Production of Activated Carbons from Agricultural Waste Corn Cob by Chemical and/or Physical Activations: an Overview
全文
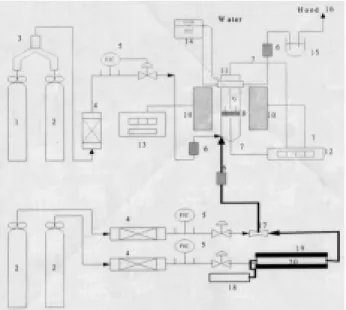
(2) 136. Journal of the Chinese Institute of Environmental Engineering, Vol. 13, No. 2 (2003). Table 1. Proximate and elemental analysis of corn coba. Proximate analysis (wt. %) Elemental analysis Moisture 4.3 C Volatile 78.7 H Fixed carbon 16.1 N Ash 0.9 O (balance) a Reference of Tsai et al. [7].. (wt. %) 46.8 6.0 0.9 46.3. objective of this work is to present an overview on the characteristics of a series of resulting activated carbons prepared by corn cob, comparing the performance of both activation methods, and offering comprehensive understanding for the activated carbon production from corn cob.. PRECURSOR MATERIALS Precursor corn cob mainly contains four parts: pitch of 2 wt.%, woody ring of 60 wt. %, coarse chaff of 34 wt.% and light chaff of 4 wt. % [12]. The main parts of corn cob for activated carbon production are the portions of woody ring and coarse chaff. The corn cob was ground and sieved to the size of 12-16 mesh and dried at 378 K overnight as the pretreatment. In addition, the wind force was used to remove the light parts of the samples during the pretreatment procedure. A typical analysis of corn cob is shown in Table 1. The results show that corn cob has high carbon but low ash content, which is very suitable for the production of activated carbon. Compared with other biomass such as soft/hard wood, lignin, nutshells and moringa oleifera, the carbon content of corn cob is similar to those of others while the volatile of that is the highest among all [2, 4-5]. After pretreatment, the samples were ready for physical activation. The samples for chemical activation were prepared as following with zinc chloride (ZnCl2) or potassium salts (KOH or K2CO3) as activating agents. Corn cob of fixed amount was mixed with one of these agents in a glass flak, filled of 200 cm3 solution of various concentrations, in order to obtain various impregnation ratios based on mass percent (wt. %). The impregnation was carried out at around 353±5 K on a hot plate with boiler-reflux condenser for 1-2 hours. The solid samples were prepared well after filtrated and dried at 383±10 K overnight.. EXPERIMENTAL APPARATUS AND PROCEDURES The experimental apparatus to produce activated carbon is shown in Fig. 1. After completing the preparation for carbonization and chemical/physical activation, the stainless steel crucible loaded with the sample of 10 gram was put into the reaction net. The heat-. Fig. 1. Schematic diagram of experimental system for the preparation of activated carbon. 1. CO2 cylinder; 2. N2 cylinder; 3. three-way valve; 4. molecular sieve; 5. mass flow controller with PIC; 6. fast connectors; 7. thermocouple; 8. reaction net; 9. thermocouple; 10. heating furnace; 11. tubular fixed-bed reactor; 12. temperature recorder; 13. temperature controller; 14. cycle water system; 15. oil collector; 16. vent to hood; 17. mixture device; 18. water syringe; 19. quartz column; 20. heating belts.. ing rate and temperature were controlled, monitored and recorded by the temperature controller, thermocouple and temperature recorder, respectively. The gases of N2 (purity of 99.99 %) and CO2 (purity of 99.99 %) from the steel cylinder was supplied by the commercial company (Ching-Feng-Harng Co. Ltd. in Taipei, Taiwan). The flow rates of gases were controlled by mass flow rate controllers (Model HFC202, Hastings Instruments). The steam-N2 mixture was produced by the use of water syringe pump (Model 355, Sage Instruments) combined with heating belt (HT353, Electrothermal Engineering Ltd.) and the commercial N2 gas, as reported previously [11]. For characterization measurements, the resulting chars and activated carbons were mixed with 3 N HCl to free the organic and mineral residues attached to the surface and hid in the pore of the particles. After filtration, the samples were washed with deionized water and then dried in a vacuum oven at 378 K overnight. CHARACTERIZATION MEASUREMENT The physical characteristics, such as specific surface areas and pore volumes, of the resulting chars and activated carbons were determined by means of N2 gas adsorption at 77 K with an automated adsorp-.
