Prodigiosin inhibits gp91
phox
and iNOS expression to protect mice against the
oxidative/nitrosative brain injury induced by hypoxia
–ischemia
Chia-Che Chang
b,f,j,k, Yea-Hwey Wang
d,1, Chang-Ming Chern
c,⁎
,1, Kuo-Tong Liou
e,1, Yu-Chang Hou
g,h,i,1,
Yu-Ta Peng
b, Yuh-Chiang Shen
a,b,⁎⁎
a
National Research Institute of Chinese Medicine, Taipei, Taiwan
b
Institute of Biomedical Sciences, National Chung-Hsing University, Taichung, Taiwan
c
Division of Neurovascular Disease, Neurological Institute, Taipei Veterans General Hospital and School of Medicine, National Yang-Ming University, Taipei, Taiwan
d
Department of Nursing, College of Medicine and Nursing, Hungkuang University, Taichung, Taiwan
eDepartment of Chinese Martial Arts, Chinese Culture University, Taipei, Taiwan f
Graduate Institute of Basic Medical Science, China Medical University, Taichung, Taiwan
g
Department of Chinese Medicine, Taoyuan General Hospital, Department of Health, Taiwan
h
Department of Nursing, Yuanpei University, Hsinchu, Taiwan
i
Department of Bioscience Technology, Chuan-Yuan Christian University, Taoyuan, Taiwan
j
Agricultural Biotechnology Center, National Chung-Hsing University, Taichung, Taiwan
k
Center of Infectious Disease and Signaling Research, National Cheng Kung University, Tainan, Taiwan
a b s t r a c t
a r t i c l e i n f o
Article history: Received 28 June 2011 Revised 29 August 2011 Accepted 29 August 2011 Available online 7 September 2011 Keywords:gp91phox
Hypoxia iNOS
Middle cerebral artery occlusion/reperfusion Oxygen–glucose deprivation
Prodigiosin
This study aimed to explore the mechanisms by which prodigiosin protects against hypoxia-induced oxidative/ nitrosative brain injury induced by middle cerebral artery occlusion/reperfusion (MCAo/r) injury in mice. Hypoxia in vitro was modeled using oxygen–glucose deprivation (OGD) followed by reoxygenation of BV-2 microglial cells. Our results showed that treatment of mice that have undergone MCAo/r injury with prodigiosin (10 and 100μg/kg, i.v.) at 1 h after hypoxia ameliorated MCAo/r-induced oxidative/nitrosative stress, brain infarction, and neurological deficits in the mice, and enhanced their survival rate. MCAo/r induced a remarkable production in the mouse brains of reactive oxygen species (ROS) and a significant increase in protein nitrosylation; this primarily resulted from enhanced expression of NADPH oxidase 2 (gp91phox), inducible nitric oxide synthase (iNOS), and the infiltration of CD11b leukocytes due to breakdown
of blood–brain barrier (BBB) by activation of nuclear factor-kappa B (NF-κB). All these changes were significantly diminished by prodigiosin. In BV-2 cells, OGD induced ROS and nitric oxide production by up-regulating gp91phox
and iNOS via activation of the NF-κB pathway, and these changes were suppressed by prodigiosin. In conclusion, our results indicate that prodigiosin reduces gp91phox and iNOS expression possibly by impairing
NF-κB activation. This compromises the activation of microglial and/or inflammatory cells, which then, in turn, medi-ates prodigiosin's protective effect in the MCAo/r mice.
© 2011 Elsevier Inc. All rights reserved.
Introduction
Stroke is still ranked as the third common cause of death in devel-oped countries. Hypoxia–ischemic stroke induces brain injury through energy deficiency-related inappropriate activation of ionotropic N-methyl-D-aspartate receptors, which is caused by excessive gluta-mate release exciting neurons to death via induced overproduction of
free radicals. This so-called oxidative stress involves recruited leukocytes, active microglial cells, damaged neurons and astrocytes, all of which are present in the ischemic stroke-damaged tissues (Lo et al., 2003; Shen
et al., 2008). Among the free radical producing-enzyme systems, the
activation of NADPH oxidase 2 (gp91phox) and inducible-nitric oxide synthase (iNOS), in response to various proinflammatory mediators (e.g., interleukin-1β (IL-1β)) produced through ischemia/hypoxia induction, represents a major pathophysiological mechanism for stroke-induced brain injury (Argaw et al., 2006; Nagel et al., 2007;
Borutaite et al., 2006).
During cerebral ischemia, activation of various transcriptional factor(s) such as nuclear factor-kappa B (NF-κB) plays a pivotal role in mediating oxidative stress-induced cell injury and in regulating post-ischemic inflammation. This occurs possibly through up-regulation of the inflammatory genes and proteins that contribute to cell death
Abbreviations: BBB, blood–brain barrier; gp91phox, NADPH oxidase 2; iNOS, inducible
nitric oxide synthase; MCAo/R, middle cerebral artery occlusion/reperfusion; NF-κB, nuclear factor-kappa B; OGD, oxygen–glucose deprivation; ROS, reactive oxygen species.
⁎ Corresponding author.
⁎⁎ Correspondence to: Y.C. Shen, National Research Institute of Chinese Medicine, 155-1 Li-Nung Street, Sec. 2, Shih-Pai, Taipei 112, Taiwan. Fax: + 886 2 28264266.
E-mail address:yuhcs@nricm.edu.tw(Y.-C. Shen).
1These authors contributed equally in this study, respectively.
0041-008X/$– see front matter © 2011 Elsevier Inc. All rights reserved. doi:10.1016/j.taap.2011.08.027
Contents lists available atSciVerse ScienceDirect
Toxicology and Applied Pharmacology
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / y t a a p(Harari and Liao, 2010). In addition, the accumulation of hypoxia-induc-ible factor-1 (HIF-1) during cerebral ischemia triggers expression of the target genes involved in various adaptive responses after injury
(Semenza, 2000; Sharp and Bernaudin, 2004). In this context, both
pro-death and pro-survival roles for HIF-1 have been discussed
(Chen et al., 2009). For example, HIF-1 has been reported to be
neuro-protective(Correia and Moreira, 2010), while on the other hand two HIF-1 target genes (iNOS and cyclooxygenase 2) are known to contribute to brain injury via inflammation induction(Mi et al., 2008). Furthermore, we have showed that the NF-κB and/or HIF-1α dependent pathways are both underlying the hypoxia–ischemic stroke-induced iNOS and/or gp91phoxexpression in microglial cells(Chern et al., 2011). Prodigiosin (2-methyl-3-pentyl-6-methoxyprodiginine) is a tripyrrole red pigment produced by microorganisms such as Serratia marcescens(Chang et al., 2011). This bacterial secondary metabolite is featured by a diverse range of bioactivities with therapeutic benefits, including antibacterial, antimalarial (Demain, 1995; Williams and
Quadri, 1980), immunosuppressive(Han et al., 1998, 2001, 2005),
anti-cancer (Pérez-Tomás et al., 2003; Pérez-Tomás and Viñas, 2010) and antimetastatic(Williamson et al., 2006). Intriguingly, prodigiosin at non-cytotoxic doses functions as an immunosuppressant, while inducing apoptosis selectively against malignant cells at higher concen-trations (Williamson et al., 2006). In addition to acting on T cells, it is worth to note that prodigiosin was recently shown to suppress lipopolysaccharide-initiated inflammatory responses of macrophages, including nitric oxide (NO) production, through inhibiting the activa-tion of p38 MPAK, JNK and NF-κB (Huh et al., 2007). However, the potential of exploiting the anti-inflammatory effect of prodigiosin on inflammation-related disorders (e.g., ischemic stroke brain injury) has never been reported yet and therefore remains to be elucidated.
