Homologous and Heterologous Neutralization
Antibody Responses After Immunization With
Japanese Encephalitis Vaccine Among
Taiwan Children
Chia-Chi Ku, Chwan-Chuen King, Ching-Yuang Lin, Huei-Chu Hsu, Li-Yen Chen, Yi-Yuen Yueh, Gwong-Jen J. Chang
Institute of Public Health, National Taiwan University (C.-C.K., C.X.K.), National Institute of Preventive Medicine (Y.-Y.Y.), Departments of Medical Research and Pediatrics, Veterans General Hospital (C.-Y.L.), Taipei and Lambay Islet Health Center (H.X.H.), and Tungkang Health Center (L.-Y.C.), Pintung, Taiwan, Republic of China; Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, Centers for Disease Control and
Prevention, Public Health Service, U.S. Department of Health and Human Services, Fort Collins, Colorado (G.
J.
J.C.)Because 21 immunized children (13%) among the 162 confirmed Japanese encephalitis (JE) cases during 1986-1991 occurred in Taiwan, we collected 320 serum samples from Taiwan chil- dren aged 15-31 and 27-44 months immediately before the 1st dose (n = 41) and 1-3 months af-
ter the 2nd dose (n = 78, 27 pairs), and immedi- ately before (n = 58) and 1-3 months after the 3rd dose (n = 143,44 pairs) to determine neutral- ization antibody (Nt Ab) against the Nakayama (N) and Beijing-I (6) strains and two Taiwan wild type JE viruses (JEV): CC-27 and CH-1392. Our Nt results showed that (1) B vaccine stimulated a better homologous Ab response than N vaccine for Nt Ab seropositivity rate (NASR), produced a higher level of Nt titer after the primary immuni- zation [2 doses = 1 0 0 % ~ ~ . 91%, geometric mean
titer (GMT) = 115 vs. 221, had a greater booster effect (3 doses: 100% vs. 95%; GMT = 320 vs. 33), and showed a better capability to neutralize two local Taiwan JEV strains, particularly only
after 3 doses (ave. NASR for B vs. N = 90% vs. 10%; and GMT for B vs. N = 154 vs. 1); (2) the
two wild type JEV strains had different plaque morphology and antigenic variation and the CC-27 strain was not neutralized as well as the CH-1392 strain after 3 doses of vaccine (BBB or
NNN or NNB); and (3) 30% of the children had lost JEV Nt Ab one year after the 2nd dose of N vaccine and natural infection with JE virus did occur among those children after immunization. In conclusion, (1) three doses of mouse-brain vaccine are the minimum requirement to protect children against the local Taiwan JEV; (2) the best strain for a JE vaccine depends on level of Nt Ab
it induced, the molecular epidemiology and anti- genic variation of the JEV in each local area; and 0 1994 WILEY-LISS, INC.
(3) future vaccine must produce better B- and T-cell memory.
o
1994 Wiley-Liss, Inc.KEY WORDS: Japanese encephalitis virus, vaccine memory, immunization, antigenic variation
INTRODUCTION
Japanese encephalitis (JE) virus, the most important cause of epidemic arboviral encephalitis in Asia, has a wide clinical spectrum, ranging from asymptomatic in- fection to permanent neurologic sequelae with a high case fatality rate of 30%-70% [Umenai et al., 1985; Monath, 19881. A mouse-brain vaccine has successfully controlled J E viral infection among human populations in Japan, Korea, and Taiwan since 1968 [Oya, 19881. The increased incidence rates of J E in India, Nepal, Sri Lanka and northern Thailand in recent years has raised public health attention and stimulated a search for effective prevention and control strategies [Vaughn and Hoke, 19921.
Hsu et al. [1971] conducted the first randomized con- trol trial of J E vaccine in Taiwan in 1965 and demon- strated its 80% vaccine efficacy with 2-dose immuniza- tion. Therefore, the Department of Health in the Republic of China in Taiwan area implemented a na- tionwide immunization program for 2-year-old chil- dren, using the J E vaccine (Nakayama strain) pro- duced by the National Institute of Preventive Medicine
Accepted for publication August 22,1993.
Address reprint requests to Dr. Chwan-Chuen King, Institute
of Public Health, National Taiwan University, 1 Jen-Ai Road Sec.
123 TABLE I. Strains of J E Vaccine Delivered in Various
Taiwan Geographic Areas During 1990-1992
Vaccine strains used in various areas
Year Taan Hsintien Tungkang Lambay Islet
1991 Nakayama Nakayamak NR NR
1992 Nakayama Beijing-1 Beijing- 1 Beijing- 1 NR = no record on which strain of J E vaccine (Nakayama andior Beijing-1) was used. The health personnel in Tungkang and Lambay Islet did not document the vaccine strain used in the first two doses of J E vaccine for each child that they delivered in 1990 and 1991 but they did document “the Beijing-1 vaccine” during immunization in 1992.
Beijing-1
(NIPM) since 1968. The high J E vaccine coverage rates (73%-92% during 1970-1991) had decreased the dis- ease incidence rate from 81100,000 in 1967 to 0.171 100,000 in 1991 [Okuno et al., 1975; National Quaran-
tine Service in R.O.C., 19921. However, 21 immunized children were reported among 162 (13%) J E confirmed cases during 1986-1991 (NIPM, 1992, unpublished data). This aroused strong concern about the effective- ness of the Nakayama strain mouse-brain vaccine.
