COMMUNICATIONS
Depending on the donor ligand the average F e - 0 distances
in the double octahedron are about 1.93
A
(FpP(Mes)O,),
2.011(7)
A
(MesPO,), and 2.123(7)
8,
(p3-OH). The equatorial
angles 02-Fe2-010 and 08-Fe2-08a were determined to be
98.9(3) and 81.7(2)", respectively. The atoms Fe2,08, Fe2a and
0 8 a lie in one plane and form a rhombus with the acute angle
at the iron atoms; thus, a bonding interaction between Fe2 and
Fe2a i s
not possible (distance Fe2-Fe2a
=3.212 Aj. The trigo-
nal-bipyramidal coordination of Fe4 is slightly distorted. The
longest F e - 0 distance in
2 was determined for Fe4-08(apical)
to be 2.168(7)A, the shortest for Fe4-0, (equatorial) to be
about 1.895
A.
The equatorial bridges
04,05,
and O l a are bent
slightly out of the ideal plane. The Fe4-Cl1 distance is
2.346(3)
A.
The bond angles at the
0 bridges lie between
124.5(4) and 146.7(4)", those at the P atoms (103.8(4)-
113.1(3)") have values close to the tetrahedron angle. The sum
of angles Fe-0-Fe a t the trigonal-pyramidal pu,-OH
bridges is
345.3'; the p,-OH hyrogen atom does not participate
in any
hydrogen bonding.
Temperature-dependent magnetic measurements for
2
re-
vealed antiferromagnetic behavior with
a Nee1 temperature of
40 K ; the bond lengths (Fe-0) and angles (Fe-0-Fe) of the
four central Fe"'
ions (Fe2, Fe2a, Fe4, Fe4a) allow super-
exchange and spin orientation. At 300K the
peff
value was
5.48 BM.[61
The mild aerial oxidation of the diferriophosphonium salt
l a
leads to many (sometimes drastic) changes in the functionality
at both the iron and phosphorus atoms: We find both retention
and elimination
of the CpFe(CO), groups as well as complete
oxidation to give iron(in) centers, and oxidation of the phospho-
nium building blocks to give p,-phosphinato and p3-phospho-
nato ligands. This wide palette of new building blocks finally
readily combines to form
2.
The new molecular framework of
2
is illustrated by the polyhedral representation in Figure 2. The
structure contains a central double octahedron (FeO,(OH)),
,
two trigonal bipyramids ( F e 0 3 ( 0 H)Cl), and six tetrahedrons
(four Fp(Mes)PO, and two MesPO,). Compound
2
is an ex-
ample for the unusual combination of classic (in the corej and
organometallic complex chemistry (at the periphery). We are
currently investigating similar organometalated sulfonium
salts,['' which also may be converted to similar coordination
polyhedrons with sulfenato, sulfinato, and sulfonato ligands by
aerial oxidation.
Experimental Section
Synthesis of l a see reference [3].
2: l a (200 mg, 0.37 mmol) was dissolved in acetonitrile (12 mL) and this solution was exposed to air for three days. Small yellow crystals formed on the inside glass surface. They were collected and washed with acetone. Yield: 30 mg (22.4%). Ad- ditional quantities of 2 remained ~n the mother liquor; however, these could not be isolated in a pure f o m ; correct elemental analysis. Compound 2 is soluble only in dimethyl sulfoxide (DMSO)
Received: May 20. 1996 Revised version: September 26, 1996 [Z9132IE] German version: Angeir.. Chem. 1997, 109, 55-56
Keywords: cage compounds
*iron
-
magnetic properties
P ligands
-
self-assembly
[I] A. Miiller, K. Hovemeier, E. Krickemeyer, H. Bocke, Angew. Chem. 1995,107, 856; Angew. Chem. In[. Ed. Engl. 1995,34,779; further examples for molecular self-assembly can be found in J.-M. Lehn, Supermolecular Chemisrry: Concepts
and Perspectives, VCH, New York, 1995.
[2] a) W. Pohl. I.-P. Lorenz, H. Noth, M. Schmidt, Z. Naturforsch. 5 1995, SO, 1485; b) I.-P Lorenz, P. Miirschel, W. Pohl,
K .