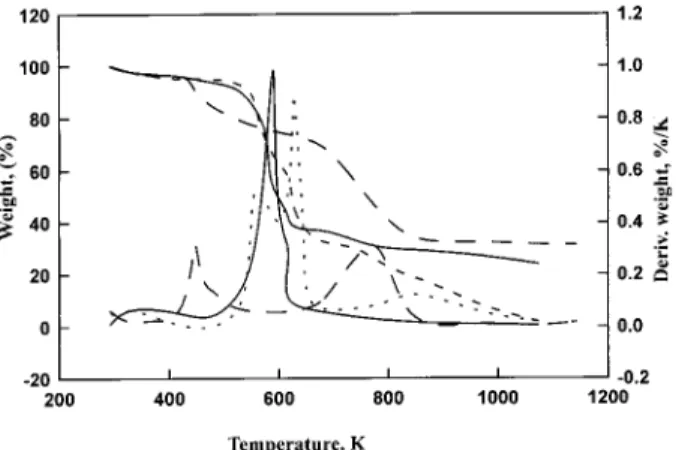
(3) C.F. Chang et el. : Production of Activated Carbons from Agricultural Waste Corn Cob by Chemical and/or Physical Activations: an Overview. Fig. 2. Thermogrvimetric analysis diagram of corn con under N2, gas stream of 60 cm3/min at a fixed heating rate of 10 K/min. ---: original sample without impregnation. —, and ——: impregnated with weight ratios of 15 wt. % KOH for1 hour, and 175 wt. % ZnCl2 for 2 hours, respectively.. tion instrument (ASAP 2000, Micromeritics). Prior to measuring the physical characteristics, samples were degassed for 24 hrs at 573 K in a vacuum of about 105 mm Hg to eliminate moisture and other volatiles from the sample. The standard BET method applied in a relative pressure range from 0.06 to 0.2 was used to determine the specific surface area (SB) of the samples. The total pore volume (Vt) was obtained by converting the amount of N2 (expressed in cm3/g STP) at a relative pressure of 0.99 into the volume of the liquid adsorbate. By assuming that the pores are all straight and cylindrical with the same diameter and depth, and not interconnected [13], the average pore diameter may be calculated by 4Vt/SB. The micropore surface area (Si) and volume (Vi) were determined by the tplot method. In addition, the mesopore (Ve) and macropore (Va) volumes were estimated by Vt and Vi combined with the proportion, which is obtained from Barrett-Joyner-Hanlenda (BJH) adsorption pore distribution, of mesopore to macropore volumes.. CARBONIZATION Pyrolysis analyses were examined by a thermogravimetric analyzer (TGA, Du Pont 9900 Thermal Analysis System) under N2, gas stream of 60 cm3/min at a constant heating rate of 10 K/min from room temperature to 1273 K, shown in Fig. 2. There are three obvious stages for unimpregnated corn cob during the pyrolysis, which are 298-523, 523-673 and 673-1273 K. It may be due to dehydration, devolatilization, and consolidation of the resulting char obtained from the previous stage, respectively [14]. The weight loss shape of the sample impregnated with weight ratio of 15 wt. % KOH for 1 hour is similar to that of the unimpregnated one before 640 K but has higher residue,. 137. whereas the derivational weight curves show great difference between these two preparation ways. Based on differential thermogravimetry (DTG) analysis, two major and one minor peaks were existed for the unimpregnated samples representing the different reactions during the carbonization. The samples impregnated with weight ratios of 15 wt. % KOH for 1 hour and 175 wt. % ZnCl2 for 2 hours mainly have one peak at about 590 K, and two peaks at 447 K and 772 K [15], respectively. For the samples impregnated with weight ratio of 15 wt. % KOH for 1hour, when the temperature is higher than 610 K, the carbonization process proceeds slowly so that it provides more time to develop the pore structure, resulting in producing chars with more micropores. For the sample impregnated with weight ratio 175 wt. % ZnCl2 for 2 hours, its weight loss shape is much different from those of unimpregnated and impregnated samples with KOH. Comparing the thermogravimetric behavior of the case with ZnCl2 with those of the cases without impregnation and with impregnation with KOH, the pyrolytic reactions proceed moderately for a wider range resulting in a higher yield. The changes of the thermal decomposition behavior during the carbonization may be due to the utilization of the chemical agents, which can reach the interior of the corn cob, generate the hydrolysis reactions of various levels during impregnation, and promote the aromatic condensation reactions to various extents [16]. In addition, an increase of the impregnation ratio of ZnCl2 increases the temperature for the appearance of the second peak [15]. As the heating temperature increases, the organic matter of the precursor was firstly destroyed. The consolidation process of the precursor was then in progress to obtain the compact solid product during the carbonization. The product is by name of char or the