Laser Trapping and Crystallization Dynamics of
L
‑Phenylalanine at
Solution Surface
Ken-ichi Yuyama,
†Teruki Sugiyama,*
,‡and Hiroshi Masuhara*
,††Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung University, Hsinchu 30010, Taiwan ‡Instrument Technology Research Center, National Applied Research Laboratories, Hsinchu 30076, Taiwan
*
S Supporting InformationABSTRACT: We present laser trapping behavior ofL-phenylalanine (L-Phe) at a surface
of its unsaturated aqueous solution by a focused continuous-wave (CW) near-infrared (NIR) laser beam. Upon the irradiation into the solution surface, laser trapping of the liquid-like clusters is induced concurrently with local laser heating, forming an anhydrous plate-like crystal at the focal spot. The following laser irradiation into a central part of the plate-like crystal leads to laser trapping at the crystal surface not only forL-Phe molecules/
clusters but also for polystyrene (PS) particles. The particles are closely packed at crystal edges despite that the crystal surface is not illuminated by the laser directly. The molecules/clusters are also gathered and adsorbed to the crystal surface, leading to crystal growth. The trapping dynamics and mechanism are discussed in view of optical potential formed at the crystal surface by light propagation inside the crystal.
SECTION: Surfaces, Interfaces, Porous Materials, and Catalysis
L
aser trapping of dielectric particles by a tightly focused laser beam was first demonstrated by Ashkin in 1986.1 Ever since, this technique has been widely employed as optical tweezers to trap and manipulate single micrometer-sized object in manyfields of physics, optics, and biology.2In chemistry, it has been applied to J-aggregates,3 polymers,4−6 amino acids clusters,7 micelles,8 quantum dots,9 as well as various nanoparticles,10−12 but individual molecules are too small to be trapped in solution at room temperature. Single J-aggregate spectroscopy,3 confined chemical reactions,4 patterning of nanoparticles,10and assembly dynamics of polymers, molecular clusters, and nanoparticles5−7,11,12 are representative results. One advantage of laser trapping is its performance to trap, manipulate, and assemble target objects three-dimensionally, so that most experiments have been conducted inside the solution. On the other hand, it is worth noting that numerous novel chemical phenomena have been observed at a solution surface.13−15 Therefore, we conceived an idea that laser trapping at a solution surface should induce new phenomena different from those inside solution, which will open a new door for laser trapping-induced chemistry.Actually, we have succeeded not only in assembling molecules/clusters but also in inducing phase transition: crystallization and liquid−liquid phase separation (LLPS) by focusing the laser at air/solution and glass/solution interfaces of a glycine solution, respectively.16−18 The increase in local concentration due to laser trapping of molecules/clusters are coupled with molecular alignment characteristic of an interface as well as surface deformation and convection, leading to crystallization and liquid droplet formation. Interestingly, the
dense liquid droplet formed in a glycine solution through LLPS never evolves to its crystal in spite of the considerably high concentration. This unique success strongly pushes us to study laser trapping phenomena at air/solution and glass/solution interfaces in molecular systems.
In this paper, we present laser trapping behavior of L
-phenylalanine (L-Phe) at a surface of its unsaturated aqueous
solution. A plate-like crystal is formed by laser trapping even under unsaturated conditions which causes no spontaneous nucleation. Furthermore, laser irradiation into a central part of the plate-like crystal leads to laser trapping of polystyrene (PS) particles andL-Phe molecules/clusters at the crystal surface, and
the latter trapping induces crystal growth in unsaturated solution. We consider that this work is an important milestone for establishing a new laser trapping technique to manipulate and assemble nanoparticles, polymers, molecular clusters, and proteins at a solution surface.
