Association of Globular
-Actin with Intracellular Lipid
Droplets in Rat Adrenocortical Cells and Adipocytes
Tsorng-Harn Fong,*
,1Ching-Hsiang Wu,† E-Wen Liao,* Chiu-Yun Chang,*
Man-Hui Pai,* Ruei-Jen Chiou,* and Ai-Wei Lee*
*Department of Anatomy, Taipei Medical University, Taipei, Taiwan 110; and †Department and Institute of Biology and Anatomy, National Defense Medical Center, Taipei, Taiwan 114
Received October 26, 2001
Proteins located on the surface of lipid droplets may mediate intracellular lipid metabolism. In the present study, immunofluorescent staining and polyacryl-amide gel electrophoresis demonstrated that actin (43 kD) is associated with isolated intracellular lipid droplets of rat adrenocortical cells and adipocytes. Two-dimensional gel electrophoresis and immunoblot analysis further confirmed that the lipid droplet-associated actin is the beta isoform. In cultured adre-nocortical cells, stress fibers and the surface of intra-cellular lipid droplets were labeled with anti-beta-actin monoclonal antibody, whereas FITC-phalloidin staining did not mark the rim of lipid droplets. The present results provide the first morphological evi-dence that globular beta-actin is associated with intra-cellular lipid droplets. This significant association of actin with the surface of lipid droplets suggests that beta-actin might be involved in the regulation of in-tracellular lipid metabolism, particularly providing insight into the important transport of lipid constit-uents. © 2001 Elsevier Science
Key Words: -actin; lipid droplet; adrenocortical
cells; adipocytes.
Actin, a cytoskeletal protein, exists in vertebrates as
six different isoforms (1).
- and ␥-isoforms of actin are
abundant in many vertebrate nonmuscle cells (2, 3).
Interactions between actin and lipids of cell
mem-branes have been well documented to play important
roles in many cell activities such as maintenance of cell
shape, cell motility, and cell adhesion (4, 5). In general,
actin is anchored to the membrane by actin-associated
proteins such as
␣-actinin (6), vinculin (7), talin (8), etc.
Purified actin can also interact directly with liposomes
composed of pure lipids without the need of a linker
protein (9, 10). That is, actin can bind directly or
indi-rectly to lipids of plasma membranes. It is unclear
whether actin is also associated with intracellular lipid
droplets, which are constituted by lipids.
Several lipid droplet-associated proteins have been
investigated. Perilipins, hormonally regulated
phos-phoproteins, have been found on the periphery of lipid
droplets in adipocytes (11, 12), adrenocortical cells, and
Leydig cells (13). In addition, recent studies reported
that ‘capsular proteins’ are also located on the surface
of lipid droplets of rat adrenocortical cells (14, 15),
hamster Leydig cells (16), and 3T3-L1 adipocytes (17).
As to their potential functions, these lipid
droplet-associated proteins may be involved in mediating lipid
metabolism such as lipid packaging or lipid hydrolysis
in response to hormone stimulation. However, the
mo-lecular processes that govern either the deposition or
catabolism of the lipid droplets are still a mystery (18).
It is possible that some other lipid droplet-associated
proteins have not been yet discovered.
In this study, we examine the proteins associated
with intracellular triglyceride-rich lipid droplets of
adi-pocytes and the cholesterol-rich lipid droplets of
adre-nocortical cells by immunofluorescence, polyacylamide
gel electrophoresis, and immunoblotting methods. The
present data demonstrate the binding of
-actin with
intracellular lipid droplets. The significant roles of
-actin on intracellular lipid droplets are also
dis-cussed.
