting the need for improving and extending the applicability range of the model of Anselmi et al. (1984), this analysis considers the ozone decomposition rate expression of general reaction orders and the enhancement factor, which varies with time during the course of gas-liquid contacting. The refined model presented herein is of special importance for ozone mass transfer in the cases of basic solutions and of low mass transfer coefficients, in which the effect of decomposition on absorption is significant, and in the system with varied liquid phase ozone concentration.
Theoretical Analysis
Consider the mass transfer of ozone absorption and decomposition in a semibatch stirred gas-liquid contactor with the decomposition rate expression of the general form as Equation [4]. Referring to the work of Anselmi et al. (1984) which indicated that, owing to mechanical mixing, bubbles circulate in the reaction vessel randomly and break and coalesce continuously, one may assume that the liquid phase as well as the gas phase are
442 C.Y. Chiu et al.
perfectly mixed as in continuous stirred tank reactors. Thus, the system equations in dimensionless forms describing the concentrations of ozone in the bulk liquid ( C ^ ) , the hold-up gas (CACS), and the free volume above the liquid surface (CAG{), which are derived
from the mass balance and the absorption equilibrium, are as follows:
[6]
[7]
[8]
[9]
The system variables and parameters in dimensionless forms are defined as:
[10]
In the previous study on ozone mass transfer in a semibatch stirred vessel, Anselmi et al. (1984) also presented a three-equation model similar to Equations [6]-[8]. However, they employed a second order kinetics of ozone decomposition which may not necessarily be applicable for other kinetics reported by many investigators (for example, Sotelo et al., 1987; Nadezhiden, 1988). Furthermore, they did not consider the effect of ozone decomposition on the mass transfer which may be reflected by the enhancement factor Er
The value of E, is dependent on the bulk liquid concentration C^ which varies with time during the course of gas-liquid contacting. The present analysis thus compensates for the lack of the model of Anselmi et al. (1984).
The decomposition reaction may affect the mass transfer rate of ozone. The influence can be reflected by the enhancement factor Er in Equations [6] and [7]. Therefore, it is
necessary to evaluate the enhancement factor Er in advance for solving Equations [6]-[9].
According to the film model, one may describe the ozone absorption with a decomposition reaction as (Chang et al., 1995) :
[ H ]
[12]
[13]
with 6A=CA/(CAGi°/He) and z = x/xM = x kL°/DA. Equations [11]-[13] can be solved for
d8A/dz :
[14]
When z = 0, -d6A/dz = ( 6 A,, - O^JE,, with Er = UJNA (ratio of mass flux of A with r to that without r) and Equation [14] holds, one can evaluate the constant of integration, C^,. Thus:
[15] Substituting Equation [15] into [14], one has :
444 C.Y. Chili et al.
[16]
Integration between z = 0, 8A = 8 ^ and z =1, 8A = 8 ^ then gives:
[17]
Evaluating the value of Ef from Equation [17] and solving for Equations [6]-[9] by the
fourth order Runge-Kutta method, one then can obtain the ozone concentrations at successive absorption times for any specific case.
A number of kinetic expressions of decomposition of ozone in aqueous solutions have been reported by some investigators ( for example, Sullivan and Roth, 1980; Sotelo et al., 1987 and 1989a; Mehta et al., 1989; Miyahara and Hirokawa, 1994). In general, the kinetic expressions are dependent on the following operating variables as indicated previously : temperature, pH, type of salt, ionic strength, and hydroxyl radical scavenger concentration. The computations of the present model thus depend on the applicable kinetic expression. There may be suggested different forms of kinetic expressions describing ozone decomposition in aqueous solutions and yielding the simulation results of the model. This, of course, deserves further study in the future. For illustration, the kinetic expression in the form of Equation [2] as reported by Sotelo et al. (1987) is employed. They reported that:
[Î8] [19] The units of k^, kßS and T in Equations [18] - [19] are min*1, L mol'1 min"1, and K,
respectively. As regards the purely physical mass transfer coefficient kL° and the specific
interfacial area a, many measurements and correlations are available. The value of kL°a
changes with variables: gas flow rate (Q) or superficial gas velocity (uG), stirring rate (N)
or power input, and shape factor of vessel (Lopes De Figueiredo et al., 1979; Van't Riet, 1979; Yocum, 1980; Anselmi et al., 1984; Kawase and Moo-Young, 1988). Sherwood et al. (1975) reported kL° with a range of 0.001 to 0.1 cm s'1. Other investigators gave the
following kL° values: 0.00826 cm s'1 (Sotelo et al., 1989a), 0.0142 cm s"1 (Bollyky,
1981), 0.025 cm s*1 (Roustan et al., 1981), and 0.04 cm s"1 (Reith, 1968).