Figure 1a shows a schematic illustration of our experimental procedure for laser trapping of L-Phe, whose details are
described in the Supporting Information 1 (SI 1). A thinfilm (120−160 μm) of the unsaturated aqueous solution (super-saturation degree (SS); 0.83) was prepared in a handmade sample bottle, and the spigot was hermetically closed. Slow solvent evaporation at room temperature (25°C) by opening the spigot generated many needle-like monohydrate crystals as given in Figure 1b. On the other hand, laser irradiation into the Received: May 31, 2013
Accepted: July 11, 2013
Published: July 11, 2013
solution surface led to the formation of a plate-like crystal at the focal spot and the succeeding crystal growth, as shown in Figure 1c (see movie 1 in the SI). In order to measure the thickness of the formed crystal, we tried to observe light reflection from the top and bottom crystal faces by moving the objective lens manually in a vertical direction. However, it was impossible to distinguish the two crystal faces well with each other, which possibly indicates that the crystal thickness was thinner than the focal depth of a few micrometers. In the captured images of the grown large crystal, we found trail-like patterns inside the crystal extending from the focal spot to crystal edges. The observation of these optical trails are possibly ascribed to the difference in the optical condition in the crystal, implying that top and bottom surfaces of the crystal are not perfectlyflat and that the thickness at the trails is different from other parts. Incidentally, when the laser was irradiated inside the solution, only a particle-like assembly was formed at the focal spot without crystallization (see SI 2), which is similar to the result reported for several amino acids by Tsuboi et al.7
Surprisingly, laser trapping at the solution surface inducedL
-Phe crystallization even under unsaturation, although super-saturation is necessary in general for crystal nucleation. It is considered that this crystal formation was realized through local increase in SS by laser trapping of the liquid-like clusters consisting of solutes and solvents weakly linked with intermolecular interactions.19 It is known that L-Phe has two
kinds of stable pseudopolymorph of crystalline monohydrate (needle-like) and anhydrate (plate-like), and that the transition temperature is about 37 °C in H2O.
20,21
Namely, the monohydrate needle-like form is obtained at a temperature below the transition point, while the plate-like one is above the point. Our infrared-spectroscopic measurement also revealed that the plate-like crystal obtained here was ascribed to the anhydrate form (see SI 3).22Therefore, we consider that laser trapping is accompanied with temperature elevation over the transition point at the focal spot. Actually, the elevation under the current experimental condition was estimated to be 24−27 °C (see SI 4).23
Although the temperature elevation decreases the SS to 0.56 in view of the solubility,24 the laser trapping
accompanied by the local heating successfully leads to the plate-like crystal formation.
One of the most interesting results is that the crystal became larger and larger outside the focal spot and grew continuously beyond thefield of view of the charge-coupled device (CCD) camera (80 × 60 μm2) even under unsaturation. The crystal
growth under unsaturation wasfirst reported for laser trapping-induced crystallization of glycine in 2010.25 The generated glycine crystal grew to a size of about 30 μm during the irradiation, and then started dissolving. We explained the mechanism of the growth and dissolution as follows. Before nucleation, a local high concentration domain is formed in the focal volume by laser trapping of the liquid-like clusters and extensively expands to its outside. The crystal nucleation is induced at the focal point, and the following spontaneous crystal growth is realized by use of the surplus solutes in the domain. When the surplus solutes are completely used up, the crystal starts dissolving because of the unsaturation condition of the surroundings. In this experiment, the formed L-Phe crystal
kept growing continuously, but never showed the dissolution under laser irradiation. So, we consider that the concentrated domain is not only prepared before nucleation, but also extended from the crystal edge during the irradiation. In other words, laser trapping of the molecules/clusters around the formed crystal is still realized by the direct irradiation into a central part of the crystal through the following possible mechanisms.