Because of the possibility that antigenic variation in different J E virus (JEV) strains may influence vaccine efficacy [Okuno et al., 1968; Kobayashi et al., 1984; Calisher et al., 1989; Chen et al., 19901, Japanese virol- ogists prepared a J E vaccine made with Beijing-1 virus strain to induce a more immunogenic antibody with a wider neutralizing spectrum against other strains of JEV [Kitano et al., 19861. However, neutralization ca- pability of the J E vaccine in Taiwan for either the vac- cine or local wild JEV strains has never been evalu- ated. To provide information needed to improve the J E immunization programs, we measured the levels and duration of neutralization antibody (Nt Ab) among Tai- wan children after immunization with J E Nakayama (N) or Beijing-1 (B) vaccine and its protective efficacy from natural infection by cross-neutralization against wild type JEV.
MATERIALS AND METHODS Japanese Encephalitis Vaccine
An inactivated, liquid, purified mouse-brain J E N vaccine produced by the NIPM in Taiwan [Hsu, 19691 was routinely used in local health centers until 1988. Since the lyophilized-powdered form of B vaccine (BIKEN) began to be imported from Japan in 1989, two strains of J E vaccine were randomly distributed in Tai- wan areas (Table I). The potency test for each prepara- tion of the two vaccines had been performed according to a standard method before human usage [Takaku et al., 1971; Chow et al., 19861. The immunogenic compo- nent of antigen in these J E vaccines had not been docu- mented. However, the optical density values of the total protein (wave length is 280 nm) in stock solution of the two vaccines were 1.05 for the N vaccine and 0.96 for the B vaccine.
Immunization Schedule and Records
All children aged 15-26 months in Taiwan are re- quired to receive J E immunization during the period March through June of every year. Two shots of one- half dose (0.5 ml for N vaccine or 0.25 ml for B vaccine)
are given on days 0 and 14 for the primary immuniza- tion. A full dose (1 ml for N vaccine or 0.5 ml for B vaccine) is administered one year after the 2nd dose as a booster immunization.
Each child’s name, age, sex, dates at each immuniza- tion, and strain of J E vaccine were obtained from the immunization records a t local health centers. The health personnel in Tungkang and Lambay Islet did not document the vaccine strain used in the first 2 doses during vaccination in 1990 and 1991, but they did doc- ument “the Beijing-1 vaccine” during immunization in 1992. For children in Taan and Hsintien, only those who had valid computerized immunization records on dose and vaccine strain given in 1991 and 1992 were recruited (Table I).
Serum Samples
We chose four local health centers located in north- ern (Taan and Hsintien, metropolitan areas) and south- ern Taiwan (Tungkang, a rural area) as well as an isolated island (Lambay Islet with high emigrant popu- lation) during 1991-1992 for evaluation. All parents of children gave informed consent to participate in the study. One to two milliliter blood samples were col- lected from each subject a t various time points. The blood samples were centrifuged a t 3,000 rpm for 10 mins and the serum was stored at -20°C for measuring Nt Ab.
Humoral immune responses after the primary and booster immunizations were evaluated simultaneously by taking serum samples from children before and after vaccination. Samples were collected a t either of two time periods relative to immunization. Samples from group I were taken immediately before the first dose of J E vaccine (n = 41) and 1-3 months after the second dose (n = 78, 27 pairs). Samples from group I1 were taken immediately before (n = 58) and 1-3 months af- ter (n = 143, 44 pairs) the third dose (Table 11). Nt Ab tests against the B and N strains were done simulta- neously. Serum samples from Lambay Islet in 1992 were tested against N strain alone for evaluating their heterologous neutralizing response because there was an insufficient volume of sera (n = 29). If enough serum was left after being tested against the N strain, serum samples from Lambay Islet were tested for Nt Ab against CC-27 strain of JEV. Only children with com- plete immunization records and confirmed serologic data were included in the analysis (Table 111).
Viruses
The N strain, provided from the NIPM in Taiwan, was further passaged twice in BHK cells and three times in porcine kidney cells (obtained from the CDC,
124
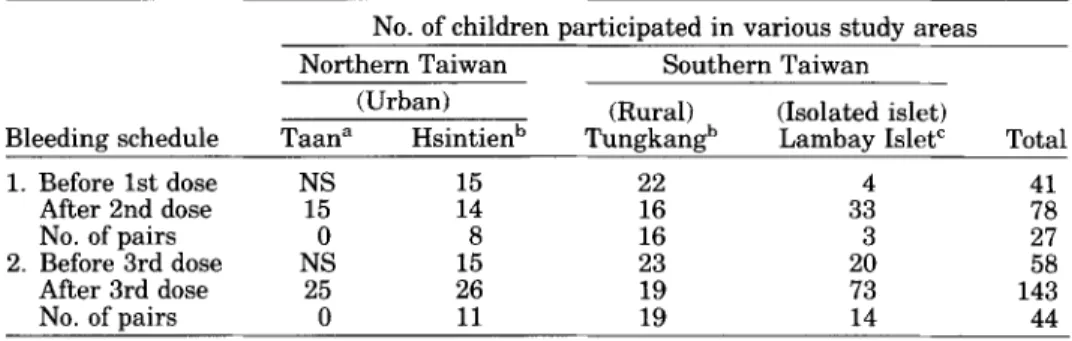
TABLE 11. Subjects and Bleeding Schedules in JE Vaccine Evaluation Study in Taiwan Areas During 1991-1992*
No. of children participated in various study areas Northern Taiwan Southern Taiwan
(Rural) (Isolated islet) (Urban)
Bleeding schedule Taana Hsintienb TungkanPb Lambav Islet' Total
1. Before 1st dose NS 15 22 4 41 After 2nd dose 15 14 16 33 78 No. of pairs 0 8 16 3 27 2. Before 3rd dose NS 15 23 20 58 After 3rd dose 25 26 19 73 143 No. of pairs 0 11 19 14 44
*NS = no serum samples were taken.