resulting char, of which the initial porous structure is weak. Its pores are often filled with decomposition products and tar, and blocked with amorphous carbon [1]. Carbonization was carried out at constant heating rate of 10 K/min under inert atmosphere condition until reached the specific final carbonization temperature (Tcf) in this series of studies, of which the results are shown in Table 2. More detailed information about the effects of Tcf on the properties of the resulting chars may be referred to the previous studies [6-11, 15]. The utilization of chemical agents (such as KOH and K2CO3) is favorable for creating more specific areas of the resulting chars. Compared with the BET specific surface areas (SB) of the resulting chars under the various flow rates of N2, gas, the SB values are about the same, interpreting that the flow rate of N2, gas almost does not affect the carbonization process when KOH is used as the chemical agent. As for the results at various final temperatures, the SB of the resulting char obtained at 1173 K (549 m2/g) is about six times more than that at 1073 K (91 m2/g), showing that the final temperature of the carbonization is one of the.
(4) 138. Journal of the Chinese Institute of Environmental Engineering, Vol. 13, No. 2 (2003). Table 2. BET surface of the resulting chars under various carbonization conditions.a Condition. Specific surface area, SB. Precursor Impregnated with weight ratio of 15 wt. % KOHb b. Impregnated with weight ratio of 15 wt. % KOH Impregnated with weight ratio of 15 wt. % KOHb Unimpregnated Unimpregnated. Carbonization. (m2/g). N2, gas = 200 cm3/min. 369c. 3. N2, gas = 500 cm /min N2, gas = 800 cm3/min N2, gas = 200 cm3/min N2, gas = 200 cm3/min. 327c 348c 91c 549d. a: Sole carbonization starting from 298 K while being stopped at final carbonization temperature. b: Impregnation time = 2 hours. c, d: Final carbonization temperatures = 1073 K, 1173 K.. Table 3. List of BET specific surface areas of resulting activated carbons with chemical and physical activations. Specific surface area, SB Vi/Vt, Vt 4Vt/SB (m2/g) (%, cm3/g) Å P-1 0 wt. %-0-1073 K-1 hr-CO2b 608 79, 0.30 19.5 P-2 0 wt. %-0-1073 K-2 hr-CO2 670 83, 0.34 20.4 P-3 0 wt. %-0-1073 K-1 hr-steamc 897 75, 0.45 20.2 P-4 0 wt. %-0-1073 K-2 hr-steam 998 75, 0.51 20.5 P-5 0 wt. %-0-1173 K-1.3 hr- CO2 1705 51, 0.88 20.7 P-6 0 wt. %-0-1173 K-0.83 hr-steam 1315 74, 0.66 20.0 C-1 15 wt. % KOH-1 hr-1073 K-1 hr-N2 608 — — C-2 15 wt. % KOH-1 hr-1073 K-1 hr-CO2 1806 61, 0.87 19.2 C-3 37.5 wt. % K2CO3-2 hr-1073 K-1 hr-N2 506 — — C-4 37.5 wt. % K2CO3-2 hr-1073 K-1 hr-CO2 1541 68, 0.74 19.3 C-5 175 wt. % ZnCl2-2 hr-773 K-0.5 hr-N2 1410 60, 0.70 19.9 a: Impregnation ratio - impregnation time - final temperature of carbonization - activation time - activating agent. b: The purity of CO2 is 99.99 %. c: Steam in N2 with a concentration of 40 vol. %. d: ρs, ρp, εp: true density, apparent density, and porosity of particles. e: For C-2, ρs = 2.47 g/m3, ρp = 0.78 g/m3, εp = 0.68; for C-4, ρs = 2.42 g/m3, ρp = 0.86 g/m3, εp = 0.64. Sample. Production conditiona. most important parameters of the processes. From the previous study [10], the precursor size is also an important factor in the process. The procedure of impregnation with the chemical agent (e.g., KOH), which makes the volatile to evolve earlier and provides more time to establish the porous structure, promotes the development of the SB of the resulting chars to a certain extent. In addition, the carries gas can be changed from the inert gas (e.g., N2) to any reactive gases (e.g., steam, for one single-stage steam physical activation) for the specified goals. The CO2 used as the carbonization gas was also investigated in the previous study, supporting that it helps the development of the microporous structure of the resulting char [10].. ACTIVATION For chemical activation, the precursor was impregnated with the activating agents in the concentrated solution by mixing. The goal of impregnation with chemical agents is to restrict the formation