Polborn, Chern. Ber. 1995,128, 441; c) I.-P. Lorenz, W. Pohl,K.
Polborn, ihid. 1996, 129, 11.[3] ILP. Lorenz. W. Pohl, H. Noth, M. Schmidt. .IOrganornet. Chem. 1994, 47S, 211.
[4] W. Pohl, PhD Thesis, University of Miinchen, 1995.
[ S ] X-ray structure analysis of 2: C8,H8,CI,Fe,02,P,, brown plates, 0.30 x 0.26 x
0.1 mm', triclinic, space group P , u = 12.991(7), b = 14.660(7), c = 14.7?6(7)
A,
1.581 p =1.476mm-l, F(OO0) =1104, diffractometer: Siemens P4,Mo,, (1. = 0.71073 A), T = 213 K, 28 = 3.24-47.00, w scan, scan range (0)
1.2'. reflections: 5241 measured, 4967 of which were independent (R(int) = 0.1251), 3022 observed [F>4o(F)]. The structure was solved with the programs XS (Siemens) and SHELXL (G. M. Sheldrick, Gottingen, 1993) by direct methods and refined by full-matrix least squares refinement. The hydro- gen atoms were refined as riding atoms with fixed isotropic temperature parame- ters. 553 refined parameters, R = 0.0693, nR2 = 0.1308, GOOF = 1.049. Fur- ther details of the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (Germany), on quoting the depository number CSD-405885.
161 The measurements of magnetic susceptability were made between 10-300 K with 20 mg of microcrystalline 2 on a Quantum Design MPMS Squid magne- tometer at a field strength of 2 T Calculations (without diamagnetic correction) were made with one molecule of CH,CN per molecule of 2.
[?I
1:P. Lorem, K . Thurow, J Orgunornrc. Cliem. 1995,496, 191.x=119.25(1), 8=106.41(2), ~=94.88(1)', 2 = 2 , V=2270(2)A3, pcSicd=
Linear Pentanuclear Complexes Containing a
Chain of
Metal Atoms: [Co:'(pu,-tpda),(NCS),]
und [Ni:'(p5-tpda),C1,1**
Shen-Jye Shieh, Chin-Cheng Chou,
Gene-Hsiang Lee, Chih-Chieh Wang, and
Shie-Ming Peng*
Metal-metal bonding
in dinuclear metal complexes is well
understood.['
-
31Our efforts to extend dinuclear metal complex-
es to form trinuclear metal complexes with a
syn-syn
bis-
[*I
Prof. S.-M. Peng, Dr. S.-J. Shieh, C.-C. Chou, G.-H. Lee, Dr. C.-C. Wang Department of ChemistryNational Taiwan University
Taipei, Taiwan, 107 (Republic of China) Fax: Int. code
+
(2)3636359(H,tpdd = N,N'-bis(i-pyridyl)-2,6-diaminopyridine). Figure 2. Polyhedral representation of 2, consisting of two edge-shared octa-
hedrons ([FeO,(OH)],), two trigonal bipyramids (FeO,(OH)CI), and six tetra- hedrons (four Fp(Mes)PO, and two MesPO,).
["I
This research was supported by the National Science Council of Taiwan.COMMUNICATIONS
(wpyridy1)amido ligand have been r e~ 0r t e d. I ~ .
51In order
to achieve a further extension to pentanuclear metal com-
plexes a new ligand,
N,N'-bis(a-pyridyl)-2,6-diaminopy-
ridine (the common name in our system is tripyridyldi-
amine abbreviated as H,tpda), was synthesized. It was
found that the ligand can bind metal
ions
in two confor-
mations, the anti-anti-anti-anti
form
I and the
syn-
syn-syn-syn
form
XI.
In
I the ligand is tridentate and coordinates to metal ion
through the nitrogen atoms of pyridine moieties. The ligand can
be either neutral or dianionic. [Co"(H,tpda)Cl,] illustrates this
coordination mode.
In I1 the ligands have been deprotonated
and act as bridging pentadentate ligands. The unprecedented
linear pentanuclear nickel(r1) and cobalt(1r) complexes ([M5(p5-
tpda),X,],
M
=Ni, X
=C1, and
M
=Co, X
=NCS) are re-
ported here.