"Serum samples were obtained in October 1992.
bBoth the first serum samples right before the 1st and the 3rd dose of J E immunization were taken from children in March 1992; the 2nd serum samples were obtained at one month after the 2nd and the 3rd dose.
'30 of 33 serum samples (2 months after the 2nd dose) and 28 of 73 serum samples (2 months after the
3rd dose) were taken in July 1991; the others were taken in July 1992. Serum samples taken right before the 1st and the 3rd dose were in April 1992.
TABLE 111. Number of Subjects With Complete Immunization Records and Number of Serum Samples Tested Against Four Strains of JEV by Plaque
Reduction Neutralization Test in Four Different Bleeding GrouDs
No. with
Time to complete
take blood immunization Vaccine strain Taiwan wild type JEV (sample size) record Nakayama Beijing-1 CC-27 CH-1392
40 39 13 13
A. Before 1st dose -
B. After 2nd dose 70 70 67 40 36
No. of sera tested against four JEV strainsa
b (n = 41) (n = 78) (n = 58) C. Before 3rd dose 55" _ . 48 38 17 22 D. After 3rd dose 116" 116 69 83 59 (n = 143)
"All children serum samples tested for Nt antibody against Beijing-1 strain were tested against Na- kayama strain simultaneously but the serum samples from Lambay Islet in 1992 were tested against Nakayama strain alone because of insufficient volume. Serum samples with enough volume were employed to test for NtAb against CC-27 or CH-1392 strain.
bPre-immunization.
'23/55 serum samples taken from Tungkang area and 461116 serum samples from Tungkang and Lambay Islet did not document on the strain of J E vaccine used in the first 2 doses.
USA) in the Virology Laboratory at National Taiwan University Hospital. The B strain, which was obtained originally from the National Institute of Health (NIH) in Japan in 1982, had been passaged 51 times in sheep embryo kidney cell and 38 times in adult mouse brain before it was grown once in C6/36 cells in our lab. Two local strains of wild type JEV, CC-27 and CH-1392, provided from the NIPM, were isolated in C6/36 cells from Culex mosquitoes collected in southern (1983) and central (1990) Taiwan, respectively (Table IV). All the infected culture supernatants were aliquoted to 0.1 mll vial, stored at -7O"C, and titrated by plaque assay in BHK-21 clone 15 cells (provided from the CDC, USA). These four strains of JEV, exhibiting variation in plaque size, morphology, and viral yields (plaque form- ing unit, PFU) in the same assay system, were used to evaluate Nt Ab (Fig. 1).
Plaque Reduction Neutralization Test (PRNT)
BHK-21 clone 15 cells were used for plaque assay and plaque reduction neutralization test (PRNT) [Russell and Nisalak, 19671. Briefly, cell suspensions (1.0 x
lo5
ml/well) were cultured in a 24-well polystyrene plate (Costar, Cambridge, MA, USA) for 48 h r to form a monolayer. The diluted test serum and virus mixture was added, in duplicate, to BHK-21 monolayer and then was incubated at 37°C for 1.5 h r [Morens et al., 19851. The formalin-fixed infected and uninfected cells were stained with 1% crystal violet and plaque num- bers were counted. A Nt titer 2 10 and a 70% plaque reduction, compared to the virus control, was defined as "Nt Ab seropositive." All Nt Ab titers higher than 320 were recorded as 320 to calculate the geometric mean titer (GMT), which is equal to the antilog of the mean of the logarithms of the Nt Ab titers [Bahn, 19851. In125 TABLE IV. Sources and Passages of JE Viruses Used in Evaluation of JE Vaccine.by Plaque Reduction Neutralization
Test*
JEV Year Location Source Passage
A. Vaccine strain
Att-2 BHK-3 PS"
Nakayama 1935 Japan, Nakayama Human brain
Beijing-1 1949 China, Beijing Human brain 51 SEKC-38amb-C6/36
cc-27 1983 Southern Taiwan Culex tritaeniorhynchus 3 C6/36
CH-1392 1990 Central Taiwan Culex tritaeniorhynchus 3 (26136
B. Wild strain
*Att = attenuated strain; BHK = baby hamster kidney cell; PS = porcine kidney cell; SEKC = sheep embryo kidney cell; amb = adult mouse brain.
"The Nakayama vaccine strain, provided from NIPM in Taiwan, had been attenuated in Japan and was further passaged twice i n BHK cells and 3 times in PS cells in the Virology Laboratory a t National Taiwan University Hospital.
Fig. 1. Vaccine strains (Nakayama and Beijing-1) of J E virus had more homogeneous morphology and plaque size than Taiwan wild strain (CC-27 and CH-1392) in BHK-21, clone 15 cells. Nakayama strain had larger plaque size than Beijing-1 strain; CH-1392 strain had more opaque morphology and smaller size than CC-27 strain. A Vaccine strain in a dilution, 200 pl/well (Al: Beijing-1 strain with viral yield 8 x los PFU/ml; A2: Nakayama strain with viral yield 1 X 10' PFU/ml). B: Wild type strain in a dilution, 200 &well (Bl: CH-1392 strain with
viral yield 3 x lo6 PFUlml; B2 CC-27 strain with viral yield 1 x 10'PFUlml). C Cell control (200 pl of diluent was added per well).
addition, the positive serum samples against the N (Nt titer 3 1,280) or against the B (Nt titer b 2,560)
strains, collected from mouse ascitic fluid and provided from the NIH in Japan, were employed as a n internal positive control in each test. Three different titers of the subjects' serum samples (Nt titers S 10,80, and 3 320) collected from this study and two positive serum Sam- ples obtained from the NIH, Japan, were sent to the CDC, USA, for proficiency testing J E PRNT with a
blinded code number. There was 100% agreement.