In order to compare the predicted results to the experimental data of Sotelo et al. (1989a), a Iq0 of 0.00826 cm s"1 is chosen for simulation. Furthermore, in order to examine the case
of very low kL° value, a kL° of 0.001 cm s"1 also is used. Calderbank (1958) observed the
interfacial area a in the range of 0.8 to 4 cm'1 (with N - 10.5 to 25 rps) for water in an
agitated absorber with uG of 1.50 cm s'1 and tank diameter (dT) of 19.1 cm. At N = 12 rps,
the value of a is about 1 cm"1. Reith (1968) obtained the values of a in the range 0.8 to 30
cm"1 with uo= 4.7 cm s*1,N3d,2 = 0.2 x 104 to 150 x 104 cmV3 (dl= impeller diameter)
anddT 19.1 to 120 cm. FordT=44,8cm,a= l,2cm'1atN3d,2 = 0.7x 104cm2s'3. In the
study of Sotelo et al. (1989a), an experimental system with the value of a of 0. 157 cm'1
was used. For illustration, the value of a of 1 cm"1 is employed for model simulations. The
values of some physical properties of an Oj/H2O system at 298 K are: DA = 2 x 10"5 cm2
s'1 (Sherwood et al., 1975; Perry and Chilton, 1984), CAGi° = 0.0015 mol L"1 (Anselmi et
al., 1984). The Henry's law constant (He) is dependent on temperature and ionic strength as indicated by Danckwerts (1970). The values of He of ozone-water systems reported by many investigators are:
atT = 298K:
He [kPa mol fr1] x 10"5 = 6.57 (Kuo et al., 1977), 6.41 (Roth and Sullivan, 1981),
5.24 (Kosak-Channing and Heiz, 1983), 4.6 (Perry and Chilton, 1984), 4.7 (Ouedemi, 1987), 6.57 (Langlais et al.,
1991), 5.7 (Miyahara and Hirokawa, 1994),
or I/He [Mfeas)/M(solution)] = 0.209, 0.214,0.261, 0.299, 0.291,0.209, 0.245, at T = 296 K:
He [kPamol fr1] x 10s = 6.2 (Caprio et al., 1982; Anselmi et al., 1984),
or l/He [M(gas)/M(solution)] = 0.223, atT = 293K:
He [kPa mol fr1] x 10'5 = 3.81 (Bollyky, 1981), 5.67 (Sotelo et ai., 1989a),
or l/He [M(gas)/M(solution)] = 0.335,0.242.
The differences of the properties of solutions used by these investigators may cause the above differences of the values of He, reflecting the complicated nature of ozone-water systems. For simulation illustration, I/He [M(gas)/M(solution)] of 0.223 (Caprio et al., 1982; Anselmi et al., 1984) is employed. The values of VL, VH, VF and Q are chosen by
referring to the work of Anselmi et al. (1984).
It is worth noting that the usual model describing C ^ in a stirred vessel assuming constant CAGi such as CAGi = CAGi° is as follows.
[20]
[21]
The corresponding film-model equation for evaluating Er is similar to Equation [17] with
0 A H = 1
446 C.Y. Chiu et a!.
Results and Discussion
Figures 1, 2 and 3 compare the ozone concentrations in the bulk liquid in a semibatch stirred vessel employing three-equation models of different types. The model of this analysis with Equations [6]-[9] includes the variable Er and the generalized ozone
decomposition reaction rate expression which is dependent on the pH value. The model equations of Anselmi et al. (1984) are similar to Equations [6]-[9] with Er = 1 and
neglecting the ozone decomposition reaction terms. Thus, the model of this analysis is applicable for the solutions of any pH value, while that of Anselmi et al. is useful only for the solutions of very low pH value as clearly indicated in Figures 1, 2 and 3. In fact, the model of Anselmi et al. (1984) was not valid for the cases of basic solutions.
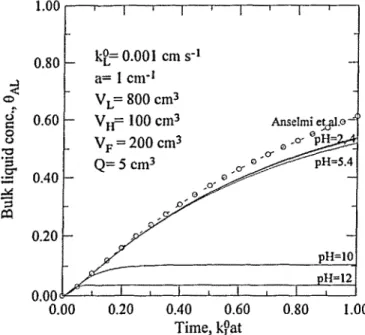
Figure 1. Comparison of 6 ^ vs time employing three-equation models. " ": this analysis (including Er and reaction terms); "o": theoretical results
using model of Anselmi at al. (1984); " " : this analysis with Er = 1
and neglecting reaction terms, a = 1 cm*1, VL = 800 cm3, V,, = 100 cm3,
VF = 200 cm3, Q = 5 cm3 s'1, kL° = 0.001 cm s*1.
The numerical results for the special case of this analysis with Et = 1 and without reaction
terms are in good agreement with the theoretical results of Anselmi et al. Figure 4 compares the predicted values of the proposed model to the experimental data of Sotelo et al. (1989a), also indicating good agreement. All these support the validity of the numerical methods and the model employed herein. However, it is noted that the value of the equilibrium liquid concentration obtained from the intercept of the plot of kcS[O3]2
+ d[Oj]/dt vs [O,] in Figure 10 of Sotelo et al. (1989a) is about 2.5 x 10"1 M, while that
calculated from Henry's law with Po3 = 2 kPa is about 1.96 x 10*4 M. The applicable
conditions of Sotelo et al. (1989a) in Figure 4 of the present study are: T = 293 K, pH =
7, PO3 = 2 kPa, Q = 60 L hf1, N = 100 rpm, salt type = sodium phosphate (buffered
solution), 1 = 0. 15 M, C0„s =0.37 M, He = 5.67 x 10s kPa mol fr"1 = 4.33
M(gas)/M(solution), kCS [NT's"1] = 7.12 x 1010 exp(-6858/T) II03/COHS, -r03 = kcS[O3]2 =
1.864 [O3f, kL° = 0.00826 cm s1, kL°a = 0.0013 s"1. The gas holdup eo is estimated to
be 0.008 by using the correlation equation for water in the stirred vessel with a four-blade paddle, eG = 0.316 (QN/o)°5 (Hassan and Robinson, 1977). The units of Q, N and a in
the e0 equation are mV1, rps and Nm'1, respectively.
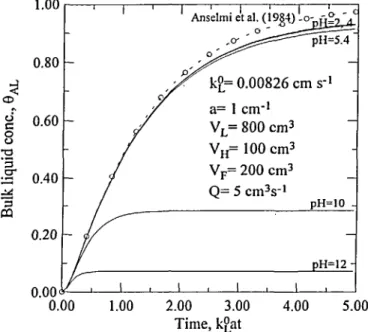
Figure 2. Comparison of 6 ^ vs time employing three=equation models. L k ° =
0.00826 cm s'1. Notation and other parameter values are the same as in
Figure 1.
Also from Figures 1,2 and 3, it can be observed that when the serni-batch reactor system approaches its steady state, a higher pH value results in a lower steady-state ozone concentration in bulk solution. This is because the rate of ozone decomposition increases with pH value. It is noted that the steady-state concentration does not correspond to the equilibrium, similar to the phenomena described by Sotelo et al. (1989a) and Miyahara and Hirokawa (1994). In fact, the steady-state concentration of C ^ is related to the equilibrium concentration of CAG/He according to the steady-state form of Equation [6]
withVLd8AL/dt = 0.