MATERIALS AND METHODS
Materials. Adult male Wistar rats (200 – 400 g) were housed in standard conditions with sufficient food and water. Type II collage-nase, poly-L-lysine, rabbit polyclonal anti-actin antibodies, mouse monoclonal anti--actin antibody, Texas-Red conjugated anti-rabbit IgG antibody, FITC conjugated anti-mouse IgG antibody that is preabsorbed with rat serum, and FITC-phalloidin were all purchased from Sigma (St. Louis, MO). Biotin-conjugated anti-mouse IgG that is preabsorbed with rat serum and streptavidin conjugated with peroxidase was purchased from Vector (Burlingame, CA). DMEM/ Ham’s F-12 (1:1 v/v) medium, horse serum, fetal bovine serum, and
1To whom correspondence should be addressed at Department of
Anatomy, Taipei Medical University, 250, Wu-Hsing Street, Taipei, Taiwan 110. Fax: 886-2-27388852. E-mail: thfong@tmu.edu.tw.
Biochemical and Biophysical Research Communications 289, 1168 –1174 (2001) doi:10.1006/bbrc.2001.6080, available online at http://www.idealibrary.com on
1168 0006-291X/01 $35.00
penicillin/streptomycin were obtained from Gibco (Grand Island, NY).
Isolation of intracellular lipid droplets. Rats were anesthetized with 7% chloral hydrate (4 ml/kg) by intraperitoneal injection. Ad-renal glands and epididymal fat pads were isolated and rinsed with phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8 mM Na2HPO4, pH 7.4).
The intracellular lipid droplets of adrenocortical cells were puri-fied as described in a previous report (19). Each adrenal gland was trimmed to remove fat and homogenized in 1 ml of a cold 0.25 M sucrose solution with a Teflon/glass homogenizer on ice. The lipid droplet fraction was isolated by discontinuous gradient centrifuga-tion. Briefly, the homogenate was layered on top of 3 ml of a 0.5 M sucrose solution that was at the bottom of a centrifuge tube. After that, 1.2 ml of a 0.125 M sucrose solution was loaded on top of the homogenate. The gradient was centrifuged at 13,000 g for 3 h at 4 °C. The white floating lipid droplets were collected and stored at –20°C. Isolation of adipocytes from epididymal fat pads has been previ-ously reported (20). Adipocytes were then hypotonically lysed in lysing medium (containing 10 mM Tris buffer, pH 7.4, 1 mM EDTA, 1 mM NaN3, and 1 mM PMSF) and then disrupted with 10 strokes in
a Teflon/glass homogenizer on ice. After centrifugation at 27,000 rpm for 30 min at 4°C, the floating fat cake containing intracellular lipid droplets was collected and stored at –20°C as described before (13).
SDS-PAGE and immunoblotting. The lipid droplet preparations from adrenocortical cells or adipocytes were assayed for protein
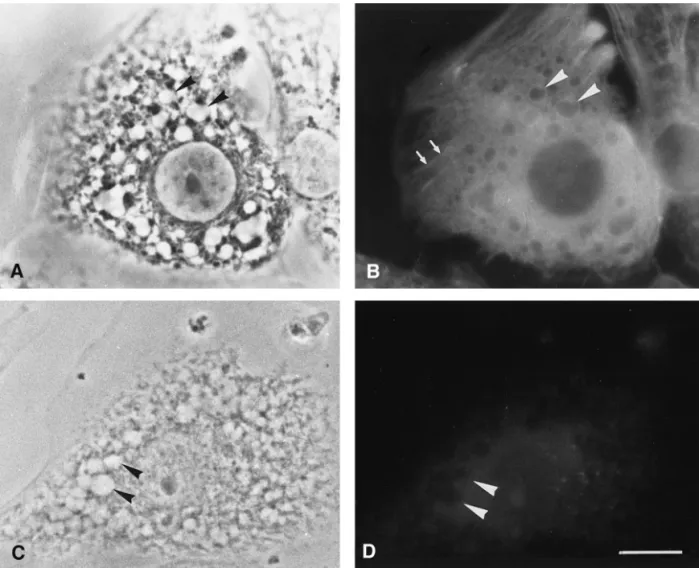
FIG. 1. Immunofluorescent staining of isolated lipid droplets from rat adrenocortical cells (A and B) and adipocytes (C and D) by anti-actin polyclonal antibodies. A and C are the phase pair of B and D, respectively. The isolated droplet from adipocytes is larger than that from adrenocortical cells. Bright rims labeled with actin can be seen encircling the lipid droplets (arrowheads). Bar⫽ 20m.
FIG. 2. SDS-PAGE and immunoblot analysis of lipid droplet
preparations. A: Coomassie blue-stained gel. B: Corresponding im-munoblot with beta-actin monoclonal antibody. C: Primary antibody blank control. Lane 1 contains myofibrillar proteins of rat soleus muscle. Lanes 2 and 3 contain proteins in lipid droplet preparations of adrenocortical cells and adipocytes, respectively. The myosin heavy chain (200 kD) and␣-actin (43 kD) of myofibrils are abundant. -actin (43 kD) (asterisks in A and visualized bands in B) is identi-fied in both lipid droplet preparations but not in myofibrils or the blank control.
concentration with Bio-Rad protein assay dye and methods. An equal volume of sample buffer (62.5 mM Tris-HCl, pH 6.8 containing 2% SDS, 10% glycerol, and 5%-mercaptoethanol) was added, and the mixture was heated to 95°C for 5 min. After centrifugation (10,000 rpm for 3 min) the upper layer of lipid was discarded; proteins (30 – 40 g/lane) in the lower layer were electrophoresed on 10% polyacrylamide gels and transferred to nitrocellulose paper (21). Strips cut from the nitrocellulose paper were blocked with 5% non-fat milk in PBS and then incubated in primary antibody at 4°C over-night. After washing with PBS-0.1% Tween 20, the strips were incubated with biotin-conjugated secondary antibody for 1 h at room temperature. After washing with PBS-0.1% Tween-20, peroxidase-conjugated streptavidin was added and incubated for another hour at room temperature. After washing with PBS, positive bands were visualized by using H2O2 as the substrate and diaminobenzidine
(DAB) as the chromogen.
Two-dimentional electrophoresis. The Bio-Rad Mini Protean 2D-cell system and methods (Bio-Rad, Richmond, CA) were used to perform isoelectric focusing of capillary gels. First-dimension gels contained 1.6% ampholytes (pH 5–7) and 0.4% ampholytes (pH 3–10). Lipid droplet preparations from adrenocortical cells and adi-pocytes were supplemented with 9.5 M urea containing 1.6% am-pholytes (pH 5–7) and 0.4% amam-pholytes (pH 3–10). Protein (5–10 g/capillary) was loaded and run at 750 V for 3.5 h. Second dimen-sion gels containing 10% polyacrylamide were electrophored and transferred to nitrocellulose paper, and then immunoblotting was performed as mentioned.
Primary culture of adrenocortical cells. Primary cultures of rat adrenocortical cells were previously described (15). Briefly, adrenal glands were isolated and cut into small fragments. Cells were ob-tained by incubating the fragments in 1 mg/ml of a collagenase solution of DMEM for 30 min at 37°C with gentle shaking, followed by mechanical dispersion by aspiration using a glass pipette. Cells were then washed, pelleted by low-speed centrifugation (1000 rpm for 5 min), resuspended, and cultured in DMEM/Ham’s F-12 (1:1 v/v)
medium supplemented with 12.5% horse serum, 2.5% fetal bovine serum, and 100 IU penicillin/streptomycin. Cells were grown and spread onto glass coverslips and maintained at 37°C in an atmo-sphere of 95% air and 5% CO2.
Immunofluorescence. Isolated intracellular lipid droplets were placed on 10% poly-L-lysine-coated slides for 30 min at room temper-ature for adhesion, then fixed with 10% formalin in PBS for 5 min and blocked with 5% non-fat milk for 30 min. Cultured cells grown on coverslips were fixed and permeated with methanol (⫺20°C) for 10 min. Nonspecific binding sites were blocked by incubation of NaBH4
(1 mg/ml in PBS) for 30 min. Purified lipid droplets and cultured cells were then incubated with rabbit polyclonal anti-actin antibodies and mouse monoclonal anti-beta-actin antibody for 1 h at room temper-ature, respectively. After washing with PBS, the samples were re-acted with Texas-Red-conjugated goat anti-rabbit or FITC-conjugated goat anti-mouse secondary antibodies for another hour at room temperature. After PBS washing, samples were mounted with 2% n-propyl gallate and 50% glycerol in PBS (pH 8.0), sealed in place with nail polish, and examined with a Nikon epifluorescence micro-scope.
For FITC-phalloidin staining, cultured cells were fixed with 10% formalin for 10 min and then acetone (⫺20°C) treatment for another 3 min. After PBS rinsing, FITC-phalloidin was incubated for 20 min at room temperature to label the actin filaments. After PBS washing, samples were mounted and examined.
RESULTS
Actin Is Associated with Isolated Lipid Droplets
Using hypotonic lysis and discontinuous sucrose
gra-dient centrifugation, we isolated intracellular lipid
droplets from adipocytes and adrenocortical cells. The
isolated steroidogenic intracellular lipid droplets of
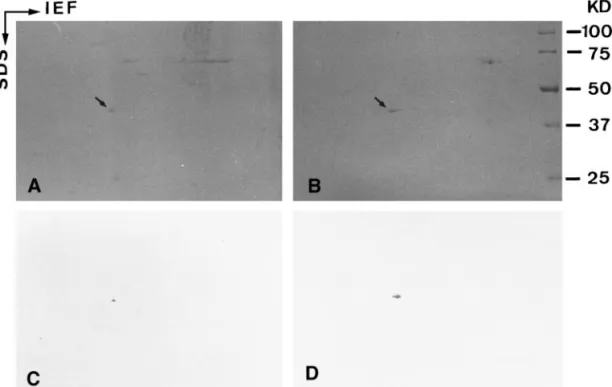
ad-FIG. 3. Two-dimensional gels and immunoblot analysis of lipid droplet preparations of adrenocortical cells (A and C) and adipocytes (B and D). A and B: Coomassie blue-stained gels. C and D: Parallel gels immunoblotted with-actin monoclonal antibody. Gels show that the actin (43 kD) in both lipid droplet preparations is only the beta isoform (arrows in A and B). Immunoblot shows the significant-actin spots in both lipid droplet preparations (C and D).
renocortical cells were small in bright field (Fig. 1A).
Immunofluorescent staining with anti-actin polyclonal
antibodies displayed peripheral staining of some of the
lipid droplets (Fig. 1B). In addition, the isolated
neu-tral lipid droplet from adipocytes was clear and large in
bright field as shown in Fig. 1C. After the same
immu-nostaining, a bright fluorescent rim structure was
ob-served surrounding the large lipid droplet (Fig. 1D).
There was no immunofluorescent reactivity without
primary antibodies in the blank control (data not
shown). These results indicate that actin was not only
co-isolated with intracellular lipid droplets but also
associated with the surface of isolated lipid droplets.
Lipid Droplet-Associated Actin Is in the
-Isoform
In the present study, we used SDS-PAGE and
im-munoblot analysis to identify the isoform of lipid
droplet-associated actin (Fig. 2). The abundant myosin
(200 kD) and alpha isoform of actin (43 kD) in
myofi-brils of rat soleus muscles were visualized by
Coomas-sie blue R-250 staining (lane 1 of Fig. 2A). The proteins
co-isolated with intracellular lipid droplets of rat
adre-nocortical cells (lane 2 of Fig. 2A) and adipocytes (lane
3 of Fig. 2A) were also separated by SDS-PAGE.