RESULTS
Pre-Immunization and Effects of JE
Primary Immunization
The serum samples obtained before the first dose of J E vaccine were negative for Nt Ab against both of the vaccine strains [N and B, n = 401 or both of the Taiwan wild J E V strains (CC-27 and CH-1392, n = 13) in all subjects (n = 40). This indicates that none of the sub- jects had ever acquired J E V infection at the beginning
126
80
60 -
-
(a) 2 doses of Nakayama vaccine
40 - #?
-
2 0 -z
c.-
- -z
r 0(c) 3 doses of Nakayama vaccine
.-
5
5 60 80 60 2040il
0 <lo 10-20 40-80 m o - - Nt titer(b) 2 doses of Beijing-1 vaccine
load
“-8
2 0 010-20 40-80 *-I60
40
(d) 3 doses of Beijiw-1 vaccine
40
t
2ou
0 40 10-20 40-60Nt titer
Virus strain
0
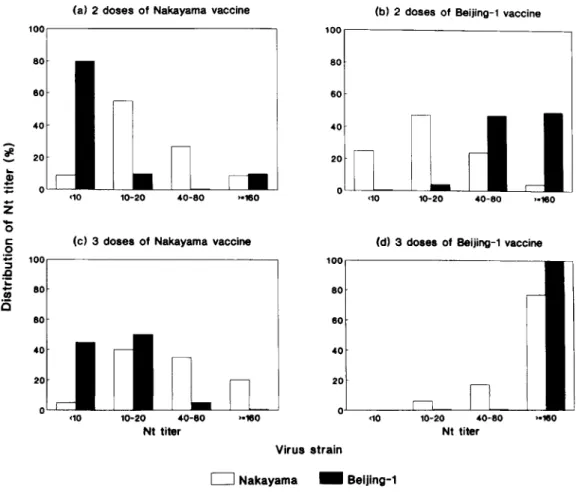
Nakayama Beijing-1Fig. 2. Distribution of Nt titers against the homologous and the heterologous strains of J E V among Taiwan children whose serum samples were collected at 1-3 months after 2 doses of JE (a) Nakayama (serum 2N) and (b) Beijing-1 (serum 2B) vaccines; 1 3 months after 3 doses of J E ( c ) Nakayama and (d) Beijing-1 vaccines against the Nakayama (open bars) and the Beijing-1 virus (filled bars), respectively.
Children immunized with the N (serum 2N, n = 11) or B (serum 2B, n = 59) vaccine a t 1 3 months after their second dose had 91% (10/11) and 100% (57/57) Nt Ab seropsitivity rates against the homologous virus, respectively. Eighty-two percent (9/11) of the serum 2N Nt Ab titers ranged from 10 to 80, whereas all of the serum 2B titers were 2 20 and 49% (28/57) of them were higher than 160. However, the Nt Ab seropositiv- ity rates to the heterologous JEV antigens, using serum 2N against the B strain and serum 2B against the N strain, were 20% (2110) and 75% (44/59), respectively (Fig. 2; Table V). Nt titers against the heterologous virus strain were lower than Nt titers against the ho- mologous virus strain for both serum 2N and serum 2B.
Effects of JE Booster Immunization
Of 58 subjects who had sufficient volume and com- plete immunization records, 48 serum samples were collected at one year after the second dose (e.g., immedi- ately before the 3rd dose) and were tested for JEV Nt Ab. The Nt Ab seropositivity rates against the N and B strains in 25 children, living in Hsintien and immu- nized with 2 doses of B vaccine, were 88% (22/25, GMT = 16) and 100% (15115, GMT = 79), respectively.
The Nt Ab seropositivity rates against the N and B
strains among the remaining 23 children living in Tungkang, for whom there was no documentation on the JEV strains, were 70% (16/23, GMT = 5) and 4% (1/23, GMT =
11,
respectively, suggesting that theywere immunized with 2 doses of N vaccine (Table V). Of 143 serum samples taken a t 1-3 months after the third dose of J E vaccine, 116 were analyzed. The Nt Ab seropositivity rates in the 46 Tungkang and Lambay Islet children, who were given an unknown vaccine strain in the primary immunization and bled in 1991 after the booster dose with B vaccine, were 100% (46146, GMT = 85) against the N strain and 86% (25/29, GMT = 27) against the B strain (Table V). In contrast, all of the 50 children known to be immunized with 3 doses of B vaccine had a
loo%,
against either the N (n = 50) o r B (n = 20) strain. The Nt titers in this group showed that 96% of them ranged from 40 to higher than 160 against the N virus (GMT = 1821, and all of thesechildren had Nt titers 2 320 against the B virus (Fig. 2d). The 20 children immunized with all 3 doses of N vaccine had 95% (19/20) and 55% (11/20) Nt Ab seropos- itivity rates against N and B virus, respectively, with Nt titers clustered around 10-80 (75%, GMT=33) against the N strain and 10-20 (50%, GMT = 4)
the children in Tungkang and Lambay Islet for whom no documentation of the vaccine used in the primary immunization was available, had been given with 2 doses of the N vaccine.
Of the 44 paired serum samples collected immedi- ately before and 1-3 months after the 3rd vaccine dose, 30 pairs had both complete vaccination records and a sufficient serum volume remaining after the previous Nt Ab testing to be analyzed for the effectiveness of the booster immunization. Among the 18 paired serum samples collected from Tungkang children, the pre- booster serum samples against the N strain had Nt titers of 33% (n = 6) at
<
10,56% (n = 10) a t 10-20 and 11% (n = 2) a t 40-80. In contrast, the pre-booster se-rum samples against the B strain had Nt titer of 94% (n = 17) a t
<
10 and 6% (n = 1) at 10 (Fig. 3a). All 18 of these children were given a booster vaccine with one dose of B strain. The serum samples taken after the booster dose showed seroconversion and an increase in Nt titers against both the N [6%, (n = 1) a t 10-20, 11% (n = 2) a t 40-80 and 83% (n = 15) a t 2 1601 and the Bstrains 128%, (n = 5 ) at 10-20, 55% (n = 10) at 40-80 and 17% (n = 3) at 2 1601 for all 18 children (Fig. 3a). Again, these data reconfirmed that Tungkang children had received the N vaccine in their primary immuniza- tion.
Of the remaining 12 paired serum samples, which were taken from Hsintien and Lambay Islet children in 1992, two pairs were seronegative against the N strain a t one year after the first two vaccine doses with an unknown strain and both showed seroconversion after the 3rd dose of B strain when we tested against the same virus. Serum pairs from the other 10 children were Nt seropositive after the pre-booster vaccine doses, Nt titers of 67% (n = 8) a t 10-20 and 16% (n = 2)
a t 40-80 against the N strain. All these Nt titers against the N virus, in either the seroconverted or se- ropositive before the booster dose (n =12), increased to 25% (n = 3) a t 10-20 and 75% (n = 9) at a 160 after the booster immunization. In addition, these 12 children were positive for Nt Ab against the B virus in the pre- booster serum samples [Nt titers were 33% at 10-20 (n = 4), 40-80 (n = 41, and 2 160 (n = 41, respectively], and showed a significant increase in Nt titers against the B virus after the booster immunization [8% (n = 1) at 10-20 and 92% ( n = 11) at 3 160, P = 0.007,
Wilcoxon rank sum test1 (Fig. 3b).
Neutralization Antibody Responses Against Two Taiwan Wild Type JEV in Serum Samples From
Children Immunized With JE Vaccine
The Nt Ab responses and GMTs against wild type JEV (CC-27 and CH-1392) varied in children receiving the different immunization protocols. Children immu- nized with 2 doses of either the N or B vaccine had similar neutralizing responses in both Nt seropositivity rate and GMTs against the CC-27 (CC) (N = 20% vs. B = 10%; GMT: 2 vs. 1) or against the CH-1392 (CH) JEV strains (N = 30% vs. B = 35%; GMT: 2 vs. 4). However, the Nt seropositivity rates and GMTs against
2
E
.- Y [I)>
2
2
2
2
*
Y .r( m al cd N r l O r l t - o m2
rl N I n r l U a t - ~ O rl t - N 01 rl m128
(a) Tmgkang chlldren (n.18)
pro-booat
1 0 0 1
lo-20 40-80
Nt titer
L
,=mo
(b) Haintien and Lambay chlldren (n-12)
pro-boort 10 10-20 40-80 Nt titer
1
-mo Virus strain Nakayama=
Beijing-1Fig. 3. Changing of N t titers in paired children serum samples collected at right before and at 1-3 months after the booster immunization. a: 18 paired serum samples collected from Tungkang children who were boosted with one dose of Beijing-1 vaccine a t one year after they had 2 doses of Nakayama vaccine for the primary immunization. b: 12 paired serum samples collected from Hsintien and Lambay Islet children who were immunized with 3 doses of Beijing-1 vaccine (i.e. boosted with the homologous strain of JEV).
both these two wild JEV strains were poor a t one year after the primary immunization, either with N (CC vs. CH = 0% vs. 10%; GMT: 0 vs. 1) or B vaccine (CC vs. CH = 11% vs. 67%; GMT: 1 vs. 7) because of antibody
waning. In addition, we found t h a t 6 out of 15 Lambay Islet children who were Nt Ab negative after their 2nd dose became seropositive against the N strain immedi- ately before the booster dose, probably because of a natural infection during 1991-1992. The serum sam- ples of these 6 children also had increased Nt titers against the N and CC strains (GMT for N strain = 40,
GMT for CC strain = 17) (data not shown).
Children who received a booster immunization had more cross-reactive neutralizing response with wild type JEV. Moreover, the children who were immunized with 3 doses of B vaccine had a greatest increase in Nt Ab seropositivity rates against the CC and CH strains (CC = 95% vs. CH = 100%; GMT: 123 vs. 195) com- pared to the children immunized with 3 doses of N vaccine (CC = 0% vs. CH = 22%; GMT: 0 vs. 2) and the children immunized with 2 doses of N and one dose of B
vaccine (CC = 84% vs. CH = 90%; GMT: 17 vs. 46) (Ta-
ble V).
DISCUSSION
This serologic evaluation of mouse-brain J E vaccine exhibited four significant findings: (1) B vaccine stimu- lated a better humoral immune response than Na- kayama (N) vaccine in Nt Ab titers (GMT: 115 vs. 22) and Nt Ab seropositivity rate (100% vs. 91%) after the primary immunization; had a better booster effect (GMT: 320 vs. 33) and a greater capability to neutralize local Taiwan strains of J E V (GMT: 154 vs. 1). (2) Three doses of J E vaccine was the minimum requirement for generating higher and durable Nt Ab against heterolo- gous J E V strains (Fig. 3). (3) There was a n antigenic variation in local Taiwan J E V strains (CC and CH) because 3 doses of N or B vaccine elicited different Nt Ab seropositivity rates (N: CC = 0% vs. CH = 22%) and GMT (B: CC = 123 vs. CH = 195) against these two
strains of J E V (Table V). (4) Original antigenic sin of J E V did exist. Two-dose immunization with either N or
129 B vaccine only conferred a narrow spectrum of neutral-
izing responses against two local Taiwan JEV strains (CC and CH) and Nt Ab waned very quickly during a one-year period. In addition, serum samples from chil- dren immunized with 3 doses of N vaccine neutralized these two Taiwan wild type JEVs only at the borderline level. Therefore, the strains of JEV used in vaccine and the booster immunization in the second year were im- portant factors in maintaining protection in the com- munity [Kanamitsu et al., 1970; Okuno et al., 1987; Poland et al., 19901. Our results emphasize that local immunologic data are more important than vaccine ef- ficacy in evaluation because the ecology of J E viral infection varies in different geographic areas over time and each encephalitis case has such a high probability of progression to a fatal outcome.
JEV strains vary in their neurovirulence, peripheral multiplication in mice, stability to heat and titers of hemagglutinin (A) [Huang, 19821. Our neutralization results demonstrate that the antigenic variation among different strains of JEV appears not only at the level of inducing Nt Ab, but also in the capability to neutralize local strains of JEV. Although in uitro virus Nt Ab test may not measure protection in uiuo, Kimoto
et al. [1968] indicate that humoral immunity is very important in preventing JEV infections of neurons af- ter a short period of viremia. We found that the higher the Nt Ab titers generated by the B vaccine, the more likely it is to neutralize the wild type JEV, implying more effective protection against human encephalitis by blocking a t the early stages (attachment, penetra- tion and uncoating) of the virus replication cycle. The higher neutralizing Ab in the B vaccine strain may be attributed to (1) the presentation of more cross-reactive epitopes, (2) the better T-helper cell ability to induce a B-cell response [Abbas et al., 19911, and (3) higher im- munogenic effect after the interaction of adjuvant and B strain JEV [Ogata et al., 1970; Gregoriadis et al., 19891. It means that the B-cell clone primed in the primary immunization has high affinity and specificity and that the humoral immunity generated after the booster immunization is synergistic and more durable. Therefore, while the protein content is standardized between N and B vaccine, the definition and detail structure of B- or T-cell epitopes that will give the best neutralization of different JEV strains remain to be elucidated [Colman et al., 19871. Alternatively, if sig- nificant mutations in JEV immunogenicity emerge in some areas, it may lead to a large-scale encephalitis epidemic such as occurred in Korea in 1982 [Umenai et al., 19851. Since viruses with changed antigenicity that may escape immune elimination, continued careful monitoring of viral antigen is always needed [Clements and Narayan, 1984; Wilson and Cox, 19901.
The major problem with a killed mouse-brain J E vac- cine is the lack of long-term immunity (memory). Our results also demonstrated a quick decline in JEV Nt Ab titer by one year after the 2nd dose. Thus, the booster
3rd dose is very important for increasing “herd immu-
nity” as well as in generating the capability to neutralize heterologous JEV strains (Table V). It is unclear why the J E vaccine is not able to elicit more durable immunologic memory after the 2nd dose as, like the Salk polio vaccine does [Zanetti et al., 19871. The memory problem may be with the T or B cell only, or with both B- and T-memory cells, or with the regula- tion of B-cell activity by T cells. Whether the B-cell memory for J E vaccine antigens is due to a lower bind- ing affinity for low dose antigen is unknown, but a higher J E viral antigen doses enhanced immunogenic- ity [Kanamitsu et al., 19701. It is also very likely the T-suppressor cells induce low avidity memory B cells that do not mature efficiently [Okumura et al., 19761. Alternatively, the lack of adhesion or activation mole- cules on memory T cells or without J E viral cryptic epitopes presented to T cells may lead to less durable JEV Nt Ab [Mackay, 1991; Gammon et al., 19911. Fur- thermore, our unpublished data suggested that the cur- rently used J E N vaccine also stimulated a lower titer of hemagglutination inhibition Ab [its seropositivity rates against the N strain were 17% (2/12) after 2-dose immunization and 20% (5/25) after 3-dose immuniza- tion] and resulted in a shorter duration (8 months) of Nt Ab even after the 4th dose of JE vaccine. The fact that in Japan the number of J E cases peaks in the over 40 age group (Igarashi at Nagasaki University, personal communication), and that 13% of reported J E cases dur- ing 1986-1991 in Taiwan had been immunized, indi- cated the urgent need to improve memory response of J E vaccine.
Because children who were immunized with the first two doses of N vaccine and boosted with B strain gener- ated a higher seropositivity rate and greater levels of Nt Ab against the first encountered strain, the “origi- nal antigenic sin” which is demonstrated in other vi- ruses also exists in JEV [Francis, 1953, 1955; Kana- mitsu et al., 1970; Halstead et al., 19831. In other words, the JEV strain used for the primary immuniza- tion provides higher immunogenicity and an epitope that has been conserved in many different JEV strains will give rise to even better immune response during repeated exposures through life. Since RNA fingerprint analysis and sequence data have documented different strains of JEV in China, India, and Sri Lanka, careful evaluation of the most appropriate strain for use in vaccine preparation must also rely on an understand- ing of JEV molecular epidemiology in the area [Baner- jee and Ranadive, 1989; Chen et al., 19901.
Immunization is the most effective prevention mea- sure against J E in Taiwan and elsewhere [Hoke et al., 1988; Poland et al., 19901. Several investigators have developed a new generation of J E vaccines to express the relevant immunogens, particularly in envelope gly- coprotein (E), a membrane protein (MI, and its glycosy- lated precursor (PrM) [Haishi et al., 1989; Yasuda et al., 1990; Konishi et al., 19921. Optimal vaccine design of J E requires: (1) an understanding of local viral strain genetic heterogeneity and immunotyping of the viral
130
infection, (2) identifying viral proteins containing pep- tides able to form antigenic complexes with prevalent class I and class I1 major histocompatibility complex alleles in the population for generating a higher level and wider spectrum of Nt Ab and cell-mediated immu- nity, and (3) elicitating the co-stimulatory activity of antigen presenting cells or adhesion molecules for a better memory response. In addition, we recommend developing a diagnostic test to differentiate natural in- fection from immunization for better evaluating vac- cine efficacy.
ACKNOWLEDGMENTS
We greatly appreciate Drs. Joan Levine, Chin-Yun Lee, Wei-Fu Chen, Mei-Shang Ho, Wei-June Chen, Hour-Yuang Chen, Rong-Hua Lin, Mr. Chuan-Liang Kao, Mr. Ying-Chang Wu, Ms. Anne Mather, and scien- tists from the CDC, USA and Japan (Dr. Akira Iga-
rashi, Dr. Akira Oya, and Dr. Tadahiko Kitano) for their invaluable discussion. Sincere gratitude is also extended t o Mr. Yu-Hsiang Hsieh and Mr. Chen-Fu Su; nurses at four local health centers (Lambay Islet, Tung- kang, Hsintien and Taan) and at Fu-Ying Hospital in Pintung; and doctors at the Department of Pediatrics a t National Taiwan University Hospital for their enthusi- asm in collecting blood. We also thank the NIPM in Taiwan for providing Beijing-1 and Taiwan local JEV sterins, the National Quarantine Service for providing J E epidemiologic data, and Dr. A. Oya and Dr. T. Ki- tan0 at the NIH in Japan for providing standard sera of JEV (Beijing-1 and Nakayama strains). We are also indebted to the American Bureau for Medical Advance- ment in China and Mrs. Hope N.F. Phillips for their strong financial support for our 1991 summer learning at CDC, Fort Collins, Colorado. We also appreciate Dr. D. Gubler for his critical review of this manuscript and his team members for their outstanding technical assis- tance, quality control of our Nt Ab data, and for provid- ing BHK-21 cells.
REFERENCES
Abbas AK, Lichtman AH, Pober JS (1991): B cell activation and anti- body production. In Wonsiewicz MJ (ed): “Cellular and Molecular Immunology.” W.B. Saunders Company, pp 187-203.
Bahn AK (1985): Description of a quantitative sample: comparison of a sample mean with a n expected mean. In “Basic Medical Statistics.” pp 127.
Banerjee K, Ranadive SN (1989): Oligonucleotide fingerprint analysis of Japanses encephalitis virus strains of different geographical origin. Indian Journal of Medical Research 89:201-216.
Calisher CH, Karabatsos N, Dalrymple J , Shope RE (1989): Antigenic relationship between flaviviruses as determined by cross-neutral- ization tests with polyclonal antisera. Journal of General Virology 70:37-43.
Chen WR, Tesh RB, Rico-Hesse R (1990): Genetic variation of Japa- nese encephalitis virus in nature. Journal of General Virology 71:2915-2922.
Chow FY, Chang SN, Lu CH (1986): Comparison of plaque reduction and active mouse protection test for estimating the potency of Japanese encephalitis vaccine. Chinese Journal of Microbiology and Immunology 19(3):21-26.
Clements J E , Narayan 0 (1984): Immune selection of virus variants. In Notkins AL, Oldstone BA (eds): “Concepts in Viral Pathogene-
sis.” New York Springer-Verlag, pp 152-157.
Colman PM, Air GM, Webster RG, Varghese J N , Baker AT, Lentz MR, Tulloch PA, Laver WG (1987): How antibodies recognize virus proteins. Immunology Today 8(11):323-326.
Francis T (1953): Influenza: the newe acquayantance. Annals of Inter- nal Medicine 39(2):203-221.
Francis T (1955): The current status of the control of influenza. Annals of Internal Medicine 43:53&538.
Gammon G, Sercarz EE, Benichou G (1991). The dominant self and the cryptic self shaping the autoreactive T-cell repertoire. Immunol- ogy Today 12(6):193-195.
Gregoriadis G, Allison AC, Poste G (eds) (1989): “Immunologic Adju- tants and Vaccines.” Plenum Press.
Haishi S, Imai H, Hirai K, Igarashi A, Kato S (1989): Expression of envelope glycoprotein (E) of Japanese encephalitis virus by recom- binant vaccinia virus. Acta Virologica (Praha) 33:497-503. Halstead SB, Rojanasuphot S, Sangkawibha N 11983): Original anti-
genic sin in dengue. American Journal of Tropical Medicine and Hygiene 32(1):154-156.
Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, Kotchasenee SO, Gingrich JB, Latendresse J , Fukai K,
Burke DS (1988): Protection against Japanese encephalitis by in- activated vaccine. New England Journal of Medicine 319(10):60& 614.
Hsu ST (1969): Production of Japanese encephalitis vaccine i n Tai- wan. Japan Journal of Tropical Medicine 10:214-217.
Hsu TC, Chow LP, Wei HY, Chen CL, Hsu ST, Huang CT, Kitaoka M, Sunaga H (1971): A completed field trial for a n evaluation of the effectiveness of mouse-brain Japanese encephalitis vaccine. In Hammon WMcD, Kitaoka M, Downn WG (eds): “Immunization for Japanese Encephalitis.” Baltimore: Williams & Wilkins, pp 258- 265.
Huang CH (1982): Studies of Japanese encephalitis in China. Ad- vances in Virus Research 27:71-101.
Kanamitsu M, Ashimoto N, Urasawa S, Katsurada M, Kimura H (1970): A field trial with a n improved Japanese encephalitis vac- cine in a nonendemic area of the disease. Biken Journal 13:313- 328.
Kimoto T, Yamada T, Ueba N, Kunita N, Kawai A, Yamagami S, Nakajima K, Aka0 M, Sugiyama S (1968): Laboratory diagnosis of Japanese encephalitis comparison of the fluorescent antibody tech- nique with virus isolation and serologic tests. Biken Journal 11: 157-168.
Kitano T, Yabe S, Kobayashi M, Oya A, Ogata T (1986): Immunoge- nicity of J E Nakayama and Beijing-1 vaccines. J E & HFRS Bulle- tin 1:37-41.
Kobayashi Y, Hasegawa H, Oyama T, Tamai T, Kusaba T (1984): Antigenic analysis of Japanese encephalitis virus by using mono- clonal antibodies. Infection and Immunity 44(1):117-123. Konishi E, Pincus S, Paoletti E, Shope RE, Burrage T, Mason PW
(1992): Mice immunized with a subviral particle containing the Japanese encephalitis virus PrMiM and E proteins are protected from lethal JEV infection. Virology 188:714-720.
Mackay CR (1991): T cell memory: the connection between function, phenotype and migration pathways. Immunology Today 12(6): 189-192.
Monath T P (1988): Japanese encephalitis A-plague of the orient. New England Journal of Medicine 319(10):641-643.
Morens DM, Halstead SB, Pepik PM, Putvatana R, Raybourne N (1985): Simplified plaque reduction neutralization assay for den- gue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutral- ization. Journal of Clinical Microbiology 22(2):250-254.
National Quarantine Service (1992): Statistics on Japanese encephali- tis control in Taiwan Areas pp 4.
Ogata M, Nagao Y, Jitsunari F, Kikui R, Kitamura N (1970): Vaccina- tion with complete adjuvant-added inactivated virus vaccine of Japanese encephalitis to swine for preventing viremia (with spe- cific reference to the effects of vaccination on viremia; epidemiolog- ical study on Japanese encephalitis 35). Acta Medica Okayama 24579-587.
Okumura K, Metzler CM, Tsu ‘IT, Herzenberg LA, Herzenberg LA (1976): Two stages of B-cell memory development with different T-cell requirements. Journal of Experimental Medicine 144:345- 357.
Okuno T, Okada T, Suzuki M, Kobayashi M, Oya A (1968): Immuno- typing of different strain on Japanese encephalitis virus by anti-
body absorption, hemagglutination-inhibition and complement- fixation tests. Bulletin of the World Health Organization 38547- 563.
Okuno T, Tseng FT, Hsu ST, Huang CT, Kuo CC, Lin CC (1975): Japanese encephalitis surveillance in China (province of Taiwan) during 1968-1971. I. Geographical and seasonal features of case outbreaks. Japan Journal of Medical Science and Biology 28:235-
253.
Okuno Y, Okamoto Y, Yamada A, Baba K, Yabuuchi H (1987): Effect of current Japanese encephalitis vaccine on different strains of Japanese encephalitis virus. Vaccine 5128-132.
Oya A (1988): Japanese encephalitis vaccine. Acta Pediatrica Japon- ica 30(2):175-184.
Poland JD, Cropp CB, Craven RB, Monath TP (1990): Evaluation of the potency and safety of inactivated Japanese encephalitis vac- cine in US inhabitants. Journal of Infectious Disease 161:878-882. Russell PK, Nisalak A (1967): Dengue virus identification by the plaque reduction neutralization test. Journal of Immunology 99(2):
291-296.
Takaku K, Yamashita T, Yoshida I, Osanai T, Goda H, Akashi M,
Sugahara Y, Kagawa H, Amano T, Kunita N, Inoue K, Toyoshima K (1971): Vaccine potency testing methods and their significance. In Hammon WMcD, Kitaoka M, Downn WG (eds): “Immunization for Japanese Encephalitis.” Baltimore: Williams & Wilkins, pp Umenai T, Krzysko R, Bektimirov TA, Assaad F (1985): Japanese encephalitis: current world status. Bulletin World Health Organi- zation 6314h625-631.
Vaughn DW, Hoke CH (1992): The epidemiology of Japanese enceph- alitis: prospects for prevention. Epidemiologic Review 14:197-221. Wilson IA, Cox NJ (1990): Structural basis of immune recognition of Influenza virus hemagglutinin. Annual Review of Immunology
8737-771.
Yasuda A, Kimura-Kuroda J , Ogimoto M, Miyamoto M, Sato T, Taka- mura C, Kurata T, Kojima A, Yasui K (1990): Induction of protec- tive immunity in animals vaccinated with recombinant vaccinia viruses that express encephalitis virus. Journal of Virology 6 4
Zanetti M, Sercarz E, Salk J (1987): The immunology of new genera-
258-265.
2788-2795.