In this study, we performed an ischemic stroke murine model to elucidate whether treatment with prodigiosin (10 and 100μg/kg, i.v.) 1 h after ischemia is able to protect mice against middle cerebral artery occlusion/reperfusion (MCAo/r)-induced oxidative/nitrosative brain injury. In addition, hypoxia in vitro was modeled using oxygen– glucose deprivation (OGD) followed by reoxygenation of BV-2 micro-glial cells in order to explore the molecular mechanism(s) of action involved when prodigiosin protects against hypoxia/ischemia-induced microglial cell activation.
Materials and methods
Animals and induction of transient middle cerebral artery occlusion All animal procedures and protocols were conducted in accor-dance with The Guide for the Care and Use of Laboratory Animals (NIH publication, 85–23, revised 1996) and were reviewed and approved by our Animal Research Committee at National Research Institute of Chinese Medicine (approval number: NRICM-IACUC-96-A-11). Male ICR mice weighing 17–22 g (National Laboratory Animal Breeding and Research Center, Taipei, Taiwan) were anesthetized with a mixture of isoflurane (1.5–2%), oxygen, and nitrogen. Transient focal cerebral ischemia was performed using a heat-blunted nylon monofilament surgical suture (6–0) coated with silicone, which was introduced into the exposed external carotid artery, advanced to the internal carotid artery, and wedged into the circle of Willis to obstruct the origin of the right middle cerebral artery (RMCA). Thefilament was left in place for 40 min and then withdrawn. This procedure leads to reproducible infarcts similar in size and distribution to those reported by others using transient RMCA occlusion of a comparable duration
(Kim et al., 2008). Blood samples collected from femoral artery before
RMCA occlusion and 30 min after reperfusion were used for immediate arterial blood gas analysis. Mice were randomly assigned to the various groups. These consisted of one sham-operated group (sham, n = 10), two prodigiosin (PG10 or PG100μg/kg)-treated groups (n=10, for each dose), a reference drug undecylprodigiosin (UP100μg/kg)-treated
group and one vehicle-control CI/R group (CI/R only, n = 10). The sham-operated mice underwent the same surgical procedures without RMCA occlusion.
Drug preparation and administration
Prodigiosin and undecylprodigiosin were isolated and purified from Serratia marcescens as previously described (Wei and Chen,
2005; Wei et al., 2005). The purity and quantity of prodigiosin and
undecylprodigiosin were determined using high-performance liquid chromatography equipped with a UV detector (Hitachi D-2000 system, Tokyo, Japan) according to a reported protocol(Song et al., 2006). Purified prodigiosin and undecylprodigiosin first dissolved in 100 μL of ethanol (≥99.5% purity, Sigma-Aldrich, St. Louis, MO, USA) to make a stock solution of 1.0 mg/mL and was then diluted with normal saline to a range offinal concentrations (1–10 μg/mL). Mice were injected via the tail vein with 0.3 mL of prodigiosin (PG, 10–100 μg/kg, i.v.) or undecylprodigiosin (UP, an analog of PG) (100μg/kg, i.v.), or normal saline with 0.01% of ethanol as a vehicle control in the CI/R only and sham-operated groups. Other chemicals used in this study including pyrrolidine dithiocarbamate (PDTC,≥99% purity, a NF-κB inhibitor), diphenyleneiodonium chloride (DPI,≥98% purity, a NADPH oxidase inhibitor) and L-NAME (≥98% purity, a non-specific NOS inhibitor) and these were all purchased from Sigma-Aldrich (St. Louis, MO, USA). For the in vitro study, all drugs were dissolved in dimethyl sulfoxide (DMSO,≥99% purity). The concentrations of prodigiosin and reference drugs used in vitro, except as indicated, were used in the range from 5 to 20μM.
Evaluation of infarct volume after MCAo/r injury
Twenty-four hours after reperfusion, the mice were terminated by rapid decapitation under deep anesthesia. The whole brain was rapidly removed and sliced into 1-mm-thick coronal sections for staining with 2,3,5-triphenyltetrazoliumchloride (TTC) (Sigma-Aldrich, USA) and all brain slices were photographed to determine infarct volume as described in our previous report(Shen et al., 2008).
Assessment of the neurological deficit and analysis of survival rate The neurological deficit score for the mice was measured just before termination(Kofler et al., 2006). The following neurological deficit scoring (NDS) system was used: 0, no motor deficits (normal); 1, forelimb weakness and torso turning to the ipsilateral side when held by tail (mild); 2, circling to the contralateral side but normal posture at rest (moderate); 3, unable to bear weight on the affected side at rest (severe); and 4, no spontaneous locomotor activity or barrel rolling (critical). For survival rate analysis, mice were kept in individually ventilated cage systems after stroke induction. Survival rates were calculated immediately (day 0), 24 h (day 1), or 48 h (day 2) after stroke induction.
Immunohistochemical staining
Twenty-four hours after RMCA occlusion, the brains were prepared for confocal imaging as described in our previous report(Chern et al.,
2011). Six consecutive brain sections (thickness, 20μm) were cut and
collected at the same rostrocaudal levels (bregma−1.7 to −1.9 mm) from each group. Afterfixation, permeabilization and blocking, the brain slices were randomly selected to be incubated with appropriate first antibodies against nitrotyrosine (1:50, Upstate, Lake Placid, NY, USA), gp91phox (NOX2, 1:50, Santa Cruz Biotechnology, Santa Cruz,
CA, USA), CD11b (1:100, Biolegend, San Diego, CA, USA), iNOS (1:50) and p65NF-κB (1:50) (all from BD PharMingen, San Diego, CA, USA) in PBS containing 3% albumin at 4 °C overnight. After washing, the sections were incubated with fluorescein isothiocyanate (FITC)-conjugated
second antibodies. All coverslips were mounted with mounting medi-um containing 4′,6-diamidino-2-phenylindole (DAPI) to counterstain the DNA in the nuclei. The sliced tissues were examined using a laser-scanning confocal microscope (Leica TCS SPII; Heidelberg, Germa-ny). The distribution and numbers of immuno-positively stained cells were determined, and averaged in the entirefield of the image after sampling in the peri-infarct region under high magnification (×40 or ×63 objective) over five independent experiments. In some experiments, dihydroethidium (DHE; Molecular Probes, Eugene, OR, USA; 2 mg in 200μL PBS) was given i.v. to animals just before the onset of reperfusion to monitor superoxide production. DHE is oxidized by superoxide to ethidium (redfluorescence) which can be detected by fluorescence microscopy as described byChern et al. (2011). To eluci-date blood–brain barrier (BBB) vascular permeability, an enhanced leak-age of rhodamine isothiocyanate (RhITC) described by Kaur et al.
(2007)was followed. Briefly, an intraperitoneal (i.p.) injection of RhITC
(5μL of 1% RhITC per gram body weight) dissolved in normal saline was administered to hypoxic animals at 24 h after the MCAo/r exposure. Cell culture of microglial cells and measurements of NO and intracellular ROS production under oxygen–glucose deprivation (OGD)
Microglial cell line BV2 cells were cultured in Dulbecco's modified Eagle medium (Gibco Laboratories, NY, USA). The production of NO or ROS was measured after 8 h of oxygen–glucose deprivation (OGD) followed by 16 h of reperfusion(Chern et al., 2011). The accumulation of nitrite in the culture medium was measured by the Griess reagent and used to assess NO production. Intracellular ROS accumulation was measured using dichlorofluorescin diacetate (DCFH-DA) as an indicator for ROS(Shen et al., 1998).
Western immunoblot analysis
Equal amounts (50μg) of protein were subjected to 12% of sodi-um dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transferred to a hydrophobic polyvinylidene difluoride membrane. After blocking, the membrane was incubated overnight at 4 °C with an antibody against gp91phox (Santa Cruz Biotechnology), IκBα,
p65NF-κB (BD Transduction Laboratories, BD Biosciences, San Diego, CA, USA), iNOS (BD Bioscience Pharmingen), orβ-actin (Sigma-Aldrich). After incubation with a properly titrated second antibody, the immuno-blot on the membrane was visible after development with an enhanced chemiluminescence (ECL) system and was quantified using an imaging program.
Statistical analysis
All results in the text, tables, andfigures are presented as the mean ± S.E.M. (standard error of the mean). Data were analyzed by one-way analysis of variance (ANOVA) followed by the post hoc Student–Newman–Keuls (S–N–K) t-test for multiple comparisons. The difference in neurological deficit score (NDS) was determined by the Mann–Whitney analysis and χ2 test. Survival curves were
computed using the Kaplan–Meier method. Differences in survival rates were assessed using the Log-Rank test followed by Holm–Sidak method for all pairwise multiple comparisons. Values of pb0.05 were considered significant.
Results
Effects of prodigiosin on changes in cerebral infarction and neurological deficits (at 24 h), and survival rates (over 2 days) after cerebral ischemic/ reperfusion injury
In this study, the MCAo/r injury (stroke) was found to induce a remarkable cerebral infarction (65 ± 8 mm3) corresponding to around
46.5% of the whole brain. Importantly, treatment with prodigiosin (10 and 100μg/kg, i.v.) 1 h after ischemia dose-dependently reduced the MCAo/r-induced brain infarction by 45%–58% (Fig. 1A, one-way ANOVA, pb0.05, n=6 for each group). On the other hand, treatment with undecylprodigiosin (100μg/kg, i.v.), an analogous of prodigiosin as a reference drug, did not significantly reduce brain infarction (one-way ANOVA followed by S–N–K t-test). The blood gas data (pH, pCO2,
and pO2) and physiological data (blood pressure and heart rate) were
all monitored, and no significant difference could be observed among the various groups (data not shown). In parallel with the cerebral infarction, MCAo/r injury also induced severe neurological deficits
(Fig. 1B) as compared to the sham-operated mice (NDS = 0). Treatment
with prodigiosin (100μg/kg, i.v.) also significantly reduced these MCAo/r-induced neurological deficits (Fig. 1B; Mann–Whitney analysis andχ2-test, pb0.05, n=6–9 for each group) and improved the survival
rate (Fig. 1C; Log-Rank test for the survival curves, p = 0.015, followed by all pairwise multiple comparisons using Holm–Sidak method, pb0.05, n=10 for each group).
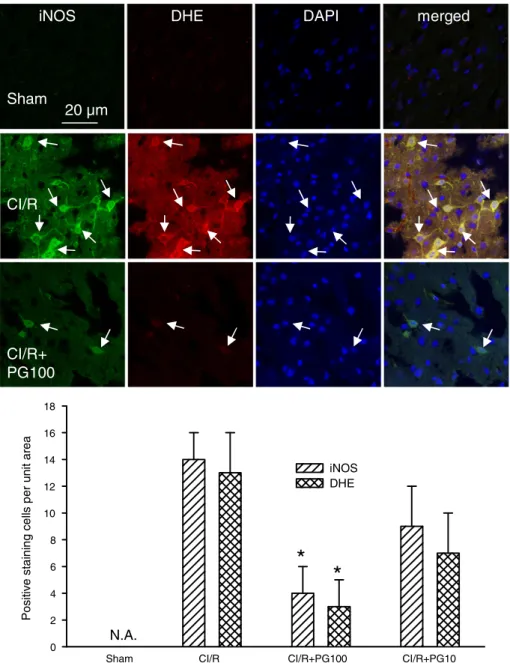
Effects of prodigiosin on changes in nitrotyrosine formation and superox-ide formation at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice
MCAo/r was also shown to provoke a significant increase in oxidative/ nitrosative-mediated tissue damage in the peri-infarct region (Fig. 2A), as validated by the induction of protein tyrosine nitrosylation (nitro-tyrosine) (Fig. 2B, upper panel) at 24 h after ischemic injury. It is noteworthy that formation of nitrotyrosine was largely colocalized with the production of superoxide anions in the damaged tissues, which was revealed by intensive staining with dihydroethidine (DHE) (Fig. 2B, upper panel). Of note, treatment with prodigiosin (100μg/kg, i.v.) extensively reduced MCAo/r-induced nitrotyrosine formation (Fig. 2B, one-way ANOVA, pb0.05, n = 5 for each group), as well as the production of superoxide anions (DHE staining) in the ischemic/damaged tissues (Figs. 2, 4, and 6, one-way ANOVA, pb0.05).
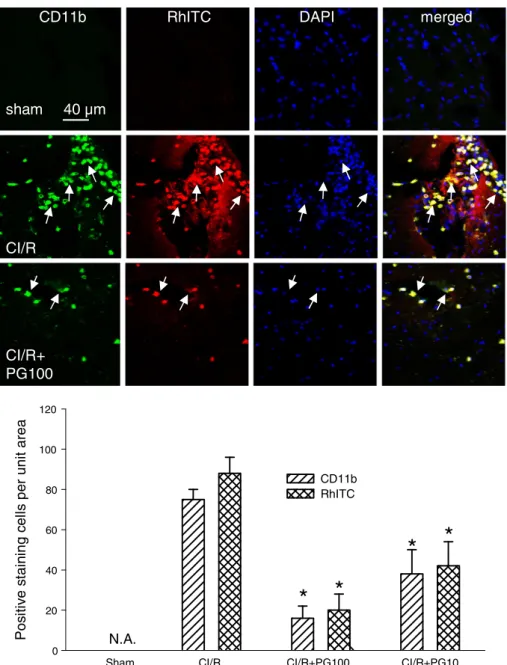
Effects of prodigiosin on changes in gp91phox(NOX2), iNOS, CD11b leukocytes
and BBB leakage at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice
Stroke-induced brain injury is often associated with enhanced inflammatory responses, leading to increased expression and activa-tion of gp91phox (NOX2) and iNOS. In line with this notion, the
expression of gp91phox(NOX2) (Fig. 3), iNOS (Fig. 4), and the in
fil-tration of CD11b-staining leukocytes (Fig. 5) were all increased by MCAo/r injury at 24 h after ischemia. The increase in gp91phoxand
iNOS expression and CD11b-positive cell infiltration was most possibly due to blood–brain barrier (BBB) damage, as evidenced by the enhanced leakage of rhodamine isothiocyanate (RhITC) (Figs. 3
and 5). Treatment with prodigiosin (100μg/kg, i.v.) significantly
reduced the levels of gp91phox, iNOS and CD11b immunoreactivity
as well as the RhITC-staining cells (Figs. 3 and 5, one-way ANOVA, pb0.05, n = 5 for each group). Moreover, it is noteworthy that the expression of gp91phoxand iNOS and the infiltration of CD11b cells
were largely colocalized with RhITC and/or DHE staining, a marker of superoxide production, which was also significantly diminished by prodigiosin (Figs. 2, 4 and 6, one-way ANOVA, pb0.05, n =5 for each group). Taken together, our results indicate that prodigiosin treatment is capable of reducing MCAo/r-induced activation and accumulation of superoxide-producing cells, suppressing the up-regulation of two pro-oxidative enzymes (gp91phoxand iNOS) and protecting BBB integrity, which in turn reduce the levels of CD11b cell infiltration.
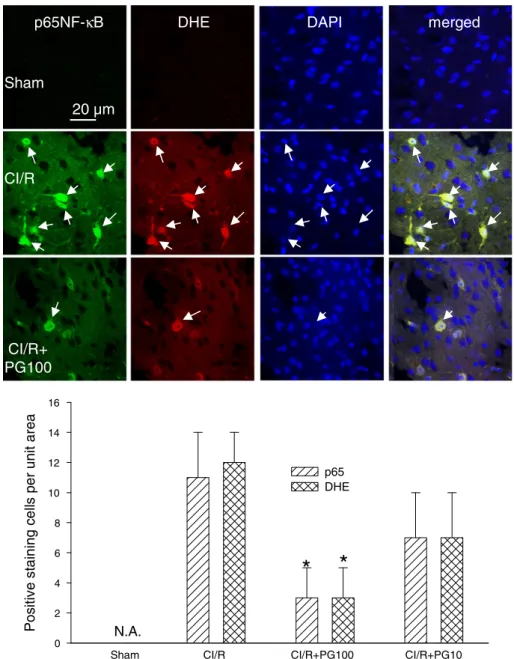
Effects of prodigiosin on changes in p65NF-κB up-expression, nuclear translocation, and superoxide production (DHE, red) at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice
It is well established that NF-κB is a central transcription factor to elicit inflammatory responses. In accordance with the promoting role of inflammation in stroke-induced brain injury, we observed that ischemic stroke increased substantially the expression of p65NF-κB, which was primarily located in the nucleus (DAPI staining) and largely colocalized with ROS-producing cells (DHE staining) (Fig. 6). Important-ly, the levels of nuclear translocation of p65NF-κB and ROS-producing cells were both significantly diminished by prodigiosin treatment
(Fig. 6B, one-way ANOVA, pb0.05, n=5 for each group), indicating
that the proinflammatory transcriptional factor-mediated ROS produc-tion was lowered by prodigiosin.
Effects of prodigiosin on the production of NO and ROS, expressions of inflammation-related proteins and signals after oxygen–glucose depriva-tion (OGD) inducdepriva-tion in microglial cells (BV2)
To further substantiate the inhibitory role of prodigiosin in stroke-induced neurological injury and the underlying modes of action, we examined the protective effect of prodigiosin on an in vitro hypoxia model established by induction of BV-2 microglial cells under oxygen– glucose deprivation (OGD) condition followed by reoxygenation
(Chern et al., 2011). Our results revealed that OGD dramatically
increased NO and ROS production after 8 h of hypoxia induction followed by 16 h of reperfusion in BV-2 cells (Table 1, one-way ANOVA, pb0.05, n=4–5 for each group). In contrast, treatment with
prodigiosin (10–20 μM) evidently inhibited the production of OGD-induced NO and ROS in a concentration-dependent manner (Table 1). In addition, NO production induced by OGD was also significantly inhibited by PDTC (an NF-κB inhibitor) and L-NAME (a non-specific NOS inhibitor); among these prodigiosin and PDTC being more potent than L-NAME (Table 1). This indicates that NF-κB activation is involved in OGD-induced NO production. Furthermore, ROS production induced by OGD was also significantly inhibited by DPI (a gp91phox
inhibitor) but not by PDTC, demonstrating that OGD-induced ROS production mainly involves the activation of gp91phox(Table 1). . In line with its
promoting effects on the activation of NF-κB and gp91phox
, OGD was found to increase the protein levels of gp91phoxand iNOS in BV-2 cells,
possibly through inducing the degradation of IκBα and the activation/ nuclear translocation of p65NF-κB (Fig. 7). Of note, prodigiosin (20μM) as well as PDTC (10 μM) both significantly inhibited the expres-sion of gp91phoxand iNOS with a concomitant increase in the levels of
IκBα as well as a decreased ratio of nuclear to cytosolic p65NF-κB
(Fig. 7, one-way ANOVA, pb0.05, n=4–5 for each group).
Discussion
Prodigiosin has been reported to exhibit anti-inflammatory effect on macrophages(Huh et al., 2007), but its effect in vivo (e.g., mice) and the mechanisms of action when a brain undergoes hypoxia/ ischemia are not fully understood. We demonstrated that prodigiosin treatment in a pharmacologically applicable range (10–100 μg/kg, i.v.) at 1 h after cerebral ischemia is able to ameliorate MCAo/r injury (both brain infarction and neurological deficits) in mice. We further proved that this protective effect of prodigiosin is achieved by
Infarction (TTC staining mm 3 ) 0 20 40 60 80 100 N.A.
*
*
Sham CI/R CI/R+ CI/R+ CI/R+
PG10 PG100 UP100
Sham CI/R CI/R+ CI/R+ CI/R+
PG10 PG100 UP100
B
A
S u rvi v al r a te (%) 0 20 40 60 80 100 120 CI/R CI/R+PG100 CI/R+PG10 CI/R+UP100 sham Fre quen c y o f neu rolog ic a l de fici t (%) 0 20 40 60 80 100 score 4 score 3 score 2 score 1C
Day0 Day1 Day2
*
Sham CI/R CI/R+ CI/R+ CI/R+ PG10 PG100 UP100
*
N.A.
Fig. 1. Effects of prodigiosin on changes in cerebral infarction and neurological deficits (at 24 h), and survival rates (over 2 days) after cerebral ischemic/reperfusion injury. (A) Upper panel, typical TTC staining of the infarct tissue damaged by cerebral ischemic/reperfusion (CI/R) injury from mice treated with vehicle (CI/R), with prodigiosin (10 or 100μg/kg, i.v.; CI/R+PG10 or CI/R+PG100) or with undecylprodigiosin (100 μg/kg, i.v.; CI/R+UP100) or from sham operated mice (Sham). Lower panel, the statistical results were calculated as the mean ± S.E.M. (n = 6–9 for each group). *pb0.05, compared with the CI/R group only by one-way ANOVA followed by S–N–K t-test. N.A., not available. (B) Statistical results of the neurological deficits score analysis (NDS), a noncontinuous variable (from NDS 1 to NDS 4; n=6–9 for each group). *pb0.05, as compared with CI/R group by the Mann–Whitney analysis and χ2
test. N.A., not available. (C) Survival curves (within 2 days) after CI/R, these were assessed by the Log-Rank test followed by the Holm–Sidak method for all pairwise multiple comparisons (n=10 for each group), * pb0.05, as compared with CI/R group.
diminishing the massive oxidative/nitrosative brain injury and BBB damage associated with the insult, and also by inhibiting the proin flam-matory responses through reducing the expression of iNOS and gp91phox via inhibition of NF-κB activation. To our best knowledge, this is the first report to demonstrate the therapeutic anti-stroke activity of prodigiosin on an in vivo mouse disease model and implicate
the potential of exploiting prodigiosin's anti-inflammatory effect to alleviate stroke-induced brain injury.
Both prodigiosin and undecylprodigiosin show immunosuppres-sive activity, which is mainly attributed to their inhibitory effect on T cell proliferation. Intriguingly, a prominent disparity between these two prodiginines lies at their effect on macrophages. In particular,
Positive staining cells per unit area
0 10 20 30 40 50 60 NT DHE
N.A.
*
*
*
A
B
Nitrotyrosine
DHE
merged
Sham
40 µm
CI/R
CI/R+
PG100
CI/RSham CI/R+PG100 CI/R+PG10
Fig. 2. Effects of prodigiosin on changes in nitrotyrosine formation and superoxide formation at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice. (A) A typical brain slice (bregma−1.7 to −1.9 mm) from a normal mouse to demonstrate the peri-infarct region (opened square) selected for confocal imaging. (B) Upper panel, confocal images of nitrotyrosine formation (green) and superoxide production (oxidized dihydroethidium (DHE, red)). The arrows indicate the colocalization (yellow) of greenfluorescence (nitro-tyrosine) and redfluorescence (DHE) in the merged columns. Lower panel, statistical results were calculated as the mean±S.E.M. (n=5 for each group). *pb0.05, compared with the corresponding CI/R group only by one-way ANOVA followed by S–N–K t-test. N.A., not available. PG100: Prodigiosin (100 μg/kg, i.v.).
undecylprodigiosin was known to have limited effect on macrophages
(Lee et al., 1998, 2000), while prodigiosin's suppressive effect on the
pro-inflammatory action of macrophages has been well defined(Huh
et al., 2007). In line with this notion, prodigiosin is known to
repress NF-κB activation in macrophages(Huh et al., 2007), whereas the anti-inflammatory activity of undecylprodigiosin and the effect of undecylprodigiosin on NF-κB activation has never been demonstrated. This could be due to that undecylprodigiosin has been found to produce too severe side effects in vivo(Songia et al., 1997)which could compro-mise its protective effect in animal study. Accordingly, given that the brain injury in our experimental system is primarily caused by the pro-inflammatory action of microglial cells, which share the same line-age as macrophline-ages, it is plausible to argue that undecylprodigiosin's effect on microglial cells is most likely limited in vivo (animals) and consequently leads to the less efficacy of undecylprodigiosin to alleviate brain infarction. Moreover, according to their chemical structures, it reveals that undecylprodigiosin is relatively a non-polar molecule
with higher molecular weight (393) than that of prodigiosin (323). The difference of the chemical properties between undecylprodigiosin and prodigiosin could be one of the key factors that determine the absorption, distribution and the bioavailability across BBB for brain protection. Besides, according to our preliminary study, we have performed three doses (10, 50 and 100 (μg/kg)) of prodigiosin in the MCAo/r mice. But the efficacy between dose of 10 and 50 (μg/kg) did not show significant difference in the reduction of infarction (TTC stain-ing) and extension of the survival rate based on statistical analysis. Therefore, we decided to elucidate the protective effect and the mecha-nisms of action underlying prodigiosin using doses of 10 and 100 (μg/ kg) in this study.
In this study, prodigiosin effectively ameliorated MCAo/r-induced brain damage by diminishing protein nitrosylation induced by peroxy-nitrite (ONOO−), a reaction product of superoxide anions with NO, which is generated by gp91phoxand iNOS in inflammatory cells. This
effect is most important with respect to CD11b-positive staining cells
Sham
40 µm
CI/R
NOX2
(gp91
phox)
d
e
g
r
e
m
C
T
I
h
R
DAPI
CI/R+
PG100
P o s iti ve s tai ni ng c e ll s p e r un it a rea 0 5 10 15 20 25 30 35 gp91 RhITCN.A.
CI/RSham CI/R+PG100 CI/R+PG10
*
*
Fig. 3. Effects of prodigiosin on changes in gp91phox
(NOX2) and BBB damage at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice. Upper panel, confocal images of gp91phox(green) and an enhanced leakage of rhodamine isothiocyanate (RhITC) (RhITC, red), a marker for BBB damage in the ipsilateral peri-infarct region. The nuclei of these
cells were visualized by DAPI staining (blue). The arrows indicate the colocalization (yellow) of greenfluorescence (gp91phox) and redfluorescence (RhITC) in the merged columns.
Lower panel, statistical results were calculated as the mean ± S.E.M. (n = 5 for each group). *pb0.05, compared with the corresponding CI/R group only by one-way ANOVA followed by S–N–K t-test. N.A., not available. PG100: Prodigiosin (100 μg/kg, i.v.).
including microglial cells and leukocytes. A reduction in the infiltration of these inflammatory cells could explain why prodigiosin (100 μg/kg, i.v.) was effective in significantly enhancing the survival rate, which was paralleled to the cerebral protective effect. Previous studies have shown that prodigiosin is able to effectively inhibit the activation of mouse peritoneal macrophages(Huh et al., 2007). In this study, we found that prodigiosin was able to prevent OGD-induced ROS and NO production in a concentration-dependent manner, as well as to reduce the expression of gp91phoxand iNOS expression in BV-2 cells. This
sug-gests that ROS and NO are primarily produced by inflammatory cells, most likely microglial cells in the brain under hypoxia and the infiltrated CD11b leukocytes following reperfusion conditions. Considering ROS and NO further mediated MCAo/r-induced oxidative/nitrosative injury, it is likely that prodigiosin's protective effects during hypoxia and reper-fusion is ascribed to modulating the production of ROS and NO through reducing gp91phoxand iNOS expression, respectively. In line with this
notion, previous reports have demonstrated that a non-specific NOS
inhibitor (L-NAME) and various gp91phox inhibitors (e.g., DPI and
apocynin) displayed potent anti-oxidative/nitrosative capacity and are able to effectively reduce stroke injury (Nagel et al., 2007; Margaill
et al., 1997). Other studies have emphasized a dual-key mechanism, in
which microglial gp91phoxor glial iNOS activation alone play a relatively
minor role in hypoxic neuronal injury or cell death. However, simulta-neous activation of gp91phoxand iNOS synergistically induce neuronal
injury and/or cell death during hypoxia by the generation of peroxyni-trite (Borutaite et al., 2006; Li et al., 2009). Therefore, given its dual effect whereby it inhibits both gp91phoxand iNOS expression,
pro-digiosin may be more potent at ameliorating hypoxia-mediated inflammatory brain injury than, for example, a gp91phox
inhibitor such as DPI. Such a simultaneous reduction in ROS and NO production, in turn, ought to lessen peroxynitrite-mediated amplification of MCAo/r injury.
Most of the inflammatory-related proteins (e.g., iNOS) are down-stream gene products of various transcriptional factors, which are
P o s it iv e st ai ni ng c e lls pe r u n it ar ea 0 2 4 6 8 10 12 14 16 18 iNOS DHE
N.A.
d
e
g
r
e
m
E
H
D
S
O
N
i
DAPI
Sham
20 µm
CI/R
CI/R+
PG100
*
*
CI/RSham CI/R+PG100 CI/R+PG10
Fig. 4. Effects of prodigiosin on changes in iNOS expression and superoxide formation at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice. Upper panel, confocal images of iNOS and superoxide production (DHE, red) in the ipsilateral peri-infarct region. The nuclei of these cells were visualized by DAPI staining (blue). The arrows indicate the colocalization (yellow) of greenfluorescence (iNOS) and red fluorescence (DHE) in the merged columns. Lower panel, statistical results were calculated as the mean±S.E.M. (n = 5 for each group). *pb0.05, compared with the corresponding CI/R group only by one-way ANOVA followed by S–N–K t-test. N.A., not available. PG100: Prodigiosin (100μg/kg, i.v.).
activated during hypoxia/ischemia, particularly NF-κB (Harari and
Liao, 2010; Wang et al., 2006). Here, we found that prodigiosin
treat-ment decreased the activation/nuclear translocation of NF-κB in MCAo/r-injured mouse brains as well as in OGD-activated microglial cells. This suggests that prodigiosin suppresses NO production and reduces the increase in the inflammation-related proteins such as gp91phoxand iNOS via a NF-κB-dependent mechanism rather than by direct interfering with NOS activity. This observation is further supported by a previous report showing that prodigiosin is able to inhibit expression and transactivation of NF-κB in inflammatory cells
(Huh et al., 2007).
Here we have demonstrated that prodigiosin, like the NF-κB inhib-itor PDTC, is able to inhibit the activation of NF-κB, and thus the expression of iNOS and NO production on induction by OGD. These re-sults suggested that the NF-κB may be responsible for the increased
expression of iNOS under hypoxia and/or OGD conditions. Further-more, prodigiosin is effective in the inhibition of the ROS production and gp91phoxexpression induced by OGD, which suggests that reduction
of gp91phoxin vivo (MCAo/r mice) may be involved in the ameliorative
effect of prodigiosin against MCAo/r-induced brain injury through reducing the nitrotyrosine formation and impediment of BBB leakage, and in turn, the infiltration of CD11b leukocytes. Further experiments are needed to elucidate the involvement of these signaling pathways.
In conclusion, our results demonstrate that prodigiosin signi fi-cantly ameliorates MCAo/r-induced mouse brain damage by reducing the enormous ROS and NO production as well as protein nitrosylation by limiting the expression of gp91phoxand iNOS in ischemia injured
tissue, most likely in microglial cells and CD11b leukocytes. This is achieved, at least in part, by reducing the activation of the NF-κB path-way. Our report therefore provides new insights into the therapeutic
CD11b
RhITC
DAPI
merged
sham
CI/R
CI/R+
PG100
40 µm
P
o
s
iti
v
e
s
tai
n
ing c
e
ll
s
pe
r unit area
0 20 40 60 80 100 120 CD11b RhITCN.A.
*
*
*
*
CI/RSham CI/R+PG100 CI/R+PG10
Fig. 5. Effects of prodigiosin on changes in CD11b staining and BBB damage at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice. Upper panel, confocal images of CD11b (green) and an enhanced leakage of rhodamine isothiocyanate (RhITC) (RhITC, red), a marker for BBB damage in the ipsilateral peri-infarct region. The nuclei of these cells were visualized by DAPI staining (blue). The arrows indicate the colocalization (yellow) of greenfluorescence (CD11b) and red fluorescence (RhITC) in the merged columns. Lower panel, statistical results were calculated as the mean ± S.E.M. (n = 5 for each group). * pb0.05, compared with the corresponding CI/R group only by one-way ANOVA followed by S–N–K t-test. N.A., not available. PG100: Prodigiosin (100μg/kg, i.v.).
action and the underlying mechanism of prodigiosin to ameliorate brain injury in ischemic stroke mice. As a potent anti-inflammatory drug with neuroprotective activities, the beneficial effects of prodigiosin when used to treat acute ischemic stroke in humans deserve further basic and clinical investigations.
Conflict of interest
All authors declare no conflict of interest. Acknowledgments
We thank Mr. C.Y. Shih for all his help in the preparation of animals. This study was supported, in part, by grants from the National Science Council, R.O.C. (NSC-97-2320-B-077-004-MY3 and NSC-98-2314-B-010-025) and the National Research Institute of Chinese Medicine
Po
si
ti
v
e
s
ta
in
ing
ce
lls
pe
r
u
n
it
a
rea
0 2 4 6 8 10 12 14 16 p65 DHEN.A.
*
p65NF-
κ
B
DHE
D
A
P
I
m
e
r
g
e
d
20 µm
Sham
CI/R
CI/R+
PG100
*
CI/RSham CI/R+PG100 CI/R+PG10
Fig. 6. Effects of prodigiosin on changes in p65NF-κB up-expression, nuclear translocation, and superoxide production (DHE, red) at 24 h after cerebral ischemic/reperfusion (CI/R) injury in mice. Upper panel, confocal images of p65NF-κB up-expression and nuclear translocation in the ipsilateral peri-infarct region. The nuclei of these cells were visualized by DAPI staining (blue). The arrows indicate the colocalization (yellow) of greenfluorescence (p65NF-κB) and red fluorescence (DHE), and nuclear (DAPI) translocation of p65NF-κB in the merged columns. Lower panel, statistical results were calculated as the mean ± S.E.M. (n = 5 for each group). *pb0.05, compared with the corresponding CI/R group only by one-way ANOVA followed by S–N–K t-test. N.A., not available. PG100: Prodigiosin (100 μg/kg, i.v.).
Table 1
Effects of prodigiosin on oxygen–glucose deprivation (OGD) induced nitric oxide (NO) and reactive oxygen species (ROS) production in murine microglial cells.
NO (μM) ROS (fluorescence intensity) Control N.A. 10 ± 1* OGD alone 1.5 ± 0.2 65 ± 5 + Prodigiosin (10μM) 0.7 ± 0.2* 46 ± 5* + Prodigiosin (20μM) 0.4 ± 0.3* 28 ± 6* + PDTC (10μM) 0.4 ± 0.2* 54 ± 4 + L-NAME (20μM) 0.6 ± 0.1* N.C. + DPI (0.1μM) 0.9 ± 0.1 18 ± 3*
Data are expressed as the mean ± S.E.M. (n = 6) for each data point. *pb0.05, compared with corresponding OGD only group by one-way ANOVA followed by post-hoc S–N–K (S–N–K). N.A., not available, N.C., data not collected.
(NRICM99-DBCM-09 and NRICM100-DBCM-09). This research was also supported in part by grant from the Ministry of Education, Taiwan, Republic of China, under the ATU plan.
References
Argaw, A.T., Zhang, Y., Snyder, B.J., Zhao, M.L., Kopp, N., Lee, S.C., Raine, C.S., Brosnan, C.F., John, G.R., 2006. IL-1beta regulates blood–brain barrier permeability via reacti-vation of the hypoxia-angiogenesis program. J. Immunol. 177, 5574–5584. Borutaite, V., Hope, H., Brown, G.C., 2006. Arachidonate and NADPH oxidase synergise with
iNOS to induce death in macrophages: mechanisms of inflammatory degeneration. Pharmacol. Rep. 58, 96–102 (Suppl.).
Chang, C.C., Chen, W.C., Ho, T.F., Wu, H.S., Wei, Y.H., 2011. Development of natural anti-tumor drugs by microorganisms. J. Biosci. Bioeng. 111, 501–511.
Chen, W., Ostrowski, R.P., Obenaus, A., Zhang, J.H., 2009. Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury. Exp. Neurol. 216, 7–15. Chern, C.M., Liou, K.T., Wang, Y.H., Liao, J.F., Yen, J.C., Shen, Y.C., 2011. Andrographolide
inhibits PI3K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta Med. (Apr 21. [Epub ahead of print] PubMed PMID: 21512969).
Correia, S.C., Moreira, P.I., 2010. Hypoxia-inducible factor 1: a new hope to counteract neurodegeneration? J. Neurochem. 112, 1–12.
Demain, A.L., 1995. Past perspectives and future trends. In: Hunter, P.A., Darby, G.K., Russell, N.J. (Eds.), Fifty Years of Antimicrobials. Society for General Microbiology, Cambridge, pp. 205–228.
Han, S.B., Kim, H.M., Kim, Y.H., Lee, C.W., Jang, E.S., Son, K.H., Kim, S.U., Kim, Y.K., 1998. T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int. J. Immunopharmacol. 20, 1–13.
Han, S.B., Park, S.H., Jeon, Y.J., Kim, Y.K., Kim, H.M., Yang, K.H., 2001. Prodigiosin blocks T cell activation by inhibiting interleukin-2Ralpha expression and delays progression of auto-immune diabetes and collagen-induced arthritis. J. Pharmacol. Exp. Ther. 299, 415–425. Han, S.B., Lee, C.W., Yoon, Y.D., Kang, J.S., Lee, K.H., Yoon, W.K., Kim, Y.K., Lee, K., Park, S.K., Kim, H.M., 2005. Effective prevention of lethal acute graft-versus-host disease by combined immunosuppressive therapy with prodigiosin and cyclosporine A. Biochem. Pharmacol. 70, 1518–1526.
Harari, O.A., Liao, J.K., 2010. NF-kappaB and innate immunity in ischemic stroke. Ann. N. Y. Acad. Sci. 1207, 32–40.
Huh, J.E., Yim, J.H., Lee, H.K., Moon, E.Y., Rhee, D.K., Pyo, S., 2007. Prodigiosin isolated from Hahella chejuensis suppresses lipopolysaccharide-induced NO production by inhibiting p38 MAPK, JNK and NF-κB activation in murine peritoneal macrophages. Int. Immunopharmacol. 7, 1825–1833.
Kaur, C., Sivakumar, V., Lu, J., Ling, E.A., 2007. Increased vascular permeability and nitric oxide production in response to hypoxia in the pineal gland. J. Pineal Res. 42, 338–349.
Pe rc en tag e ( % ) of c o n tro l 0 100 200 300 400 control OGD OGD+PG20 OGD+PDTC10 gp91phox iNOS IκBα p65n/ p65c
*
*
*
*
* *
* *
control
OGD
PG20
PDTC10
OGD+
OGD+
gp91
iNOS
I
κBα
p65 (n)
p65 (c)
actin
Fig. 7. Effects of prodigiosin on the expressions of inflammation-related proteins and signals after oxygen–glucose deprivation (OGD) induction in microglial cells (BV2). The protein (50μg) was prepared from cells incubated under normal conditions (control) or after being subjected to 2 h of hypoxia treatment in the presence of culture medium only (OGD) or in the presence of prodigiosin (20μM; OGD+PG20) or PDTC 10 μM (OGD+PDTC10). Upper panel, the representative immunoblots of gp91phox, iNOS, IκBα, nuclear
p65NF-κB (p65(n)), cytosolic p65NF-κB (p65(c)), and nuclear. β-actin was included as a reference for normalization. Lower panel, statistical results from the densitometric measure-ments after normalization againstβ-actin were calculated as the mean±S.E.M. (n=4–5 for each group). *pb0.05, compared with the corresponding OGD only group, respectively, by one-way ANOVA followed by S–N–K t-test.
Kim, H.H., Sawada, N., Soydan, G., Lee, H.S., Zhou, Z., Hwang, S.K., Waeber, C., Moskowitz, M.A., Liao, J.K., 2008. Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J. Cereb. Blood Flow Metab. 28, 1285–1293.
Kofler, J., Otsuka, T., Zhang, Z., Noppens, R., Grafe, M.R., Koh, D.W., Dawson, V.L., de Murcia, J.M., Hurn, P.D., Traystman, R.J., 2006. Differential effect of PARP-2 deletion on brain injury after focal and global cerebral ischemia. J. Cereb. Blood Flow Metab. 26, 135–141.
Lee, M.H., Yamashita, M., Tsuji, R.F., Yamasaki, M., Kataoka, T., Magae, J., Nagai, K., 1998. Suppression of T cell stimulating function of allogeneic antigen presenting cells by prodigiosin 25-C. J. Antibiot. 51, 92–94.
Lee, M.H., Kataoka, T., Honjo, N., Magae, J., Nagai, K., 2000. In vivo rapid reduction of alloantigen-activated CD8(+) mature cytotoxic T cells by inhibitors of acidification of intracellular organelles, prodigiosin 25-C and concanamycin B. Immunology 99, 243–248.
Li, J., Luo, L., Wang, X., Liao, B., Li, G., 2009. Inhibition of NF-kappaB expression and allergen-induced airway inflammation in a mouse allergic asthma model by andro-grapholide. Cell. Mol. Immunol. 6, 381–385.
Lo, E.H., Dalkara, T., Moskowitz, M.A., 2003. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4, 399–415.
Margaill, I., Allix, M., Boulu, R.G., Plotkine, M., 1997. Dose- and time-dependence of L-NAME neuroprotection in transient focal cerebral ischaemia in rats. Br. J. Pharmacol. 120, 160–163.
Mi, Z., Rapisarda, A., Taylor, L., Brooks, A., Creighton-Gutteridge, M., Melillo, G., Varesio, L., 2008. Synergystic induction of HIF-1alpha transcriptional activity by hypoxia and lipopolysaccharide in macrophages. Cell Cycle 7, 232–241.
Nagel, S., Genius, J., Heiland, S., Horstmann, S., Gardner, H., Wagner, S., 2007. Diphenyle-neiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral is-chemia of the rat. Brain Res. 1132, 210–217.
Pérez-Tomás, R., Viñas, M., 2010. New insights on the antitumoral properties of prodi-ginines. Curr. Med. Chem. 17, 2222–2231.
Pérez-Tomás, R., Montaner, B., Llagostera, E., Soto-Cerrato, V., 2003. The prodigiosins, proapoptotic drugs with anticancer properties. Biochem. Pharmacol. 66, 1447–1452.
Semenza, G.L., 2000. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 88, 1474–1480.
Sharp, F.R., Bernaudin, M., 2004. HIF1 and oxygen sensing in the brain. Nat. Rev. Neurosci. 5, 437–448.
Shen, Y.C., Sung, Y.J., Chen, C.F., 1998. Magnolol inhibits Mac-1 (CD11b/CD18)-dependent neutrophil adhesion: relationship with its antioxidant effect. Eur. J. Pharmacol. 343, 79–86.
Shen, Y.C., Wang, Y.H., Chou, Y.C., Liou, K.T., Yen, J.C., Wang, W.Y., Liao, J.F., 2008. Dimemorfan protects rats against ischemic stroke through activation of sigma-1 receptor-mediated mechanisms by decreasing glutamate accumulation. J. Neurochem. 104, 558–572. Song, M.J., Bae, J., Lee, D.S., Kim, C.H., Kim, J.S., Kim, S.W., Hong, S.I., 2006. Purification
and characterization of prodigiosin produced by integrated bioreactor from Serra-tia sp. KH-95. J. Biosci. Bioeng. 101, 157–161.
Songia, S., Mortellaro, A., Taverna, S., Fornasiero, C., Scheiber, E.A., Erba, E., Colotta, F., Mantovani, A., Isetta, A.M., Golay, J., 1997. Characterization of the new immunosup-pressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J. Immunol. 158, 3987–3995.
Wang, Y.H., Wang, W.Y., Chang, C.C., Liou, K.T., Sung, Y.J., Liao, J.F., Chen, C.F., Chang, S., Hou, Y.C., Chou, Y.C., Shen, Y.C., 2006. Taxifolin ameliorates cerebral ischemia– reperfusion injury in rats through its anti-oxidative effect and modulation of NF-kappa B activation. J. Biomed. Sci. 13, 127–141.
Wei, Y.H., Chen, W.C., 2005. Enhanced production of prodigiosin-like pigment from Serratia marcescens SMdeltaR by medium improvement and oil-supplementation strategies. J. Biosci. Bioeng. 99, 616–622.
Wei, Y.H., Yu, W.J., Chen, W.C., 2005. Enhanced undecylprodigiosin production from Serratia marcescens SS-1 by medium formulation and amino-acid supplementa-tion. J. Biosci. Bioeng. 100, 466–471.
Williams, R.P., Quadri, S.M., 1980. The genus Serratia. In: Von Graevenitz, A., Rubin, S.J. (Eds.), CRC Press Inc., Boca Raron, pp. 31–75.
Williamson, N.R., Fineran, P.C., Leeper, F.J., Salmond, G.P., 2006. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 4, 887–899.