Coo-massie blue staining showed that the proteins in the
lipid droplet preparation of adrenocortical cell differed
from those of adipocyte. Interestingly, a 43 kD protein
(asterisks in lanes 2 and 3 of Fig. 2A) having the same
migration rate as the
␣-actin of myofibrils was
visual-ized in both lipid droplet preparations. Immunoblot
analysis showed that the 43 kD protein was indeed
recognized by the anti-
-actin monoclonal antibody
(vi-sualized bands in lanes 2 and 3 of Fig. 2B), but the
␣-actin in myofibrils of rat soleus muscles was not
labeled (lane 1 of Fig. 2B). As shown in Fig. 2C, there
was a clear background when the primary anti-
-actin
antibody were omitted. Furthermore, two-dimensional
gel electrophoresis indicated that only
-type actin was
visualized in lipid droplet preparations of
adrenocorti-cal cells (arrow in Fig. 3A) and adipocytes (arrow in
FIG. 4. Immunofluorescence staining of cultured adrenocortical cells. A and C are the phase images of B and D, respectively. Intracellular lipid droplets became clear vacuoles after methanol fixation and extraction (arrowheads in A). Note that bright rims labeled with-actin monoclonal antibody enclose intracellular lipid droplets (arrowheads in B). Some stress fibers are also positive for-actin (arrows in B). No fluorescent staining can be observed in blank control (arrowheads in D). Bar⫽ 20m.
Fig. 3B) by Coomassie blue staining. Lipid
droplet-associated actin was identified to be the beta-type by
two-dimensional immunoblot of adrenocortical cells
(Fig. 3C) and adipocytes (Fig. 3D).
Globular Type of
-Actin Is Associated
with Intracellular Lipid Droplets
Immunofluorescence was used to investigate the
lo-calization of
-actin in cultured adrenocortical cells.
After methanol fixation, cultured adrenocortical cells
showed numerous intracellular lipid droplets that had
a clear appearance (arrowheads in Fig. 4A). Anti-
-actin monoclonal antibody not only labeled the
fimentous stress fibers (arrows in Fig. 4B) but also
la-beled the rim structures around the intracellular lipid
droplets (arrowheads in Fig. 4B). With omission of the
primary antibody, there was no staining of stress fibers
or the periphery of lipid droplets (arrowheads in Figs.
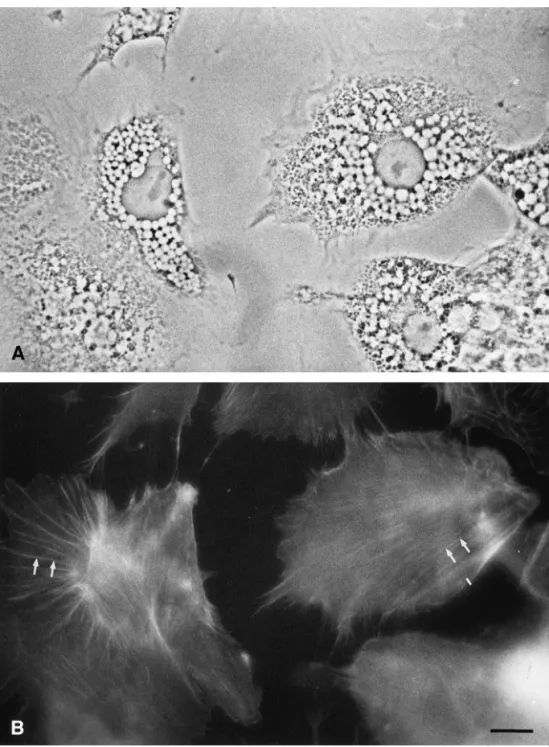
4C and 4D). On the other hand, we utilized
FITC-conjugated phalloidin to specify the characteristics of
actin filaments in cultured adrenocortical cells. Stress
fibers, the bundles of actin filaments, were strongly
stained by FITC-phalloidin (arrows in Fig. 5B), which
however did not mark the periphery of the lipid
drop-FIG. 5. FITC-phalloidin staining of cultured adrenocortical cells. A is the phase pair of B. Note that stress fibers (arrows in B) but not intracellular lipid droplets are labeled by FITC-phalloidin. Bar⫽ 20m.
let. These data suggest that actin located on the
sur-face of lipid droplet might be the globular type instead
of the filamentous type.
DISCUSSION
Lipid droplet-associated proteins are potentially
in-volved in mediating lipid metabolism. Among these
proteins, perilipins were first described and were
pro-posed to participate in lipid hydrolysis and lipid
pack-aging in adipocytes, steroidogenic cells of the adrenal
cortex, and testes (22). The adipose
differentiation-related protein (ADRP) is another lipid
droplet-associated protein that plays a role in the management
of lipid stores in a wide variety of cells including
adi-pocytes, steroidogenic Leydig cells, fibroblasts and
hep-atoma cells (23). The capsular proteins of lipid droplets
are reported to mediate lipid hydrolysis in response to
hormone stimulation in adrenocortical cells (14, 15)
and Leydig cells (16). In addition, a novel
vimentin-associated protein was proposed to protect the nascent
lipid droplets because the protein might translocate
from vimentin intermediate filaments to the surface of
nascent lipid droplets during lipid accumulation in
3T3-L1 adipocytes (17). The above-mentioned proteins
may act as barrier proteins enclosing the lipid droplets,
but lipid transport into or out of droplets remains
un-clear (18).
Previous studies have shown that intracellular lipid
droplets and mitochondria will attach to vimentin
in-termediate filament in steroidogenic adrenal cells (24,
25) and Leydig cells (26). Stimulation of acrylamide,
which specifically disrupts vimentin intermediate
fila-ments and shortens the distance between lipid droplets
and mitochondria, improves steroid production in
mouse adrenal tumor (Y-1) cells (27). Thus, the binding
of lipid droplet and mitochondria with vimentin
inter-mediate filaments provides a possible mechanism by
which the transport of cholesterol takes place from
lipid droplets to mitochondria (26, 27). On the other
hand, the nascent lipid droplet of 3T3-L1 adipocytes
was even enclosed and protected by a vimentin
inter-mediate filamentous cage during adipose conversion
(28). Vimentin, one of the intermediate cytoskeletal
proteins, seems to be another type of lipid
droplet-associated protein involved in the regulation of lipid
metabolism.
Treatment with cytochalasins induced the
disrup-tion of actin filaments and inhibited the conversion of
[
3H]cholesterol to 20alpha-[
3H]dihydroprogesteron (a
major product of the mitochondrial cleavage enzyme)
in adrenal tumor (Y-1) cells during ACTH stimulation.
Intact actin filaments were proposed to be necessary
for the transport of cholesterol from intracellular lipid
droplets to mitochondria (29, 30). Moreover, the
trans-port of cholesterol in adrenal tumor (Y-1) cells and
Leydig cells responding to trophic hormone or
dibu-tyryl cyclic AMP was also inhibited by liposomes
con-taining anti-actin antibodies (31, 32) or by erythrocyte
ghosts loaded with DNase I (33). It seems that
steroi-dogenic cells maintain a pool of monomeric actin
(glob-ular type of actin) which is available for facilitating
cholesterol transport (34, 35).
In the present study, we provide evidence that
glob-ular beta-actins are significantly associated with the
surface of intracellular lipid droplets in adrenocortical
cells and adipocytes by immunofluorescent staining
and immunoblotting analysis. However, both cells
greatly differ in their physiological functions. These
data therefore suggest that beta-actin might be a
uni-versal protein located on the surface of intracellular
lipid droplets. Based on the fact that actin filaments
have a defined polarity, by showing that the addition of
monomers occurs faster at the plus (
⫹) end than the
minus (
⫺) end, we suspect that globular beta-actins
associated with lipids may guide the direction or
accel-erate intracellular lipid transport during hormone
stimulation.
ACKNOWLEDGMENTS
This work was partly supported by grants from the National Science Council of the Republic of China (NSC 88-234-B-037-043 and NSC 89-2320-B-038-071).
REFERENCES
1. Vandekerckhove, J., and Weber, K. (1978) At least six different actins are expressed in a higher mammal: An analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J.
Mol. Biol. 126, 783– 802.
2. Garrels, J. I., and Gibson, W. (1976) Identification and charac-terization of multiple forms of actin. Cell 9, 793– 805.
3. Vandekerckhove, J., and Weber, K. (1981) Actin typing on total cellular extracts: A highly sensitive protein-chemical procedure able to distinguish different actins. Eur. J. Biochem. 113, 595– 603.
4. Geiger, B. (1983) Membrane-cytoskeleton interaction. Biochem.
Biophy. Acta. 737, 305–341.
5. Jacobson, B. S. (1983) Interaction of the plasma membrane with the cytoskeleton: An overview. Tissue Cell 15, 829 – 852. 6. Rotman, A., Heldman, J., and Linder, S. (1982) Association of
membrane and cytoplasmic proteins with the cytoskeleton in blood platelets. Biochemistry 21, 1713–1719.
7. Otto, J. J. (1990) Vinculin [Review]. Cell Motil. Cytoskel. 16, 1– 6. 8. Burridge, K., and Cornell, L. (1983) Talin: A cytoskeletal com-ponent concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 3, 405– 417.
9. Gicquaud, C. (1995) Does actin bind to membrane lipids under conditions compatible with those existing in vivo? Biochem.
Bio-phys. Res. Commun. 208, 1154 –1158.
10. Bouchard, M., Pare, C., Dutasta, J.-P., Chauvet, J.-P., Gicquaud, C., and Auger, M. (1998) Interaction between G-actin and vari-ous types of liposomes: A19
F,31
P, and 2
H nuclear magnetic res-onance study. Biochemistry 37, 3149 –3155.
11. Greenberg, A. S., Egan, J. J., Wek, S. A., Garty, N. B., Blanchette-Mackie, E. J., and Londos, C. (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein Vol. 289, No. 5, 2001 BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS
associated with the periphery of lipid storage droplets. J. Biol.
Chem. 266, 11341–11346.
12. Blanchette-Mackie, E. J., Dwyer, N. K., Barber, T., Coxey, R. A., Takeda, T., Rondinone, C. M., Theodorakis, J. L. Greenberg, A. S., and Londos, C. (1995) Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J. Lipid Res.
36, 1211–1226.
13. Servetnick, D. A., Brasaemle, D. L., Gruia-Gray, J., Kimmel, A. R., Wolff, J., and Londos, C. (1995) Perilipin are associated with cholesterol ester droplets in steroidogenic adrenal cortical and Leydig cells. J. Biol. Chem. 270, 16,970 –16,973.
14. Wang, S.-M., and Fong, T.-H. (1995) A lipid droplet-specific capsule is present in rat adrenal cells: Evidence from a mono-clonal antibody. Biochem. Biophys. Res. Commun. 217, 81– 88. 15. Fong, T.-H., and Wang, S.-M. (1997) Dissection of the signaling
mechanism for capsule detachment of lipid droplets in rat adre-nocortical cells. J. Cell. Biochem. 65, 67–74.
16. Fong, T.-H., Wang, S.-M., and Lin, H.-S. (1996) Immunocyto-chemical demonstration of a lipid droplet-specific capsule in cultured Leydig cells of the golden hamsters. J. Cell. Biochem.
63, 366 –373.
17. Wang, S.-M., Fong, T.-H., Hsu, S.-Y., Chien, C.-L., and Wu J.-C. (1997) Reorganization of a novel vimentin-associated protein in 3T3-L1 cells during adipose conversion. J. Cell. Biochem. 67, 84 –91.
18. Londos, C., Brasaemle, D. L., Schultz, C. J., Segrest, J. P., and Kimmel, A. R. (1999) Perilipin, ADRP and other proteins that associate with intracellular neutral lipid droplets in animal cells.
Semi. Cell Develop. Biol. 10, 51–58.
19. Mrotek, J. J., Mathew, J. K., Curtis, J. C., and Johansson, K. R. (1981) A method for the isolation of lipid droplet fractions from decapsulated rat adrenals. Steroids 38, 229 –241.
20. Rodbell M. (1964) Metabolism of isolated fat cells. J. Biol. Chem.
239, 375–380.
21. Towbin, H., Staehelin, T., and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacryamide gels to nitrocellulose sheets: Procedure and some application. Proc. Natl. Acad. Sci.
USA 76, 4350 – 4354.
22. Londos, C., Brasaemle, D. L., Gruia-Gray, J., Servetnick, D. A., Schultz, C. J., Levin, D. M., and Kimmel, A. R. (1995) Perilipin: Unique proteins associated with intracellular neutral lipid drop-lets in adipocytes and steroidogenic cells. Biochem. Soc. Trans.
23, 611– 615.
23. Brasaemle, D. L., Barber, T., Wolins, N. E., Serrero, G.,
Blanchette-Mackie, E. J., and Londos, C. (1997) Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38, 2249 –2263. 24. Almahbobi, G., Williams, L. J., and Hall, P. F. (1992) Attach-ment of steroidogenic lipid droplets to intermediate filaAttach-ments in adrenal cells. J. Cell Sci. 101, 383–393.
25. Almahbobi, G., Williams, L. J., and Hall, P. F. (1992) Attach-ment of mitochodria to intermediate filaAttach-ments in adrenal cells: Relevence to the regulation of steroid synthesis. Exp. Cell Res.
200, 361–369.
26. Almahbobi, G., Williams, L. J., Han, X.-G., and Hall, P. F. (1993) Binding of lipid droplets and mitochondria to intermediate fila-ments in rat Leydig cells. J. Reprod. Fertil. 98, 209 –217. 27. Shiver, T. M., Sackett, D. L., Knipling, L., and Wolff, J. (1992)
Intermediate filaments and steroidogenesis in adrenal Y-1 cells: Acrylamide stimulation of steroid production. Endocrinology
131, 201–207.
28. Franke, W. W., Hergt, M., and Grund, C. (1987) Rearrangement of the vimentin cytoskeleton during adipose conversion: Forma-tion of an intermediate filament cage around lipid globules. Cell
49, 131–141.
29. Mrotek, J. J., and Hall, P. F. (1977) Response of adrenal tumor cells to adrenocorticotropin: Site of inhibition by cytochalasin B.
Biochemistry 16, 3177–3181.
30. Hall, P. F., Osawa, S., and Mrotek, J. (1981) The actions of various cytochalasins on mouse adrenal tumor cells in relation to trophic stimulation of steroidogenesis. Biochem. Biophys. Acta
676, 338 –344.
31. Hall, P. F., Charpponnier, C., Nakamura, M., and Gabbiani G. (1979) The role of microfilaments in the response of adrenal tumor cells to adrenocorticotrophic hormone. J. Biol. Chem. 254, 9080 –9084.
32. Osawa, S., Betz, G., and Hall, P. F. (1984) Role of actin in the responses of adrenal cells to ACTH and cyclic AMP: Inhibition by DNase I. J. Cell Biol. 99, 1335–1342.
33. Hall, P. F., Charpponnier, C., Nakamura, M., and Gabbiani, G. (1979) The role of microfilaments in the response of Leydig cells to luteinizing hormone. J. Steroid Biochem. 11, 1361–1366. 34. Hall, P. F. (1995) The roles of microfilaments and intermediate
filaments in the regulation of steroid synthesis. J. Steroid
Bio-chem. Mol. Biol. 55, 601– 605.
35. Hall, P. F., and Almahbobi, G. (1997) Roles of microfilaments and intermediate filaments in adrenal steroidogenesis. Microsc.
Res. Tech. 36, 463– 479.