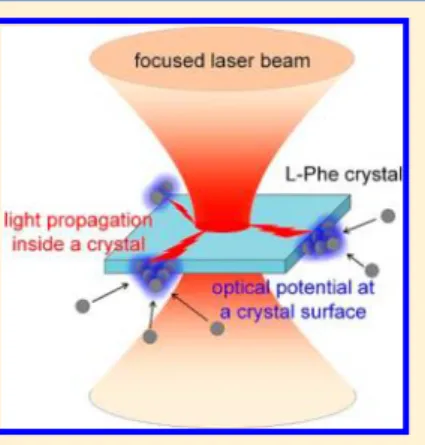
One possibility for the trapping is that heating and accompanying convection supply molecules/clusters homoge-neously toward the focal spot (Heating Effect).26,27 The molecules/clusters are adsorbed to the crystal surface through their transportation. As the second possibility, laser light incident to a central part of the plate-like crystal can propagate inside the crystal and penetrate into the surrounding solution, forming optical potential at the crystal surface (Optical Effect).28 The molecules/clusters are further trapped and attached to the crystal face. The third possibility is that the crystal surface is locally charged positively by the irradiation into the formed crystal (Electrostatic Effect). If the L-Phe
clusters are charged to be minus, they are attracted to the
Figure 1. (a) The experimental procedure for laser trapping-induced crystallization of L-Phe. (b) Needle-like monohydrate crystals generated
through spontaneous solvent evaporation at room temperature. (c) CCD images of the formation of a plate-like anhydrousL-Phe crystal and the
crystal surface by electrostatic attraction. Conversely, they would receive repulsive force if the charge relation is reverse.
In order to examine these possible explanations, we conducted a model experiment with 1 μm-sized PS particles as illustrated in Figure 2a. After the crystallization by laser trapping at 1.1 W, the laser was turned off and then a small amount of the colloidal solution was poured into the solution, when the Brownian motion of the particles was observed around the crystal. Immediately after reswitching on the laser incident to the crystal center at 1.1 W, the particles were clearly transported toward the crystal edges, indicating their directional diffusion. Interestingly, the transported particles were trapped at some specific corners of the crystal, where some optical trails were extended from the focal point (Figure 2b (1)). This result cannot be interpreted in terms of thermally induced convection, because the particles should be supplied homoge-neously to the crystal surface by the convection. Furthermore, the trapping and outward diffusion of particles at the crystal surface quickly responded to switching on and off of the laser as shown in Figure 2b (2) and (3), respectively (see movie 2 in the SI). This quick behavior seems inconsistent with the thermal diffusion, so that Heating Effect is nondominant. The laser power dependence reveals how laser irradiation attracts the particles at crystal edges, as shown in Figure 2c. The irradiation at 0.06 W loosely trapped the particles at the crystal surface, while that at 1.1 W packed them tightly. These results can be explained from the optical viewpoint that the laser incident to a central part of the crystal propagates up to the edges along the trails and forms optical potential at the crystal surface. The other possibility of Electrostatic Effect is interesting, but surface charge of the crystal should be generated by photoinduced charge separation while the crystal is growing with irradiation time.L-Phe molecules can be excited
only through four-photon absorption of the trapping laser beam, which is considered to be negligible under the present
conditions. Thus, Optical Effect is the most probable to interpret the trapping dynamics of PS particles at the crystal surface.
Another experiment on laser trapping of the formed plate-like crystal provided us a crucial insight to explain how laser irradiation into a crystal central part can propagate along the trails inside the crystal (Figure 3). When the irradiation was
stopped after the crystallization, the formed crystal migrated outward at a solution surface and never sank into solution due to surface tension. Before the crystal moves away completely from the focal position, the laser of 0.06 W was switched on again. The plate-like crystal went back to the original position, in which the focal point traced the bright trail in the image of the crystal. It is interesting to note that the focal point always
Figure 2.(a) The experimental procedure for a model experiment using PS particles with size of 1μm. (b,c) CCD images of PS particles around the crystal under laser irradiation.
Figure 3. The behavior of outward diffusion and retrapping of the plate-like crystal.
came back along the bright trail to the original central point where the initial crystal formation was started. The diffusion upon switching off and the following trapping back to the original position along the trail are reproducible. The result implies that the crystal central point is the thickest and/or has the highest refractive index at the crystal plane, and the deepest optical potential is formed when the focus is set there. In addition, the trail should be thicker and/or have higher refractive index than other parts, which generates stronger trapping force. The observation of two-dimensional distribu-tions in thickness and refractive index should be done while the crystal is kept at the focal point. Here we suppose that some parts of the trails can work as a waveguide for laser light incident to a crystal central part. The light is guided to some specific positions at the crystal surface, where PS particles are preferentially trapped. The results here revealed the interplay between the trails and the laser light incident to the crystal, which supports Optical Effect for laser trapping at the crystal surface.
It is reasonable to consider that the trapping of L-Phe
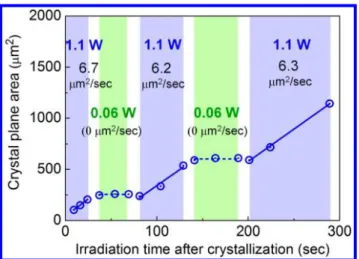
molecules/clusters at the crystal surface is similarly achieved by light propagation inside the crystal upon irradiating the laser into a central part of the crystal. Their trapping at the crystal surface was clearly confirmed through crystal growth, and their amount could be changed by tuning the laser power. The trapping behavior was quantified by examining the time evolution of the crystal plane area. Figure 4 shows the time
evolution of the plane area upon alternately varying the laser power between 1.1 and 0.06 W. The irradiation time of 0 s corresponds to the time when the small crystal became identified on a CCD image. The plane area enlarged linearly with irradiation time, and the slope can be deemed as a crystal growth rate. The crystal growth rate under the initial 1.1 W-irradiation was estimated to be 6.7μm2/sec. After that, the laser power was reduced to 0.06 W, and the laser was continuously irradiated into the crystal for 30 s. During this period, the crystal was still trapped stably at the focal spot, but its growth was almost stopped while keeping crystal size of 260 μm2. When the power was increased to 1.1 W again, the crystal immediately started growing with a growth rate of 6.2μm2/sec. Such an alternating change in the growth rate of 0 and ca. 6.4 μm2/sec was observable repetitively by the further irradiation.
Thus, the growth rate at 1.1 W was always kept to almost a
constant value (6.4μm2/sec on average), even after the interval of the 30 s-irradiation at 0.06 W. This result is extremely interesting since the growth rate during the late 1.1 W-irradiation part is expected to be slowed down gradually because the SS in the remaining solution should decrease accompanied with crystal growth.
Thus, the direct irradiation into the central part at 1.1 W can trap L-Phe molecules/clusters at the crystal edge in the similar
manner to the case of PS particles despite that the growing surfaces are not illuminated by the laser. The trapped molecules/clusters are also rearranged by the laser light guided from the central part, leading to further crystal growth as well as the resultant formation of bright trails.29Based on CCD images of the crystal, we consider that the trail structure is formed by optical anisotropic crystal growth. Of course, the conventional growth adsorbing associated clusters may take place simulta-neously around the trails, compensating inhomogeneous surface energy. It is likely that hydrate clusters of L-Phe exist
dominantly in solution around the crystal, since the monohydrate crystallization is preferentially induced at room temperature. Considering that the trapping of the hydrate clusters at a crystal surface leads to crystal growth of the anhydrous form, optical potential formed at the crystal surface might induce not only laser trapping of L-Phe molecules/
clusters but also dehydration of the hydrate clusters, which is similar to laser trapping-induced dehydration of poly(N-isopropylacrylamide).5
In conclusion, we have successfully demonstrated the trapping of PS particles and L-Phe molecules/clusters at a
surface of a plate-like anhydrous crystal by focusing a continuous wave (CW) near-infrared (NIR) laser beam into its central part at a solution surface. The PS particles were closely packed at crystal edges, while the molecules/clusters were adsorbed to the crystal surface and crystal growth was induced. Their trapping at crystal edges was achieved despite that the crystal surface was not directly illuminated by the laser, so that we explained their trapping dynamics in view of light propagation inside the crystal through some trails. The propagated light possibly generates optical potential at the crystal surface, where crystal growth proceeds by laser trapping of the clusters and their dehydration. Subsequently, the laser light can be propagated to further distant position through this optically induced crystal growth, leading to continuous crystal growth. Upon switching off the laser irradiation, the plate-like crystal gradually dissolved and became smaller because of the unsaturation condition. Considering these results from the viewpoint of crystal growth, this technique will enable us to control crystal size arbitrarily. Laser trapping at a solution surface is considered to be unique compared with laser trapping inside solution. The present findings will be a base for new interesting laser trapping-induced chemical phenomena at a solution surface and an interface.
■
ASSOCIATED CONTENT*
S Supporting InformationExperimental procedure, laser trapping experiment inside the solution, polymorph analysis by IR measurement, estimation of local temperature elevation, and movies of the formation of anhydrous L-Phe crystal and the gathering of 1 μm-sized
polystyrene beads. This material is available free of charge via the Internet at http://pubs.acs.org.
Figure 4. The time evolution of the crystal plane area under laser irradiation.
■
AUTHOR INFORMATIONCorresponding Author
*E-mail: sugiyama@narlabs.org.tw (T.S.); masuhara@ masuhara.jp (H.M.).
Notes
The authors declare no competingfinancial interest.
■
ACKNOWLEDGMENTSThe present work is partly supported by the MOE-ATU Project (National Chiao Tung University) of the Ministry of Education, Taiwan, to H.M., and the National Science Council of Taiwan to T.S. (NSC 100-2113-M-492-002-MY2) and to H.M. (NSC 100-2113-M-009-001).
■
REFERENCES(1) Ashkin, A.; Dziedzic, J. M.; Bjorkholm, J. E.; Chu, S. Observation of a Single-Beam Gradient Force Optical Trap for Dielectric Particles. Opt. Lett. 1986, 11, 288−290.
(2) Ashkin, A. Optical Trapping and Manipulation of Neutral Particles Using Lasers. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 4853− 4860.
(3) Tanaka, Y.; Yoshikawa, H.; Masuhara, H. Two-Photon Fluorescence Spectroscopy of Individually Trapped Pseudoisocyanine J-Aggregates in Aqueous Solution. J. Phys. Chem. B 2006, 110, 17906− 17911.
(4) Ito, S.; Tanaka, Y.; Yoshikawa, H.; Ishibashi, Y.; Miyasaka, H.; Masuhara, H. Confinement of Photopolymerization and Solidification with Radiation Pressure. J. Am. Chem. Soc. 2011, 133, 14472−14475. (5) Hofkens, J.; Hotta, J.; Sasaki, K.; Masuhara, H.; Iwai, K. Molecular Assembling by the Radiation Pressure of a Focused Laser Beam: Poly(N-isopropylacrylamide) in Aqueous Solution. Langmuir 1997, 13, 414−419.
(6) Singer, W.; Nieminen, T. A.; Heckenberg, N. R.; Rubinsztein-Dunlop, H. Collecting Single Molecules with Conventional Optical Tweezers. Phys. Rev. E 2007, 75, 011916-1−011916-5.
(7) Tsuboi, Y.; Shoji, T.; Kitramura, N. Optical Trapping of Amino Acids in Aqueous Solutions. J. Phys. Chem. C 2010, 114, 5589−5593. (8) Hotta, J.; Sasaki, K.; Masuhara, H. A Single Droplet Formation from Swelled Micelles by Radiation Pressure of a Focused Infrared Laser Beam. J. Am. Chem. Soc. 1996, 118, 11968−11969.
(9) Jauffred, L.; Richardson, A. C.; Oddershede, L. B. Three-Dimensional Optical Control of Individual Quantum Dots. Nano Lett. 2008, 8, 3376−3380.
(10) Ito, S.; Yoshikawa, H.; Masuhara, H. Optical Patterning and Photochemical Fixation of Polymer Nanoparticles on Glass Substrates. Appl. Phys. Lett. 2001, 78, 2566−2568.
(11) Hosokawa, C.; Yoshikawa, H.; Masuhara, H. Cluster Formation of Nanoparticles in an Optical Trap Studied by Fluorescence Correlation Spectroscopy. Phys. Rev. E 2005, 72, 021408-1−021408-7. (12) Tanaka, Y.; Yoshikawa, H.; Itoh, T.; Ishikawa, M. Laser-Induced Self-Assembly of Silver Nanoparticles via Plasmonic Interactions. Opt. Express 2009, 17, 18760−18767.
(13) Wickman, H. H.; Korley, J. N. Colloid Crystal Self-Organization and Dynamics at the Air/Water Interface. Nature 1998, 393, 445−447. (14) Zepik, H.; Shavit, E.; Tang, M.; Jensen, T. R.; Kjaer, K.; Bolbach, G.; Leiserowitz, L.; Weissbuch, I.; Lahav, M. Chiral Amplification of Oligopeptides in Two-Dimensional Crystalline Self-Assemblies on Water. Science 2002, 295, 1266−1269.
(15) Krafft, M. P.; Riess, J. G. Chemistry, Physical Chemistry, and Uses of Molecular Fluorocarbon−Hydrocarbon Diblocks, Triblocks, and Related CompoundsUnique “Apolar” Components for Self-Assembled Colloid and Interface Engineering. Chem. Rev. 2009, 109, 1714−1792.
(16) Sugiyama, T.; Adachi, T.; Masuhara, H. Crystallization of Glycine by Photon Pressure of a Focused CW Laser Beam. Chem. Lett. 2007, 36, 1480−1481.
(17) Yuyama, K.; Sugiyama, T.; Masuhara, H. Millimeter-Scale Dense Liquid Droplet Formation and Crystallization in Glycine Solution Induced by Photon Pressure. J. Phys. Chem. Lett. 2010, 1, 1321−1325. (18) Sugiyama, T.; Yuyama, K.; Masuhara, H. Laser Trapping Chemistry: From Polymer Assembly to Amino Acid Crystallization. Acc. Chem. Res. 2012, 45, 1946−1954.
(19) Chattopadhyay, S.; Erdemir, D.; Evans, J. M. B.; Ilavsky, J.; Amenitsch, H.; Segre, C. U.; Myerson, A. S. SAXS Study of the Nucleation of Glycine Crystals from a Supersaturated Solution. Cryst. Growth Des. 2005, 5, 523−527.
(20) Mohan, R.; Koo, K.-K.; Strege, C.; Myerson, A. S. Effect of Additives on the Transformation Behavior of L-Phenylalanine in Aqueous Solution. Ind. Eng. Chem. Res. 2001, 40, 6111−6117.
(21) Kee, N. C. S.; Arendt, P. D.; Goh, L. M.; Tan, R. B. H.; Braatz, R. D. Nucleation and Growth Kinetics Estimation for L-Phenylalanine Hydrate and Anhydrate Crystallization. CrystEngComm 2011, 13, 1197−1209.
(22) Lu, J.; Lin, Q.; Li, Z.; Rohani, S. Solubility of L-Phenylalanine Anhydrous and Monohydrate Forms: Experimental Measurements and Predictions. J. Chem. Eng. Data 2012, 57, 1492−1498.
(23) Ito, S.; Sugiyama, T.; Toitani, N.; Katayama, G.; Miyasaka, H. Application of Fluorescence Correlation Spectroscopy to the Measurement of Local Temperature in Solutions under Optical Trapping Condition. J. Phys. Chem. B 2007, 111, 2365−2371.
(24) Weast, R. C. CRC Handbook of Chemistry and Physics, 1st student ed.; CRC Press, Inc.: Boca Raton, FL; 1988.
(25) Rungsimanon, T.; Yuyama, K.; Sugiyama, T.; Masuhara, H. Crystallization in Unsaturated Glycine/D2O Solution Achieved by
Irradiating a Focused Continuous Wave Near Infrared Laser. Cryst. Growth Des. 2010, 10, 4686−4688.
(26) Braun, D.; Libchaber, A. Trapping of DNA by Thermophoretic Depletion and Convection. Phys. Rev. Lett. 2002, 89, 188103-1− 188103-4.
(27) Duhr, S.; Braun, D. Two-Dimensional Colloidal Crystals Formed by Thermophoresis and Convection. Appl. Phys. Lett. 2005, 86, 131921-1−131921-3.
(28) DelRe, E.; Tamburrini, M.; Segev, M.; Pergola, R. D.; Agranat, A. J. Spontaneous Self-Trapping of Optical Beams in Metastable Paraelectric Crystals. Phys. Rev. Lett. 1999, 83, 1954−1957.
(29) Gordon, R.; Blakely, J. T.; Sinton, D. Particle-Optical Self-Trapping. Phys. Rev. A 2007, 75, 055801-1−055801-4.