of tar and induce some hydrolysis reactions, which enhance the exit of volatiles, weaken the structure, and increase in elasticity of the precursor [14]. As for the. physical activation, the endothermic reactions (e.g., C+H2O→H2+CO; C+CO2→2CO) between the resulting chars and activating agents result in a slight loss of mass, create more micropores, and subsequently widen the microporosity; however, the exact mechanism has not yet fully understood [3]. The SB values and some physical properties of the resulting activated carbons by chemical and/or physical activation are listed and compared in Table 3. Compared with the SB values of P-1 (608 m2/g) and P-5 (1705 m2/g), the higher temperature has the advantage of producing the resulting activated carbons with greater SB values and pore structures of better development. Regarding the effect of activating agents in the cases of P-5 (1705 m2/g) and P-6 (1315 m2/g), using low partial pressure of steam (i.e., 40 vol. %) as the activating agent may produce a more selective attack on the carbon structure, which contributes greater mircroporous structure, compared with the case of using CO2 as activating agent [11]. Similar results were obtained by Rodriguez-Reinoso and Molina-Sabio [14] for olive stone. When potassium salts are used as activating agents for chemical activation, it is necessary to follow up the physical activation to obtain resulting activated carbons with high SB values, as seen in the cases.
(5) C.F. Chang et el. : Production of Activated Carbons from Agricultural Waste Corn Cob by Chemical and/or Physical Activations: an Overview. of C-1 (608 m2/g) and C-2 (1806 m2/g). Among the cases of P-1 (608 m2/g), P-3 (897 m2/g), C-1 (608 m2/g) and C-3 (506 m2/g), using steam-N2 mixture as the activation agent is the most effective method to produce good resulting activated carbons at 1073 K. The use of KOH with N2 does not help in the aspect of creating more specific surface areas but the yield. Comparison of SB values with P-1 (608 m2/g), C2 (1806 m2/g), and C-4 (1541 m2/g), the usage of KOH/K2CO3 with CO2 dramatically promotes the creation of surface areas. This shows that the potassium salts act like catalyst rather than chemical activating agent and effectively catalyze the C-CO2 reaction although they do alter the thermal decomposition behavior during the carbonization process. The catalytic function of the potassium may take place and be restricted within the vicinity of it, which also induces the selective attack on the limited surface similar to the case with steam-N2 mixture activation [14]. In addition, compared with Fe catalyst reported by Rodriguez and Molina-Sabio [14], potassium salts are the better catalysts to produce resulting activated carbons with more microporous structure and small pore size, and to effectively and subsequently develop the microporosity. Regarding the chemical activating agent of ZnCl2 in the case of C-5, the resulting activated carbons are well developed at much lower activation temperature. This interprets that ZnCl2 is a very good chemical activating agent for activated carbon production when corn cob is used as the precursor. During the carbonization and subsequent activation processes, the formation of tar and the porous structure are inhibited and created, respectively. The more the amount of ZnCl2 is used, the larger the SB of the resulting activated carbon is gained. In addition, the combination of ZnCl2 with the followed CO2 activation for producing activated carbon was also investigated by Torregrosa and Martin-Martinez [17]. Their results show that the followed CO2 activation enhances the supermicro and mesoporosity of the resulting char without causing any important difference in reactivity. When the metal salts are used in the production process, the acid wash is a necessary procedure because the salts may block the pores resulting in reducing about a half of the specific surface areas of the resulting activated carbons, as reported by Tsai et al. [9]. In the meantime, the recovered chemical agent from washing may be reused in the impregnation process, achieving the sustainable use. Noting the specific surface areas and pore diameters of the resulting activated carbons with chemical and physical activations in the cases of P-5, P-6, C-2, C-4, and C-5 indicates that the resulting activated carbons can compete with the commercial ones. These results also illustrate that not only chemical activation but also physical activation is a very good method to produce activated carbon from corn cob. The average pore diameters of the resulting activated carbons are near 20 Å for all the cases and. 139. near 20 Å for all the cases and slightly smaller than that of Calgon F400 (i.e., 24 Å) [11], which shows that both manufacturing methods can produce microporous activated carbons. The resulting activated carbons obtained from the physical activation possess higher proportion of microporous volumes than that from the chemical activation, interpreting that the metal salts not only effectively create the specific surface areas but also widen the micropores formed. For the cases of physical activation using steam-N2 mixture at 1173 K, the superior activated carbon can be produced. The method can also avoid the greenhouse effect mainly caused by CO2. In addition, the physical activation can be employed to replace the chemical activation, which causes more pollution problems from the metal slats, achieving the goal of clean production, which is much friendlier to the environment. More detailed information about the application of physical and chemical activations and of activation agents at various experimental conditions may be referred to the previous works [6-11, 15]. As for the detailed discussion about the activation mechanism and the comparison of using KOH and K2CO3, one may refer to the studies of Chang et al. [11] and Tsai et al. [8, 9], respectively.. CONCLUSIONS Well developed activated carbons with higher specific surface areas and more microporous structures can be obtained by using both manufacturing methods employing chemical and physical activations and compete with the commercial ones (Calgon F400). The resulting activated carbons produced by the latter method possess greater microporosity. The higher physical activation temperatures have potential and advantage of producing greater microporous activated carbons and can overcome the drawbacks of long period of activation time to obtain the activated carbons with similar specific surface areas compared with the those via chemical activation. Potassium salts act like catalyst rather than chemical activating agent although they do alter the thermal decomposition behavior during the carbonation, requiring further subsequent CO2 physical activation. Chemical activation with activating agents, such as ZnCl2, KOH, or K2CO3, has a higher yield of the resulting activated carbon. Physical activation with steam-N2 mixture at 1173 K not only produces superior microporous activated carbon but also matches the cleaner production and sustainable environment. It should be recognized that the agricultural waste corn cob is a suitable precursor to produce activated carbon competing with the commercial ones by any of the physical activation (e.g., with CO2 or steam-N2 mixture), chemical activation (e. g., with ZnCl2), and the combination of both (e. g., subsequent CO2 activation of KOH/K2CO3 activated carbon)..
(6) 140. Journal of the Chinese Institute of Environmental Engineering, Vol. 13, No. 2 (2003). ACKNOWLEDGEMENTS We express our sincere thanks to the National Science Council of Taiwan for financial support and would also like to thank the Laboratory of Particulate Technology of Department of Chemical Engineering of National Taiwan University for its technical assistance.. REFERENCES 1. Bansal, R.C., J.B. Donnet and F Stoeckli, Active Carbon, Marcel Dekker, New York (1988). 2. Bansal, N., T. Cordero, J. Rodriguez-Mirasol and J.J. Rodriguez, “Activated Carbons from Uruguayan Eucalyptus Wood,” Fuel, 75(15), 1701-1706 (1996). 3. Jankowska, H., A. