Pulsed focused ultrasound enhances boron drug accumulation in a human head and neck cancer xenograft-bearing mouse model
Running title: HIFU-enhanced boron drug accumulation in H&N cancer *Chun-Yi Wu1,2, *Pei-Chia Chan1, Lin-Shan Chou1, Chi-Wei Chang3, Feng-Yi Yang1,
Ren-Shyan Liu3,4,5, Shih-Hwa Chiou6, Yi-Wei Chen7, Sang-Hue Yen7, and Hsin-Ell Wang1,2
1Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan
2Biophotonics & Molecular Imaging Research Center, National Yang-Ming University, Taipei, Taiwan
3Department of Nuclear Medicine and National PET/Cyclotron Center, Taipei Veterans General Hospital, Taipei, Taiwan
4Department of Nuclear Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan
5Taiwan Mouse Clinic, National Comprehensive Mouse Phenotyping and Drug Testing Center, Taipei, Taiwan
6Department of Medical Research and Education, Taipei Veterans General Hospital, Taipei, Taiwan
7Cancer Center, Taipei Veterans General Hospital, Taipei, Taiwan
* Chun-Yi Wu and Pei-Chia Chan contribute equally to this work. Manuscript Category: Article
Correspondence:
Hsin-Ell Wang, Ph.D, Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, No.155, Sec.2, Li-Nong St., Taipei, Taiwan 11217. e-mail: hewang@ym.edu.tw
Phone: +886-2-28267215 Fax: +886-2-28201095
Abstract
Purpose: This study aims to demonstrate that pulsed high intensity focused ultrasound (pulsed-HIFU) may enhance the fructose-conjugated 4-borono-L-phenylalanine (BPA-Fr) accumulation in tumor lesion using 18F-FBPA-Fr microPET scans.
Procedures: To the mice bearing orthotopic SASC03 human tongue squamous carcinoma xenograft, a 2-min pulsed-HIFU was applied to tumor. Immediately after pulsed-HIFU treatment, 18F-FBPA-Fr was intravenously injected and biological characterizations including microPET imaging and biodistribution were conducted. Results: Both biodistribution studies and microPET imaging performed after intravenous injection of 18F-FBPA-Fr revealed higher tumor uptake in HIFU-treated mice than that of the control. CD31 and Ki-67 histochemical staining of tumor sections and H&E staining of nearby normal tissues revealed no significant difference between the pulsed-HIFU-treated mice and the control.
Conclusion: This study demonstrated that pulsed-HIFU was beneficial to the accumulation of boron drug in the head & neck tumor lesion, and may enhance the therapeutic efficacy of clinical BNCT.
Key words: Head and neck cancer, Pulsed high-intensity focused ultrasound, Boron neutron capture therapy, 18F-FBPA-Fr, MicroPET
Introduction
Head and neck (H&N) cancer, especially in squamous cell carcinoma (SCC) form, is a malignant disease with high prevalence in Taiwan. Despite the advance of surgery, radiotherapy, or chemotherapy, locally recurrent H&N cancer after multi-disciplined treatment is still a therapeutic challenge. Boron neutron capture therapy (BNCT) is a binary system based on the neutron capture reaction that occur when nonradioactive 10B atoms are irradiated with thermal or epithermal neutrons to produce high linear energy transfer -particles and recoiling 7Li nuclei. For successful clinical application of BNCT, 10B is required to be selectively accumulated in tumor lesion without high normal tissues uptake. L-p-Dihydroxyborylphenylalanine (BPA) has been clinically used as neutron capture agent for the treatment of various tumor types .
BPA conjugated with fructose (BPA-Fr) may appreciably increase its solubility and slightly enhance the active accumulation in tumor . A radioactive surrogate, 4-borono-2-18F-fluoro-L-phenylalanine fructose (18F-FBPA-Fr), was successfully synthesized for noninvasive evaluation of the pharmacokinetics of BPA-Fr in vivo with positron emission tomography (PET) in our previous studies . These findings demonstrated that the biodistribution and pharmacokinetics of 18F-FBPA-Fr parallel that of BPA-Fr in a glioma rat model, suggesting that the pharmacokinetics of 18
F-FBPA-Fr derived from PET scans can guide an optimal window for effective BNCT in clinical.
Vascular, stromal and interstitial barriers in solid tumor impede the tumor uptake of therapeutic agents and result in the heterogeneous distribution. High intensity focused ultrasound (HIFU) is being an clinical optionforthermal ablation of prostate, liver or breast malignancies, as well as for uterine fibroids . In contrast, pulsed-HIFU uses low duty cycle pulses to overcome the impedance of barriers while minimizing the heat generation. Traughber et al. reported that pulsed-HIFU significantly enhanced the antitumor effect of low-temperature heat-sensitive liposome-encapsulated doxorubincin in a murine mammary JC adenocarcinoma-bearing mouse model . Poff et al. also revealed that treatment of tumor with pulsed-HIFU resulted in noted tumor cytotoxicity and growth inhibition at lower dose level of bortezomib .
The current BNCT shows promise in some H&N cancers. Regarding that there is relatively little in terms of published literatures on the use of HIFU in BNCT, we demonstrate that HIFU is useful for enhancing the accumulation of BPA-Fr in tumor lesions and may achieve better therapeutic efficacy of BNCT while minimizing the off-target side effects.
Materials and Methods
Preparation of 18F-FBPA and 18F-FDG
The procedures for the preparation of 18F-FBPA-Fr have been detailed in our previous reports .
18F-FDG was prepared according to the method published by Lemair et al. using an automated 18F-FDG synthesis system (TracerLab MX, GE Healthcare, WI, USA) at the National PET/Cyclotron center in Taipei Veterans General Hospital, Taipei, Taiwan.
Cell culture and orthotopic xenograft inoculation
SASC03 tongue squamous carcinoma cells, a gift from prof. Chiou (National Yang-Ming University, Taiwan), were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Thermo, MA, USA) in a humidified atmosphere with 5% CO2 at 37˚C. The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of the National Yang-Ming University (Taipei, Taiwan). About 1×105 of SASC03 cells were injected into the buccal pouch in the right cheek to grow xenograft in an 8-week-old male nude mouse under anesthesia (ketamine, 87 mg/kg and xylazine, 13 mg/kg, intraperitoneally).
In vitro cellular accumulation
SASC03 cells (3 x 105) were seeds into six-well culture plates containing 3 mL of culture medium supplemented with 10% FBS. After 24 h of growth, a 10-L aliquot of 18F-FBPA-Fr and 18F-FDG (37 kBq ea.) was added individually to each well. The culture medium was removed and the monolayers were washed two times with 0.5 mL of cold PBS at each designated time points (15, 30, 60 and 120 min post-incubation). The cells were harvested from the culture plates by the addition of 0.5 mL of 0.5% trypsin for 5 min. The cells were re-suspended in 0.5 mL of culture medium to neutralize the trypsin. A 50-L sample was taken to assess the number of viable cells in the cell suspensions. The accumulation of radiotracer was expressed as the percentage of administered dose in the medium that had accumulated in one million cells (% AD/106 cells). The radioactivity of cell pellet and medium was measured by a gamma scintillation counter (1470 WIZARD Gamma Counter, Wallac, Finland).
Pulsed-HIFU exposures
Mice were anesthetized with isoflurane (2% in O2, 1 L/h, inhaled) throughout the
processes of HIFU exposure. To precisely irradiate the tumor xenograft, a mouse in treated-group was secured in a holder, and then placed on a 3-dimentional stage, which was used to ensure the position of tumor was in the area of focal zone of
transducer (A329S, Panametrics, Waltham, MA). This positioning system was performed by a graphic user interface. The radial and axial diameter (-6dB) of focal zone was 3 mm and 26 mm, respectively. After an intravenous injection of microbubbles, the tumor was immediately swept by HIFU beam, which moved horizontally left-to-right at a constant rate. The parameters of pulsed-HIFU sonications are listed as follows: frequency, 1 MHz; spatial average and temporal average intensity, 130 W/cm2; pulse repetition frequency, 1 Hz; duty cycle, 5% (50
ms ‘‘on’’ and 950 ms ‘‘off’’). Soon after a 2-min HIFU exposure, the mice were intravenously injected with 18F-FBPA-Fr for microPET imaging and biodistribution study.
18F-FBPA-Fr and 18F-FDG microPET imaging
Imaging studies was performed on a microPET R4 scanner (Concorde Microsystems, Inc.) with a resolution of 1.8 mm at full width of half maximum. Because it is difficult to determine the tumor growth of an orthotopic H&N tumor mouse model at the early stage, on day 10 after SASC03 cells inoculation, 18F-FDG microPET scan was conducted to visualize the growth of SASC03 xenograft. The tumor-bearing mice were randomly assigned to two groups of at least five mice in each group (HIFU-treated and the control). Static microPET imaging of the mice in HIFU-treated and the control groups was conducted for 10 min at 15, 30, 60, 120, and
240 min post-injection of 18F-FBPA-Fr. The tumor uptake of 18F-FBPA in mice of these two groups was compared to evaluate whether pulsed-HIFU can enhance the accumulation of boron drug or not. During the examination, the mice receiving anesthesia with isoflurane were placed in the prone position with the long axis parallel to the table of the scanner. Attenuation correction was carried out by transmission scans with 68Ge/68Ga rotating. Image data were constructed by using an
attenuation-corrected ordered subsets expectation maximization algorithm, which was supplied the manufacturer. Regions of interest (ROIs) were drawn over the tumor, and the average values from the pixels within ROIs were corrected by subtracting the background radioactivity, which was measured in the remote areas away from the experiment animal. Tumor radioactivity concentration is normalized by the injected dose and expressed as %ID/mL.
Biodistribution studies
After microPET imaging at designated time points, the mice were sacrificed by cervical dislocation. Tissues of blood, heart, lung, liver, stomach, small intestine, large intestine, pancreas, spleen, kidney, muscle, bone marrow and tumor were excised and parts of these organs were weighted. The accumulated radioactivity in these tissue samples was measured by a gamma scintillation counter, normalized to sample weight, and expressed as the percentage of injection dose per tissue (%ID/g).
%ID/g = Injection dose(Ci)37000A601000Eff.Organweight(mg)TCF
Where A is the radioactivity measured by gamma counter, Eff. is the counting efficiency of the counter, TCF is time corrected factor (ln(A/A0) = -0.693t/t1/2), A0 is the decay-corrected radioactivity (cpm) of tissue, t1/2 is the half-life of the radioisotope and t is the time post-injection (p.i.).
The area under curve (AUC), which corresponds to the integral of the amount of disintegrations (h·%ID/g), was calculated based on the results of biodistribution study. The AUCs were determined by using the WinNonlin program (version 6.3, Pharsight, CA, USA). Histological analysis
After microPET imaging, the SASC03 xenograft-bearing mice in each group were sacrificed for immunohistochemistry staining to assess whether HIFU may influence the angiogenesis and proliferation rate of SASC03 xenograft. Before dissecting the tissues, the mouse was perfused with 30 mL of normal saline. The dehydration, paraffin embedding, and section steps were conducted as described elsewhere . The slices were incubated with 1 mM of EDTA at 95˚C for 15 min and then blocked with goat serum at ambient temperature for 30 min. The rabbit polyclonal antibodies against CD31 (Santa Cruz Biotechnology, TX, USA) and the
mouse monoclonal Ki67 antibody (Millipore, MA, USA) was applied to the slides at a dilution of 1:50 and 1:75, respectively. The slides were incubated in liquid DAB+ substrate chromogen system (Dako, Denmark A/S, Denmark) until the brown stains was observed. Besides, the brain section was also obtained for the assessment of tissue injury after pulsed-HIFU exposure. H&E staining was performed to evaluate the morphological changes in brain.
Results
In vitro cellular uptake studies
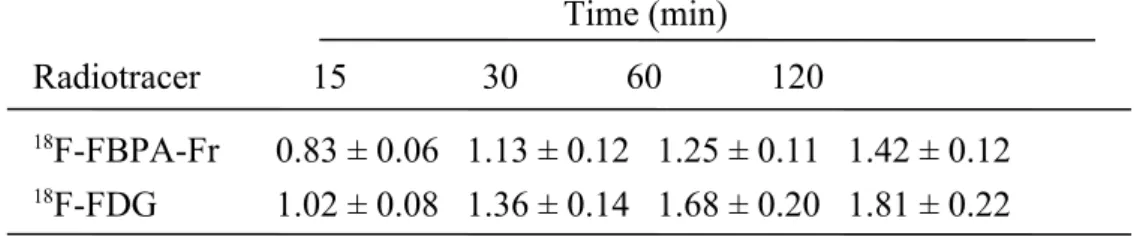
Both the cellular accumulation (expressed in %AD/106 cells) of 18F-FBPA-Fr and 18F-FDG in SASC03 cells kept increasing with time and reached 1.42±0.12 and 1.81±0.22, respectively, after 2 h of incubation (Table 1). These results indicate an active uptake of 18F-FBPA-Fr in cells, as well as that of 18F-FDG.
MicroPET imaging
Increasing but heterogeneous 18F-FDG uptake in tumor observed till 55 min post-injection witnessed the growth of orthotopic SASC03 human tongue squamous carcinoma xenograft in nude mice (Fig. 1). Compared with 18F-FDG, the tumor uptake of 18F-FBPA-Fr was more significant (Fig. 2). Apparent radioactivity accumulation in tumor lesion was observed in the HIFU-treated mice and the control post injection of 18F-FBPA-Fr, and both groups displayed similar pharmacokinetic profiles. The tumor uptake of the HIFU-treated mice derived from microPET images was 9.29±0.79, 13.58±0.47, 10.32±2.93 and 5.59±0.44 % ID/mL, respectively, at 0.5, 1, 2 and 4 h p.i., higher than that of the control (8.17±0.71, 10.13±0.34, 8.41±0.55 and 3.45±0.19 % ID/mL, respectively) in the same study period. Enhanced and prolonged radioactivity retention in SASC03 xenograft was observed, suggesting that
pulsed-HIFU was beneficial to the accumulation of BPA-Fr in tumor lesion. Biodistribution studies
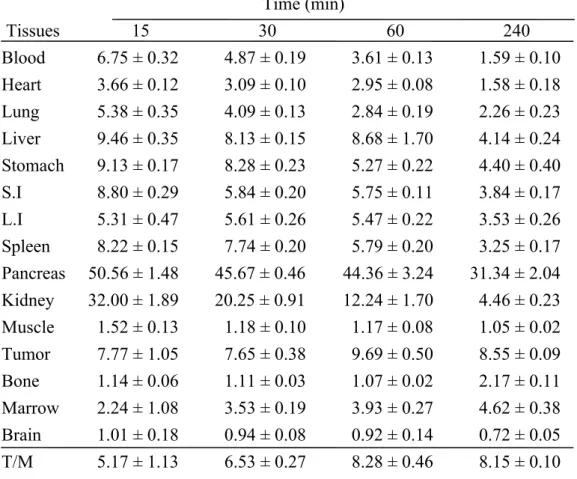
The uptake of 18F-FBPA-Fr in tumor of SASC03 xenograft was higher than that in most of normal organs, except the pancreas, at 60 min p.i. (Table 2). The tumor-to-blood and tumor-to-muscle ratios increased with time in both HIFU-treated and the control mice, indicating the preferential and specific tumor accumulation of 18 F-FBPA-Fr. The tumor uptake peaked at 60 min p.i. in the HIFU-treated mice (17.12±1.32 %ID/g, n=5, Table 2b), and was significantly higher than that of the control (9.69±0.50 %ID/g). Enhanced tumor accumulation can be observed at 30 min post HIFU exposure, and the effect retained for 240 min. The AUC of tumor derived from biodistribution study indicated a 40% increase in HIFU-exposed mice (38.81 h· %ID/g) than that of the control (27.33 h·%ID/g) for a 240-min period (p<0.05, Table 3). However, pulsed-HIFU did not affect the distribution and retention of 18 F-FBPA-Fr in most of normal organs except the brain region, which was close to the tumor during HIFU exposure (Table 3). The brain accumulation in the HIFU-treated mice also peaked at 60 min p.i., and is about 3-fold higher compared with that in the control. These findings were consistent with those observed in the microPET imaging study.
To assess the possible tissue injury due to the exposure under pulsed-HIFU, samples of tumor and brain that was close to the focused zone were sectioned. Tumor sections were immunohistochemically stained with CD31 and Ki-67 and then examined with microscopy (Fig. 3a). The histological morphology and the expression of CD31 and Ki67 in xenograft tumors of the HIFU-treated mice and the control were not significantly different, suggesting that a 2-min pulsed-HIFU exposure neither retard the angiogenesis nor slow down the growth of tumor till 4 h post HIFU treatment. The histological analysis of brain, which is partly included in the pulsed HIFU treatment zone, did not reveal any perceptible morphological change after HIFU exposure (Fig. 3b).
Discussion
BNCT is a binary therapy for the tumor treatment and it may be a promising solution to the cancers with drug-resistance. In this management, a relatively high amount of boron atoms must be delivered to the tumor lesion, while maintaining low levels in the normal tissues to guarantee a successful treatment outcome. A high response rate (60~83%) has been reported for recurrent H&N cancer after BNCT . Pulsed-HIFU is increasingly being used to improve the delivery of macromolecules, such as antibody and liposomal drug , to solid tumor in preclinical studies, while the effect of pulsed-HIFU on small molecules, especially boron-containing drug, only received less attention. Therefore, this study investigated the effect of pulsed-HIFU on the accumulation of BPA-Fr in tumor using 18F-FBPA-Fr PET imaging.
In this study, a mouse model bearing an orthotopic SASC03 xenograft tumor in the buccal pouch was established to mimic the clinical patients with H&N cancer. Orthotopic SASC03 xenograft would invade the surrounding tissues; hinder the mouse from eating food, lead to significant body weight loss and pass away within three weeks after tumor cells inoculation. However, the orthotopic tumor grows in the buccal pouch is difficult to measure using calipers, the 18F-FDG microPET was applied instead for longitudinally monitoring the SASC03 xenograft tumor growth
(Fig. 1). Active cellular uptake of 18F-FBPA-Fr in SASC03 human tongue squamous carcinoma cells (Table 1) and the apparent in vivo accumulation in orthotopic xenograft in microPET images (Fig. 2) indicate the feasibility of treating this type of H&N with BNCT.
Both microPET imaging (Fig. 2) and biodistribution studies (Table 2) revealed that preexposure of SASC03 xenograft to pulsed-HIFU followed by immediate intravenous administration of 18F-FBPA-Fr resulted in enhanced tumor accumulation. The AUC of tumor derived from biodistribution study showed that a single 2-min pulsed-HIFU exposure resulted in a 40% increased tumor accumulation within 4 h p.i. compared with the control (Table 3), while sparing the normal organs that were critical in 18F-FBPA-Fr biodistribution, such as pancreas and liver (Table 2). Kankaanranta et al. reported that BNCT with a 2-h infusion of BPA-Fr dose up to 400 mg/kg is feasible in the treatment of locally recurred, inoperable and previously irradiated H&N cancer in clinic . In this proof-of-concept study, although bolus injection of 18F-FBPA-Fr was used instead of infusion, the elevated tumor-to-normal tissues ratios in pulsed-HIFU-treated group suggested that pulsed-HIFU can enhance the delivery of BPA-Fr in the targeted region, and may provide benefit in clinical BNCT for treating H&N cancer. Further studies are needed to build the optimal protocol of HIFU-aided BNCT.
Several studies have employed drug delivery systems to achieve increased tumor accumulation of boron-containing drugs in vivo, for example, liposome , micelle , and carbon nanotube . Though these nanoparticulate drug carrier-driven strategies may provide an enhanced and prolonged tumor uptake, different distribution patterns and pharmacokinetic profiles compared with the original boron drug forced these nanosized “new drugs" to face a long-period preclinical study. For example, significant uptake of nanosized drugs in liver due to high expression of reticuloendothelial system was observed. The liver toxicity may become an emerging issue for clinical trial. On the contrary, HIFU has been routinely applied for prostate thermal ablation and hepatoma treatment. Thus, the combination of BNCT and HIFU could be applied to patients with H&N cancers without time-consuming “new drug" development processes and may be closer to clinical trial.
Two possible mechanisms that may account for HIFU-induced enhanced drug accumulation in tumor region are (1) the widening of the endothelial junction , and (2) the reduction of the interstitial fluid pressure (IFP) . High IFP is known to hinder extravasation of the therapeutics from blood vessel to lesion site despite leakier neovasculature of tumor . Since BPA-Fr is a small molecule, IFP may play a pivotal role in boron drug accumulation. Therefore, the pulsed-HIFU approach may improve the therapeutic efficacy of BNCT through physical change rather than via biological
approach. The similar tumor uptake of 18F-FBPA-Fr between the HIFU-treated and the control group at 4 h p.i. implied that the effect of HIFU on reducing IFP is only transient (Table 2). Khaibullina et al. found that A431 tumor uptake of 111In-MX-B3 monoclonal antibody peaked at 24 h p.i. in HIFU-treated mice, but decreased to the level of the control tumor at 120 h p.i. . Lin et al. also demonstrated that the concentration of liposomal doxorubicin in HIFU-treated CT-26 xenograft decreased to about the same level as that of the control at 48 h after HIFU exposure . Although the effective period of HIFU-enhanced tumor accumulation is shorter for small molecule (eg. 18F-FBPA-Fr) compared to that for macromolecules, pulsed-HIFU is just perfect for BNCT employing BPA-Fr (Table 2).
Immunohistochemical staining of the tumor xenograft sections of HIFU-exposed mice showed that pulsed-HIFU did not influence angiogenesis and proliferation of tumor within 4 h post HIFU treatment, however, the long-term monitoring should be performed in the future (Fig. 3). In addition, the HIFU-treated tumors did not display visible histological differences compared with the control at 4 h after HIFU exposure, indicating that damaged (leaky) tumor structure is not the reason of HIFU-induced enhanced boron drug accumulation. Regarding the higher brain uptake of boron drug (up to three folds at 60 min p.i.) in the HIFU-treated mice, a concern about the brain injury may arise. The focal zone of the therapeutic transducer was in the shape of an
elongated ellipsoid with a radial diameter of 3 mm and an axial of 26 mm. The relatively small size of the SASC03 xenografts, which were comparable with the focal zone of the HIFU device, may account for the increment in the pulsed-HIFU-spread brain region. Histological examination of the brain sections near the HIFU-exposed tumor region did not reveal perceptible morphological change in this study (Fig. 3). This side effect may be of less importance in clinical human trial because the effective focal zone of this HIFU system would not reach the brain area. However, focused-HIFU may have difficulties when treating patients with large or diffused tumor lesions. Furthermore, owing to the large diameter (14 cm) of epithermal neutron beam for BNCT treatment at the Tsing Hua Open-pool Reactor (THOR) in Taiwan, it is hard to mimic the clinical situation when the whole animal body was irradiated by neutron beam.
Conclusion
This study demonstrated that noninvasive and nondestructive pretreatment with pulsed-HIFU significantly enhanced the delivery of boron-containing drug in an orthotopic SASC03 human tongue squamous carcinoma-bearing mouse model, suggesting that sonication may enhance the therapeutic efficacy of clinical BNCT and was beneficial to the patients with H&N cancer.
Acknowledgement
The authors thank the financial support from Taipei Veterans General Hospital, Taipei, Taiwan (V100A-045, and VGHUST101-G1-2-3). The authors also appreciate the technical support from the Taiwan Mouse Clinic which is funded by the National Research Program for Biopharmaceuticals (NRPB) at the National Science Council (NSC) of Taiwan.
Conflict of interest
References
1. Hatanaka H, Nakagawa Y (1994) Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. Int J Radiat Oncol Biol Phys 28:1061-1066.
2. Kato I, Ono K, Sakurai Y et al. (2004) Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot 61:1069-1073.
3. Yoshino K, Suzuki A, Mori Y et al. (1989) Improvement of solubility of p-boronophenylalanine by complex formation with monosaccharides. Strahlenther Onkol 165:127-129.
4. Hsieh CH, Chen YF, Chen FD et al. (2005) Evaluation of pharmacokinetics of 4-borono-2-18F-fluoro-L-phenylalanine for boron neutron capture therapy in a glioma-bearing rat model with hyperosmolar blood-brain barrier disruption. J Nucl Med 46:1858-1865.
5. Wang HE, Liao AH, Deng WP et al. (2004) Evaluation of 4-borono-2-18 F-fluoro-L-phenylalanine-fructose as a probe for boron neutron capture therapy in a glioma-bearing rat model. J Nucl Med 45:302-308.
6. Kennedy JE (2005) High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 5:321-327.
7. Dromi S, Frenkel V, Luk A et al. (2007) Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and
antitumor effect. Clin Cancer Res 13:2722-2727.
8. Poff JA, Allen CT, Traughber B et al. (2008) Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology 248:485-491.
9. Lemaire C, Damhaut P, Lauricekka B et al. (2002) Fast [18F]FDG synthesis by alkaline hydrolysis on a low polarity solid phase support. J Label Compd Radiopharm 45:435-447.
10. Tournier I, Bernuau D, Poliard A, et al. (1987) Detection of albumin mRNAs in rat liver by in situ hybridization: usefulness of paraffin embedding and
comparison of various fixation procedures. J Histochem Cytochem 35:453-459. 11. Kankaanranta L, Seppala T, Koivunoro H et al. (2007) Boron neutron capture
therapy in the treatment of locally recurred head and neck cancer. Int J Radiat Oncol Biol Phys 69:475-482.
12. Wang S, Shin IS, Hancock H et al. (2012) Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. J Control Release 162:218-224. 13. Khaibullina A, Jang B , Sun H et al. (2008) Pulsed high-intensity focused
ultrasound enhances uptake of radiolabeled monoclonal antibody to human epidermoid tumor in nude mice. J Nucl Med 49:295-302.
microbubbles on the treatments of anticancer nanodrug in mouse tumors. Nanomedicine 8:900-907.
15. Ranjan A, Jacobs GC, Woods DL et al. (2012) Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release 158:487-494. 16. Maruyama K, Ishida O, Kasaoka S et al. (2004) Intracellular targeting of sodium
mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J Control Release 98:195-207.
17. Feng B, Tomizawa K, Michiue H et al. (2009) Delivery of sodium borocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials 30:1746-1755.
18. Sumitani S, Oishi M, Yaguchi T et al. (2012) Pharmacokinetics of core-polymerized, boron-conjugated micelles designed for boron neutron capture therapy for cancer. Biomaterials 33:3568-3577.
19. Yinghuai Z, Peng AT, Carpenter K et al. (2005) Substituted carborane-appended water-soluble single-wall carbon nanotubes: new approach to boron neutron capture therapy drug delivery. J Am Chem Soc 127:9875-9880.
20. Frenkel V, Kimmel E, Iger Y (2000) Ultrasound-induced intercellular space widening in fish epidermis. Ultrasound Med Biol 26:473-480.
21. Watson KD, Lai CY, Qin S et al. (2012) Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res 72:1485-1493. 22. Mesiwala AH, Farrell L, Wenzel H J et al. (2002) High-intensity focused
ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol 28:389-400.
23. Boucher Y, Baxter LT, Jain RK (1990) Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res 50:4478-4484.
Figure Legend
Figure 1. Dynamic microPET/CT imaging of orthotopic SASC03 tongue squamous carcinoma-bearing mice after an intravenous injection of approximately 3.7 MBq of 18F-FDG witnessed the growth of SASC03 xenograft at 10 days post tumor cells inoculation. White arrows indicate tumor lesions.
Figure 2. MicroPET imaging of the pulse HIFU-treated mice and the control (n≧5 in each group) at 15, 30, 60, 120, and 240 min after an intravenous injection of approximately 3.7 MBq of 18F-FBPA-Fr. White arrows indicate tumor lesions.
Figure 3. Histochemical staining of the tissue samples obtained from pulsed-HIFU-treated mice and the control. a SASC03 tumor was excised from mice and examined after immunohistochemical staining with Ki67 and CD31 to assess the difference in proliferation rate and angiogenesis. b The brain tissue close to tumor was also examined to evaluate the tissue injury after pulsed-HIFU exposure. H&E staining was performed to evaluate the morphological changes in brain.
Table 1. The cellular uptake of 18F-FBPA-Fr and 18F-FDG in SASC03 human tongue squamous carcinoma cells. The culture medium and cells were collected after
incubating with 37 kBq of 18F-FBPA-Fr or 18F-FDG in designated time points (15, 30, 60 and 120 min). The cellular uptake of radiotracer was expressed as the percentage of administered dose in the medium that had accumulated in one million cells (% AD/106 cells).
Time (min) Radiotracer 15 30 60 120
18F-FBPA-Fr 0.83 ± 0.06 1.13 ± 0.12 1.25 ± 0.11 1.42 ± 0.12 18F-FDG 1.02 ± 0.08 1.36 ± 0.14 1.68 ± 0.20 1.81 ± 0.22 Values were presented as mean±SD, n = 5.
Table 2. Radioactivity distribution in SASC03 tongue squamous carcinoma-bearing mice, a without pulsed HIFU exposure, and b with pulsed HIFU exposure, after an intravenous injection of approximately 3.7 MBq of 18F-FBPA-Fr. The radioactivity in these tissue samples was normalized to sample weight and expressed as the
percentage of injection dose per gram of tissue (%ID/g). (n = 4)
a Time (min) Tissues 15 30 60 240 Blood 6.75 ± 0.32 4.87 ± 0.19 3.61 ± 0.13 1.59 ± 0.10 Heart 3.66 ± 0.12 3.09 ± 0.10 2.95 ± 0.08 1.58 ± 0.18 Lung 5.38 ± 0.35 4.09 ± 0.13 2.84 ± 0.19 2.26 ± 0.23 Liver 9.46 ± 0.35 8.13 ± 0.15 8.68 ± 1.70 4.14 ± 0.24 Stomach 9.13 ± 0.17 8.28 ± 0.23 5.27 ± 0.22 4.40 ± 0.40 S.I 8.80 ± 0.29 5.84 ± 0.20 5.75 ± 0.11 3.84 ± 0.17 L.I 5.31 ± 0.47 5.61 ± 0.26 5.47 ± 0.22 3.53 ± 0.26 Spleen 8.22 ± 0.15 7.74 ± 0.20 5.79 ± 0.20 3.25 ± 0.17 Pancreas 50.56 ± 1.48 45.67 ± 0.46 44.36 ± 3.24 31.34 ± 2.04 Kidney 32.00 ± 1.89 20.25 ± 0.91 12.24 ± 1.70 4.46 ± 0.23 Muscle 1.52 ± 0.13 1.18 ± 0.10 1.17 ± 0.08 1.05 ± 0.02 Tumor 7.77 ± 1.05 7.65 ± 0.38 9.69 ± 0.50 8.55 ± 0.09 Bone 1.14 ± 0.06 1.11 ± 0.03 1.07 ± 0.02 2.17 ± 0.11 Marrow 2.24 ± 1.08 3.53 ± 0.19 3.93 ± 0.27 4.62 ± 0.38 Brain 1.01 ± 0.18 0.94 ± 0.08 0.92 ± 0.14 0.72 ± 0.05 T/M 5.17 ± 1.13 6.53 ± 0.27 8.28 ± 0.46 8.15 ± 0.10
b Time (min) Tissues 15 30 60 240 Blood 6.32 ± 0.14 5.33 ± 0.28 3.15 ± 0.16 2.65 ± 0.14 Heart 3.55 ± 0.23 3.71 ± 0.14 3.37 ± 0.24 2.61 ± 0.12 Lung 4.94 ± 0.24 4.62 ± 0.22 3.47 ± 0.29 2.53 ± 0.17 Liver 10.04 ± 0.38 8.37 ± 0.18 8.13 ± 0.16 4.74 ± 0.15 Stomach 9.11 ± 0.19 8.97 ± 0.14 4.99 ± 0.10 4.45 ± 0.15 S.I 8.69 ± 0.31 5.70 ± 0.19 5.28 ± 0.18 3.54 ± 0.19 L.I 5.47 ± 0.44 5.67 ± 0.24 5.59 ± 0.37 4.16 ± 0.37 Spleen 8.33 ± 0.25 7.78 ± 0.22 4.40 ± 0.35 3.21 ± 0.27 Pancreas 54.65 ± 5.62 43.37 ± 2.29 43.95 ± 3.13 35.56 ± 3.55 Kidney 34.19 ± 4.75 21.87 ± 1.90 13.21 ± 2.21 7.11 ± 1.28 Muscle 1.52 ± 0.23 1.24 ± 0.08 1.22 ± 0.07 1.10 ± 0.07 Tumor 6.05 ± 0.31 8.98 ± 0.30 17.12 ± 1.32 9.50 ± 0.37 Bone 1.12 ± 0.09 1.33 ± 0.08 1.87 ± 0.03 2.54 ± 0.15 Marrow 1.59 ± 0.07 3.46 ± 0.32 2.43 ± 0.24 3.87 ± 0.97 Brain 0.75 ± 0.07 2.67 ± 0.21 2.81 ± 0.11 1.71 ± 0.09 T/M 4.06 ± 0.73 7.25 ± 0.24 14.06 ± 1.18 8.64 ± 0.48
Table 3. Area under the curve (AUC) of tissues post intravenous injection of 3.7MBq of 18F-FBPA-Fr in SASC03 tongue squamous carcinoma-bearing mice with/without pulsed-HIFU treatment (n = 4). AUC values (h·%ID/g) were calculated based on the results of biodistribution study using the WinNonlin program.
pulsed-HIFU
Tissue With Without
Tumor 39.93 27.37
Brain 6.79 2.45
Liver 19.30 19.63