The ligand was synthesized by treating
2.5
equivalents
of
2-bromopyridine with
1
equivalent
of 2,6-diaminopyridine
un-
der basic conditions. Remaining 2,6-diaminopyridine and N-(a-
pyridyl)-2,6-diaminopyridine
were washed away with water.
The crude product of N,N'-bis(2-pyridyl)-2,6-diaminopyridine
was recrystallized from dichloromethane and methanol. The
cobalt complex of type
I was synthesized by treating equimolar
amounts of CoC12.6H,0 with H,tpda
in
dichloromethane. The
crystal structure of the neutral complex [Co(H,tpda)Cl,] reveals
that the coordination geometry around the cobalt@) ion is trig-
onal bipyramidal with two chloride ions and the nitrogen atom
of the central pyridine as the basal plane and two nitrogen atoms
of the terminal pyridine group as the apex. The ligand H,tpda
is planar and coordinates to cobalt ion in anti-anti-anti-anti
conformation.
The pentanuclear cobalt(I1) complex was synthesized similar-
ly to the trinuclear metal complex.[4-
']It was characterized by
various spectroscopic methods, in particular mass spectrometry
(FAB) and X-ray diffraction. The parent peak of
[Cos(ps-
tpda),(NCS),J
is
clearly assigned and peaks of the fragments of
[Co,(p-tpda),], and [Co,(p-tpda),] are observed. The structure
of the complex, obtained by X-ray single crystal diffraction
study, has several uncommon features (Figure
1).
First, the pen-
tacobalt metal chain is helically wrapped by four
syn-syn-syn-
syn
type ligands. The complex exhibits approximate
D,
symme-
try. Secondly, the five cobalt(r1) ions are collinear, linked by
multicentered
Co-Co
ISbonding. The Co-Co distances are
2.277(2), 2.232(2), 2.229(2), and 2.274(2)
A,
which are com-
parable with those of dinuclear cobalt complexes.[*] The prelim-
inary MO analyses indicate there are two
CTbonds among five
cobalt(I1) ions, and the HOMO is of nonbonding character and
singly occupied. The average Col -N(py) (1.97(1)), C02-
N(amido) (1.90( I ) ) , Co3
-
N(py) (1.93(1)), Co4-N(amido)
(1.90(1)), and Co5-N(py) distances (1.97(1)
A)
are consistent
with a
low spin
state for the cobalt@) ion
(p
=1.9
pB
and tem-
perature-independent)
.Thirdly, the negative charges
of
the an-
[CO ~(PL-~P ~~ ),(NCS )J
1[ C o & - t ~ d a ) J > [Co,(~-tpda),(NCS)1,
Figure 1. Top: Crystal structure of [Co,(ps-tpda),(NCS),l (ORTEP view) Perti- nent bond lengths
(A)
and anglesr):
Col -C02 2.277(2), Co2-Co3 2.232(2), c 0 3 - c o 4 2.229(2), Co4-CoS 2.274(2), Col -N21 2.07(1), Col --N(py) (averaged) 1.97(1), Co2-N (averaged) 1.90(1), Co3-N (averaged) 1.93(1), Co4-N (averaged) 1.90(1), CoS -N(py) (averaged) 1.97( l ) , CoS -N22 2.06( l ) , N21 -Col -C02 178.8(3), N22 179.3(3), N(py)-Col-CoZ (averaged) 86(1), N-Co2-Co3 (averaged) 89(1), N-Co3-Co4 (averaged) 90(1), N-Co4-Co3 (averaged) 89(1), N(py)-CoS-Co4 (averaged) 86(1). Center: Illustration of the helical ligands wrapped around the linear metal chain (along the metal chain axis). The three carbon atoms ofpyridine rings are omitted for clarity. Bottom: Another illustration down the metal chain axis.
COl-cO2-CO3 178.7(1), CO2-Co3-Co4 179.9(1), C O ~ - C O ~ - C O S 178 8 ( 1 ) , CO4-CO5-
ionic ligand are delocalized
on the five nitrogen atoms of the
ligand. The observed bond pattern is qualitatively consistent
with the delocalization model.
The pentanuclear nickel(I1) complex has also been synthesized
similarly to the trinuclear metal complexes.[4
71The parent
peak of [Ni,(ps-tpda),C1,]
is clearly assigned, and peaks of the
[Ni,(p-tpda),] and [Ni,(p-tpda),Cl] fragments are also ob-
served. The single-crystal X-ray diffraction study of [Niy(p5-
tpda),Cl,] reveals that the asymmetric unit contains half of the
complex and two dichloromethane molecules. The complex is
located at the crystallographic center of inversion. The atomic
positions are averaged, because right-turn and left-turn helical
complexes are disordered in the crystal. Eight nitrogen atoms
from amido groups that are coordinated to nickel ions (Ni2,
occupancy factor
0.5)
and the a-carbon atoms of the pyridyl
groups have high
anisotropic
thermal parameters.
Oneof
the helical forms
is
shown
in
Figure2. The pentanuclear
COM NI
U
N
ICATIONS
^, n C28
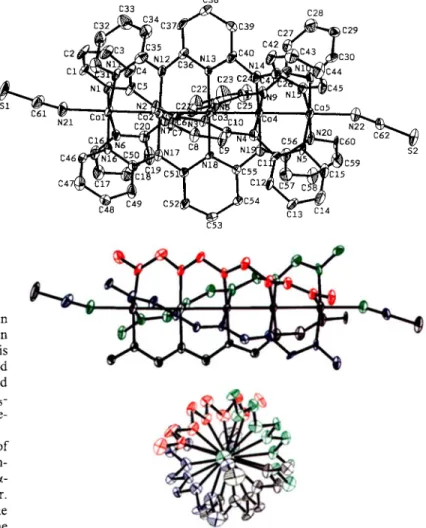
Figure 2. Crystal structure of pi,(p,-tpda),CI,] (ORTEP view) Pertinent bond lengths
(A)
and anglesr):
Nil-CI 2.346(3), Nil -Ni2 2.385(2), Ni2-Ni3 2.305(1), Nil - N (averaged) 2.11 1(9), Ni2- N (averaged) 1.897(1 S), Ni?- N 1.904(8), CI-Nil- Ni2 l79.1(1), Nil-Ni2-Ni3 179.5(1), Ni2-Ni3-Ni2 180, N-Ni3-N2 (averaged) 90. N-Ni2-Ni3 (averaged) 88(1), N-Nil-Ni2 (averaged) 82(1).linear metal chain is helically wrapped by four
syn-syn-syn-
syn type ligands. The five nickel ions and two chloride ions are
collinear (Cl-Nil-Ni2 179.1(1)", Nil-Ni2-Ni3 179.5(1)", and
Ni2-Ni3-NiY 180"). The Ni-Ni distances are very short (Nil
-Ni2 2.385(2); Ni2-Ni3 2.306(2)
A).
The latter
is the shortest
known Ni-Ni distance.[9-"] According to
MO analyses, no
bonding interactions exist between the nickel ions, which is con-
sistent with the magnetic data (see below).
The average Ni2-N (1.897(15)
A)
and Ni3-N distances
(1.904(8)
A)
are short, consistent with a square-planar, diamag-
netic arrangement of nickel(I1) ions. The terminal Ni" ions (Nil)
are in a square-pyramidal environment and exhibit a long Nil -
C1 bond (2.346(3)
A)
and long Nil -N bonds (av. 2.1 1 l(9)
A),
consistent with high-spin nickel(I1) ions ( S
=1,
p
=2.8
pB).
A
temperature-dependent magnetic study
o f the complex (Fig-
ure 3) indicates the antiferromagnetic interaction of terminal
high-spin nickel ions with
J
=-
14.6 cm-'. This interaction
is
one order of magnitude smaller than those of similar trinuclear
nickel(I1) complexes.[',
Attempts to isolate other linear pentanuclear metal complex-
es and demonstration of their potential application as molecular
wires are under way.
0.018 0 O o Z o t 016
j
0
I
0.012 xM/emu mol-' 0.010 0 008 0.006 50 1 0 0 150 200 250 300 TIK-Figure 3. Magnetic data for [Ni,(p,-tpda),CI,]. Solid curves represent calculation based on the equation
5,
= C(2e2"+
10eb=)/(l+
3eZ'+
Se'"). C = Ng2f12/ kT,x = J/kT, 2 = - 2 J S , - S , (S, = S, = S, = 0, S, = S, =1) and pert =2.84((,T)"2. o is the observed xhl and the observed perr.
Experimental Sect ion
H,tpda: Under a nitrogen atmosphere 2-bromopyridine (36 mL) was added to a solution of 2.6-diaminopyridine (10 g, 0.09 mol) and fBuOK (30.24 g. 0.27 mol) in T H F ( 1 50 mL). The reaction mixture was heated under reflux for 24 h. After cool- ing to room temperature, the solvent was removed by rotary evaporator. Then the mixture was extracted with dichloromethane, and the solvent removed under re- duced pressure. Unchanged 2.6-diaminopyridine and N-(a-pyridyl)-2,6-diaminopy- ridine were rinsed away with water. The crude product of N.N'-bis(2-pyridyl)-2.6- diaminopyridine was recrystallized from dichloromethane and hexane(Yie1d 26%). IR (KBr)
V
= 3442.31 80 cm -'
(NH). UV/Vis (CH,CI,): i.,,(6:) ,= 268 (3.76 x I 0,). 321 n m ( 4 . 1 3 ~ lo3). MS(FAB):m/~(%)264(60)[M+l]~. 186(100) [C,oNaHJ+, 77 (20) [C,H,N]'.[Co(H,tpda)CI,]: CoCI, 6H,O (23 mg, 0.09 mmol) was added to a solution of H,tpda (25 mg, 0.09 mmol) in CH,CI, (30 mL). The yellow solution was stirred for 6 h A green precipitate was formed, filtered off, washed with ether, and air-dried. Green prismatic crystals were formed by slow diffusion of diethyl ether into a CH,CN/DMF solution of the compound (yield 70%). IR (KBr): 3 = 3397, 3316, 3203 c m - ' (NH). UV/Vis ( D M F ) i,,( 6 ) ,= 330 (3.34 x lo4). MS (FAB): m/z (%) 321 (40) [ M
-
Cl]'[Co,(p,-tpda),(SCN),]: CoCI,.6H,O (0.936 g, 4 mmol) and H,tpda (1.02 g, 4 mmol) were placed in an Erlenmeyer flask, and naphthalene (7.2 g) was added. The mixture was heated (about 160-180°C) for 10min to remove water. Then n-butanol(3 mL) was added to the heated mixture, and heating was continued until the n-butanol had almost completely evaporated. A solution of potassium n-butox- ide (0.88 g, 8 mmol)n-butanol(20 mL) wasadded dropwise. Heating was continued until the remaining n-butanol had evaporated completely, after which an excess of sodium thiocyanate (1.0 g) was added. After the mixture had cooled. n-hexane was added to wash out naphthalene. The remaining solid was extracted with CH,CI, and recrystallized from CH,Cl,/n-hexane solution. Deep brown crystals were ob- tained (yield 5 % ) . I R (KBr) i = 2058 (CN), 1594, 1565, 1536 (C=C). UV/Vis (CH,CI,) i,,, ( 6 ) = 279 (1.13 x lo5), 338 (6.21 x lo4), 393 ( 5 . 8 4 ~ lo4), 513 ( 9 . 7 5 ~ lo'). 725cm-' (1.01 x lo3). MS (FAB): mi;
(YO)
1455 (2) [MI', 1397 (4)[M - SCN]+. 1339 ( I ) (Co,(p-tpda),]', 1077 (6) [Co,(p-tpda),SCN]+, 1019 (4) ICo&-tpda)J+.
[Ni,(p,-tpda),CI,]: NiCI;6H,O (0.948 g, 4 mmol) and H,tpda (1.02 g, 4 mmol) were placed in an Erlenmeyer flask, and naphthalene (7.2 g) was added. The mixture was heated (about 160-180") for 10min to remove water. Then n-butanol (3 mL) was added to the heated mixture, and heating was continued until the n-butanol had almost completely evaporated. A solution ofpotassium n-butoxide (0.88 g, 8 mmol, in 20 mL n-butanol) was added dropwise. Heating was continued until the remain- ing n-butanol had evaporated completely. After the mixture had cooled, n-hexane was added to wash out naphthalene. The remaining solid was extracted with CH,CI, and recrystallized from CH,Cl,/n-hexane solution Deep purple crystals were obtained (yield 50%). IR (KBr) V ~ 1 5 9 1 , 1562, 1541 cm-' (C=C). UV/Vis (CH,CI,) i.,,, (E) = 253(1.02 x lo5). 293(1.28 x lo5), 37311.55 x lo5), 592 nm (1.28 x lo4). MS (FAB): m/z (YO) 1408 (4) [MI', 1373 (3). [ M - Cll'. 1017 (10) [Ni&-tpda)J+, 698 (10) [Ni,(p-tpda),]'
Crystal data for [Co"(H,tpda)CI,I: Monoclinic, space group C2/c, a = 7.482(1). 2 = 4; CAD4 diffractometer with graphite-monochromated Mo,, radiation, Y - scan absorption correction (0.91
-
1 .OO); of 1406 unique reflections (20< SO0) mea- sured, 1125 with I > 2 u ( I ) were used in the refinement. R = 0.027, Rn, = 0 026 (133 variables)Crystal data for [Ni~(p,-tpda),CI,](CH,C12)4: Monoclinic, space group P2,/n,
u = 13.416(3), V = 3578(1) A3,
p = 1.623 gcm-'.Z = 4; Y-scanabsorptioncorrection(0.92-1.00);of6280unique reflections (20i50") measured, 3188 with I>Zu(I) were used in the refinement.
R = 0.072, R n = 0.071 (485 variables).
Crystal data for [Co~(pt,-tpda),(SCN),I-CH,CI,-(C~H,O), ;(H,O), 5 : Triclinic,
space group P i . a = 11.802(1), b =14.631(7), c = 20.184(4)
A,
a =73.42(3), b=77.77(1),g=87.56(3)", V = 3 2 6 4 ( 2 ) A 3 , p = 1 . 6 1 5 g c m - ' , Z = 2 ; Y-scan ab- sorption correction (0.83- 1 .OO); of 8645 unique reflections (20i45') measured, 3400 with I > 2 u ( I ) were used in the refinement. R = 0.049, R n = 0 046 (847 vari- ables)Crystallographic data (excluding structure factors) for the structure(s) reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-179-145 Copies of the data can be ob- tained free of charge on application to The Director, CCDC, 12 Union Road, Cambridge CB2 IEZ, UK (fax: Int. code
+
(1223) 336-033; e-mail: teched(rlchemcrys.cam.ac.uk).5=17.172(3), c = i 2 . 8 3 3 ( i ) A , ~ = i o 4 . i ( i ) " , v = i 5 9 8 8 ( 4 ) A 3 , =1.633gcm-',
h =16.894(3), c = 15.8807(4)
A,
= 92.54(2)",Received: July 12, 1996 Revised version: October 8, 1996 [Z9328IEJ German version: Angew. Chem. 1997. 109, 57-59
Keywords:
cobalt
*helical structures
-
magnetic propertiesnickel
.
N ligands
COMMUNICATIONS
[I] F. A. Cotton. G. Wilkinson. Advanced Inorganic Chemistry, 5th ed., Wiley,[2] F. A. Cotton. R. A. Walton. Multiple Bonds Between Meral Atoms, 2nd ed., 131 Metul-Meiul Bonds C1u.ster.s in Cl?emi.rtrv nnd Cataljsis, Plenum, New York, [4] E C. Yang, M C. Cheng. M. S . Tsai. S. M. Peng, J Chem. Soc. Chem. Com- [5] J. T Sheu. C. C . Lin. I. Chao. C. C. Wang, S. M. Peng, Chem. Commun. 1996, [6] L. P. Wu. P. Field. T. Morrissey, C. Murphy, P. Nagle, B. Hathway, J Chem.
[7] S Aduldecha. 8. Hathway. J Chem. Soc. Dalton Trans. 1991, 993. [XI F. A. Cotton. R . Poli. lnorx Chrin. 1987, 26, 3653. Co-Co = 2.265(2) 8, in
[Co','(triaz),] (rriaz = di-p-tolytriazenato)
[9] 0 Jarchow. H Schultz. R. Nast, Angeu.. Chern. 1970, 614. 43. Angew. Chem. I n ! Ed Engl. 1970, 9, 71. Ni-Ni = 2.32 8, in [Ni:(CN),]"-.
[lo] L. Sacconi. C. Mealli. D. Gatteschi. Inorg. Chem. 1974, 13, 1985. Ni- Ni = 2 42 8, in [N~:-~(napy),Br,]' (napy = 1.8-naphthyridine).
[ l l ] M. Corbett.
B.
Hoskins, Chem. Commun. 1969, 1602. Ni-Ni = 2.38 8, in [Niy(PhN,Ph),] (PhN,Ph = diphenyltriazenato).[12] C. Lin, C. Chou, S. Peng, unpublished. The J for [Ni,(p3-dpa),X,l"+ idpa = dipyridylamido ion) is - 96. -122 and - SSOcm-' for X = C1- i n = 0 ) . X = KCS- ( n = 0) and X = NCMe ( n = 2). respectively. New York. 1988, Chap. 23.
Clarendon Press. Oxford, 1993. 1989.
miin. 1994. 2377. 315.
so<..
Daiton Ti.un.\ 1990. 3835.An Interwoven Supramolecular
Cage**
Peter
R.
Ashton, Andrew N. Collins,
Matthew C. T. Fyfe, Peter
T.
Glink, Stephan Menzer,
J. Fraser Stoddart,* and David J. Williams
Dedicated to Professor Vincenzo Balzani
on the occasion of his 60th birthday
In recent times, supramolecular, noncovalent synthesis"] has
provided the chemist convenient access t o some remarkable
su-
perstructures, many of which are produced with a high degree of
architectural control and have the potential to perform specific
functions. One of the most challenging domains within this field
of
endeavor involves s e l f - a ~ s e m b l i n g [ ~ ~
discrete supramolecular
cages that are held together by intermolecular intera~tions[~1
and can act as synthetic receptors. However, none of the systems
reported to date involve interlocked o r interwoven structures.[41
We have designed such
a
system (Scheme I ) , which relies upon
the simultaneous threading of two secondary dialkylammonium
ions through the cavity of a ditopic crown ether, namely bis-p-
phenylene[34]crown-10 (BPP34C10)
.[51The trifurcated trisam-
monium ion
[1-H,I3'
has three secondary ammonium centers in
branches radiating from a central polyaromatic core. Each
branch can insert itself through the cavity
of
a
BPP34C10
molecule, but then utilizes only half of the potential receptor
[*I Prof J F. Stoddart, P. R. Ashton, M. C. T. Fyfe, Dr. P. T. Glink School of Chemistry, University of Birmingham
Edgbaston. GB-Birmingham B15 2TT (UK)
F a x : Int. code +(121)414-3531 e-mail. J f.stodddrt(a bham.ac.uk Dr. S Menzer. Prof. D. J. Williams
Chemical Crystallography Laboratory, Imperial College. London (UK) Dr. A N Collins
ZENECA Specialities, Manchester (UK)
[**I This research was sponsored in the UK by an Engineering and Physical Sciences Research Council CASE Award (to M.C.T.F) and also by the Biotech- nology and Biological Sciences Research Council.
Scheme 1. Schematic representation of the anticipated [3
![Figure 2. Polyhedral representation of 2, consisting of two edge-shared octa- hedrons ([FeO,(OH)],), two trigonal bipyramids (FeO,(OH)CI), and six tetra- hedrons (four Fp(Mes)PO, and two MesPO,)](https://thumb-ap.123doks.com/thumbv2/9libinfo/8671580.196077/1.864.53.383.804.1065/figure-polyhedral-representation-consisting-hedrons-trigonal-bipyramids-hedrons.webp)
![Figure 2. Crystal structure of pi,(p,-tpda),CI,] (ORTEP view) Pertinent bond lengths (A) and angles r): Nil-CI 2.346(3), Nil -Ni2 2.385(2), Ni2-Ni3 2.305(1), Nil - N (averaged) 2.11 1(9), Ni2- N (averaged) 1.897(1 S), Ni?- N 1.904(8),](https://thumb-ap.123doks.com/thumbv2/9libinfo/8671580.196077/3.864.58.422.92.354/figure-crystal-structure-ortep-pertinent-lengths-averaged-averaged.webp)