In dealing with the purely physical mass transfer of gas in a semibatch stirred vessel, one usually assumes a constant CAGi such asCAQi = CAa0 (Adams et al., 1981). A model similar
to Equation [20] with Er = I and without reaction terms has been applied to describe the
bulk liquid concentration in the purely physical mass transfer system. To test the validity of such model assuming CAffl = CAGi° to the chemical mass transfer of ozone (Er * 1), the
results of the model with Equation [20] are obtained and compared with those of the
448 C.Y. Chiu et al.
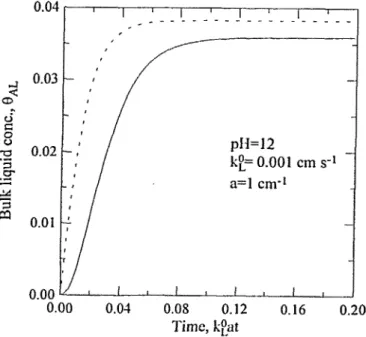
three-equation model of this analysis. The comparison results of the ozone concentrations in the bulk liquid ( 8 ^ are presented in Figures 5 and 6. For the conditions specified in the figures, at dimensionless time of 1, the relative deviations of 8 ^ employing the model with CACS = CA£K° from those employing the three-equation model of this analysis are about
6.4% and 18.4% for kL° = 0.001 and 0.00826 cm s"\ respectively.
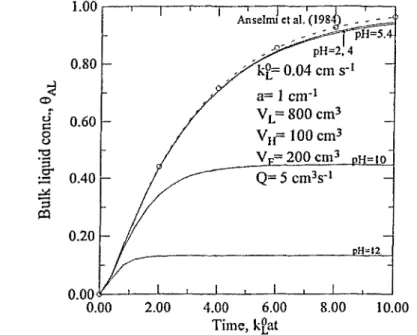
Figure 3. Comparison of 8 ^ vs time employing three-equation models. kL° = 0.04
cm s ''. Notation and other parameter values are the same as in Figure 1. The comparison results of the corresponding Er illustrated in Figures 7 and 8 also reveal
the deviations of the two models. All these again clearly indicate the significant improvements of the present model over the usual model assuming CAGi = CAa?. Further,
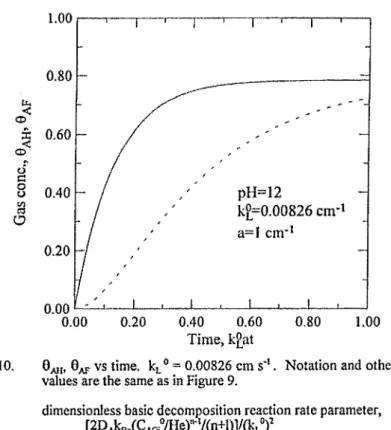
Figures 9 and 10 illustrate the difference between the concentrations of the hold-up gas (BAH) and the outlet gas in the free volume above the liquid surface (8^). In practice, one usually measures the concentrations of 8 ^ and 8^, instead of 8^, and 8 ^ . A proper model should allow one to relate 8 ^ to 6 ^ and then to 8 ^ . Thus, the refined three-equation model of this analysis is more rigorous and appropriate to describe the ozone mass transfer in a semibatch stirred vessel.
Concluding Remarks
1. The ozone decomposition reaction rates (r03) and the enhancement factor (E r)
should be included in the system equations describing the ozone mass transfer in a semibatch stirred gas-liquid contactor.
2. The refined three-equation model with rO3 and variable Er and considering the
concentrations of ozone in the bulk liquid, the hold-up gas and the free volume above the liquid surface is more rigorous and adequate to describe the system performance.
Figure 4, Comparison of ozone concentration by model prediction " " with experimental data "o" of Sotelo et al. (1989a). T - 293 K, pH = 7, Po 3
= 2kPa, Q = L h"1, N = 100 rpm, I = 0.15 M, salt type = sodium phosphate
(buffered solution), Com = 0.37 M, kL° = 0.00826 cm s"!, a = 0.157 cm"1.
Nomenclature
A ozone species
a interfacial area per unit volume of liquid, S/VL
CA concentration of A in film according to film model
CAGe gas concentration of A in free volume
CAOi concentration of A in hold up gas
CAGj° concentration of A of inlet gas
C^UJ, concentration of A in bulk liquid
C,«,0 equilibrium liquid concentration with CAGi°, = CAai°Me CAL* equilibrium liquid concentration withCAtji, = CAGi/He
Cm constant of integration given in Equation [15]
450 C.Y. Chiu et al.
Figure 5. 0 ^ vs time employing different simulation models. " " : three-equation simulation of this analysis; " " : simulation of model assuming CAGi = CAGi°. a = 1 cm"1, VL = 800 cm3, VH = 100 cm3, VF =
200 cm3, Q = 5 err? s'1, pH = 12, kL° = 0.001 cm s'1.
COHS hydroxyl radical scavenger concentration
dT tank diameter
DA diffusivity of A
Er enhancement factor with effect of self-decomposition reaction (or
reaction), "Nf/tiA
He Henry's law constant C^,* = CAGi/He
I ionic strength of solution
^Am» kßa acidic and basic decomposition rate constants in kinetic expression of Chang et al. (1995) defined in Equation [4]
^AS. ^BS acidic and basic decomposition rate constants in kinetic expression of Sotelo et al. (1987) defined in Equation [2]
kcS decomposition rate constant in kinetic expression of Sotelo et a!. (1989a)
defined in Equation [5]
kL° purely physical liquid-phase mass transfer coefficient
kL liquid-phase mass transfer coefficient for a specific case, e.g., with
chemical reaction
m, n acidic and basic decomposition reaction orders with respect to Oa in kinetic
expression
Mm dimensionless acidic decomposition reaction rate parameter,
t2D
Ak
Am(C
AGi°/Her
1/(m+l)]/(k
L0)
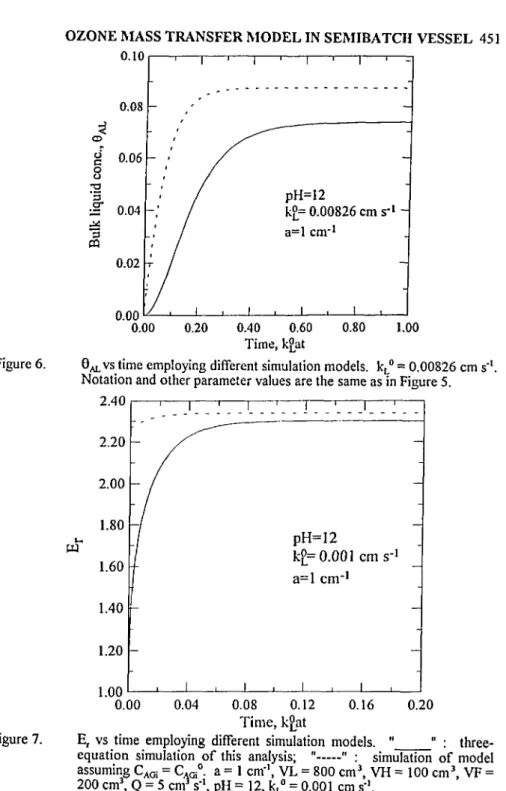
3Figure 6. ö^vs time employing different simulation models. kL° = 0.00826 cm s'1.
Notation and other parameter values are the same as in Figure 5.
Figure 7. Er vs time employing different simulation models. " " :
three-equation simulation of this analysis; " " : simulation of model assuming CAGi = C.^0. a = 1 cm"1, VL = 800 cm3, VH = 100 cm3, VF =
200 cm3, Q = 5 cm3 s'\ pH = 12, kL° = 0.001 cm s-I.
452 C.Y. Chiu et al.
Figure 8. Er vs time employing different simulation models. kLc = 0.00826 cm s "'.
Notation and other parameter values are the same as in Figure 7.
Figure 9. QMb 6 ^ vs time. " " : dMl, " " : 6 ^ (three-equation simulation of this analysis), a = 1 cm'1, VL = 800 cm3, V,, = 100 cm3, VF = 200 cm3,
Q = 5 cm3 s \ pH = 12, kL° = 0.001 cm s"1.
Figure 10. Qm, 6 ^ vs time, kL° = 0.00826 era s'%. Notation and other parameter values are the same as in Figure 9,
MB„ dimensionless basic decomposition reaction rate parameter,
[2DAkBn(CAGi°/Herl/(n+l)]/(kL0)2
N agitation speed
NA mass flux of purely physical mass transfer, kL° [(CAC/He) -CAJJ,] according
to film model
N ^ NA with self-decomposition reaction (r), -DAdCA/dx
P0 3 ozone partial pressure
Q gas flow rate r reaction rate S mass transfer area
Sh Sherwood number, kL°VL(DAS)
t time T temperature
Uo superficial gas velocity
VF free volume above liquid surface
VH gas hold up volume
VL liquid volume
x distance from gas-liquid interface into liquid film
XM thickness of liquid film representing liquid-phase mass transfer resistance,
DA/ k /
z dimensionless x, x/xM
e0 dimensionless gas hold up, VH/(VL + V,|)
6A dimensionless CA, CA/(CAOi°/He)
454 C.Y. Chiu et al.
SAP dimensionless CA G e, CA0/CAGi°
dm dimensionless CA G i, CAO/CAOi° OAL dimensionless C ^ , C A L ^ C A ^ / H C )
0 surface tension
x dimensionless time, kL° S t/ VL
A cknowledgment
This study was supported by the National Science Council of Taiwan under Grant No. NSC 84-222 l-E-002-030 and 84-222 l-E-002-044.
References
ADAMS, C.E., Jr.; FORD, D.L.; ECKENFELDER, W.W., Jr., 1981, Development of
Design and Operational Criteria for Wastewater Treatment (Nashville, TN, U.S.A.:
Enviro Press, Inc.).
ANSELMI, G.; LIGNOLA, P.G.; RAITANO, C., VOLPICELLI, G. (1984) "Ozone mass transfer in stirred vessel," Ozone: Science & Engineering, 6:17-28.
BOLLYKY, L.J., 1981, "The mass transfer of ozone into water: Energy requirements -state of the art," Ozone: Science & Engineering, 3:181-210.
CALDERBANK, P.H., 1958, "Physical rate processes in industrial fermentation part 1: the interfacial area in gas-liquid contacting with mechanical agitation," Trans. Inst. Chem. Engrs, 36:443.
CAPRIO, V.; INSOLA, A.; LIGNOLA, P.G., VOLPICELLI, G., 1982, "A new attempt for the evaluation of the absorption constant of ozone in water," Chem. Eng. Sci., 37:122-124.
CHANG, C.Y.; CHIU, C.Y.; LEE, S.J.; HUANG, W.H.; YU, Y.H.; LIOU, H.T.; KU, Y., CHEN, J.N., 1995, "Absorption of ozone in aqueous solutions with self-decomposition effect," Toxicological and Environmental Chemistry, 50:197-205. DANCKWERTS, P.V., 1970, Gas-liquid reactions (New York, NY: McGraw-Hill). FAROOQ, S., AHMED, M., 1989, "Modeling of an ozone-wastewater system's kinetics,"
Water Res., 23:809-815.
GUROL, M.D., SINGER, P.C., 1982, "Kinetics of ozone decomposition: a dynamic approach," Environ. Sci. Technol., 16:377-383.
HASSAN, I.T.M.; ROBINSON, C.W., 1977, "Stirred-tank mechanical power requirement and gas holdup in aerated aqueous phase," AIChE, 23:48-56.
KAWASE, Y.; MOO-YOUNG, M., 1988, "Volumetric mass transfer coefficients in aerated stirred tank reactors with Newtonian and non-Newtonian media," Chem. Eng. Res. Des., 66:284-288.
KOSAK-CHANNING, L.F.; HELZ, G.R., 1983, "Solubility of ozone in aqueous solutions of 0-0.6M ionic strength at 5-30°C", Environ. Sci. Technol., 17:145-149.
KUO, C.H.; LI, K.Y.; WEN, C.P.; WEEKS, J.L., Jr., 1977, "Absorption and decomposi-tion of ozone in aqueous soludecomposi-tion," AlChE Symp., 73:230-241.
KUO, C.H., 1982, "Mass transfer in ozone absorption," Environ. Progress, 1:189-195. LANGLAIS, B.; RECKHOW, D.A.; BRINK, D.R., 1991, Ozone in Water Treatment :
Application and Engineering (Chelsea, MI: Lewis Publishers, Inc.).
LOPES DE FIGUEIREDO, M.M.; CALDERBANK, P.H., 1979, "The scale-up of aerated mixing vessels for specified oxygen dissolution rates," Chem. Eng. Sci., 34:1333-1338.
MEHTA, Y.M.; GEORGE, C.E.; KUO, C.H., 1989, "Mass transfer and selectivity of ozone reactions," Canadian J. Chem. Engrg., 67:118-126.
MIYAHARA, T.; HIROKAWA, M., 1994, "Solubility of ozone into water in a bubble column," Kagaku Kogaku Ronbunshu, 20:497-503.
NADEZHDIN, A.D., 1988, "Mechanism of ozone decomposition in water. The role of termination," Ind. Eng. Chem, Res., 27:548-550.
PELEG, M., 1976, "The chemistry of ozone in the treatment of water," Water Res., 10: 361.
PERRY, R.H.; CHILTON, C.H., eds., 1984, Chemical Engineer's Handbook, 6th ed. (New York, NY: McGraw-Hill).
QUEDERNI, A., 1987, "Ozone absorption in water: mass transfer and solubility," Ozone: Sci. & Engrg., 9:1.
REITH, T., 1968, "Physical aspects of bubble dispersions in liquids," Doctor's Thesis, Delft (The Netherlands: Delftsche Uitgevers Maatschappij N. V.).
ROTH, J.A.; SULLIVAN, D.E. (1981) "Solubility of ozone in water," Ind. Eng. Chem. Fundam., 20:137-140.
ROUSTAN, M.; MALLEVIALLE, J.; ROQUES, H.; JONES, J.P., 1981, "Mass transfer of ozone to water: a fundamental study," Ozone: Science & Engineering, 2:337-344. SHEFFER, S.; ESTERSON, G.L., 1982, "Mass transfer and reaction kinetics in the ozone
- tap water system," Water Res., 16:383-389.
SHERWOOD, T.K..; PIGFORD, P.L.; WILKE, C.R., 1975, Mass Transfer, New York, NY: McGraw-Hill).
SOTELO, J.L.; BELTRÁN, F.J.; BENITEZ, F.J.; BELTRÁN-HEREDIA, J., 1987, "Ozone decomposition in water: kinetic study," Ind. Eng. Chem. Res., 26:39-43. SOTELO, J.L.; BELTRÁN, F.J.; BENITEZ, F.J.; BELTRAN-HEREDIA, J., 1989a,
"Henry's law constant for the ozone-water system," Wat. Res., 23(10):1239-1246. SOTELO, J.L.; BELTRÁN, F.J., GONZALEZ, M.; DOMINGUEZ, J., 1989b, "Effect of
high salt concentrations on ozone decomposition in water," J. Environ. Sci. Health, A24(7):823-842.
SULLIVAN, D.E.; ROTH, J.A., 1980, "Kinetics of ozone self-decomposition in aqueous solution," AlChE Symp. Ser., 76, No. 197:142-149, 1979.
TERAMOTO, M.; IMAMURA, S.; YATAGAI, N.; NISHIKAWA, Y.; TERANISHI, H., 1981, "Kinetics of the self-decomposition of ozone and the ozonation of cyanide ion and dyes in aqueous solutions," J. Chem. Eng. Japan, 14:383-388.
VAN'T RIET, K., 1979, "Review of measuring methods and results in nonviscous gas-liquid mass transfer in stirred vessels," Ind. Eng. Chem. Process Des. Dev.,
18:357-364.
WEISS, J., 1935 "Investigation on the radical HO2 in solution," Trans. Faraday Soc., 31:
668-681.
YOCUM, F.H., 1980, "Ozone mass transfer in stirred vessel," AlChE Symp. Ser.. 76, No. 197:135-141, 1979.
Key Words
Ozone; Aqueous Ozone; Decomposition of Aqueous Ozone; Mass Transfer, Semibatch Vessel;
456 C.Y. Chiu et ai.
Résumé
Le modèle mathematique proposé par Anselmi et autres (OS&E 6:17, 1984) pour un réacteur gaz-liquide agité en semi-batch est affiné pour décrire le transfert de masse de l'ozone absorbé et sa décomposition dans la solution aqueuse incluant l'ordre des réactions de décomposition de la réaction générale. Un système de trois équations est utilisé pour décrire la concentration en ozone dans le liquide (CALb)), dans les bulles de gaz (CAGi) et le
gaz s'echappant au dessus du liquide (CAGe). L'effet de la décomposition de l'ozone sur le
transfert de masse, qui est exprimé par le facteur d'amélioration (Er) est pris en compte
dans ce modèle. Le facteur d'amélioration est défini comme étant le rapport de la masse absorbée par unité de surface dans un temps t lorsqu'il y a réaction chimique (r) à la masse absorbée sans réaction chimique (absorption purement physique). En outre, le modèle amélioré prend aussi en compte les variations de Er en fonction de CALb, qui change dans
le temps au cours de contact gaz-liquide. Cette étude permet d'étendre l'applicabilité du modèle d'Anselmi et autres (1984); elle est d'importance particulière pour le transfert de masse dans le cas de solutions basiques et de faibles coefficients de transfert, pour lesquels l'effet de la décomposition sur l'absorption est significatif, et dans le cas des systèmes avec concentration variable de l'ozone.
Zusammenfassung
Das von Anselmi (OS&E 6:17, 1984) vorgeschlagene mathematische Model für einen gerührten Gas/Flüssig-Batchreaktor wird verbessert, um den Stofrübergang für die Ozonabsorption und die Ozonzersetzung in wässriger Lösung mit einem Term allgemeiner Reaktionsordnung für die Zersetzung zu beschreiben. Drei System-gleichungen werden benutzt, um die Ozonkonzentrationen in der Flüssigkeit (CAlb), dem Gas (C AGi) und dem
Abgas im Volumen über der Flüssigkeit (CAGe) zu beschreiben. Der Einfluß der
Ozonzersetzung auf den Stoffübergang, der ausgedrückt wird durch einen Verstärkungsfaktor (Er), definiert als das Verhältnis des absorbierten Stoffes, pro
Fläscheneinheit in der Zeit t mit der chemischen Reaktion (r), zu dem ohne chemische Reaktion bzw auf die rein physikalische Absorption bezogen, wird als das verbesserte Modell bezeichnet. Darüber hinaus berücksichtigt das verbesserte Modell die Schwankung von Er mit CALb, die sich mit der Zeit wärend des Gas/Flüssig-Austausches ändert. So
ermöglicht diese Analyse eine erweiterte Anwendung des Modells von Anselmi und ist besonders wichtig für den Ozonübergang bei Lösungen und niedrigen Stoffübergangs-koeffizienten, bei denen der Effekt der Ozonzersetzung auf die Absorption besonders groß ist und in Systemen mit schwankenden Ozonkonzentrationen in der Flüssigphase.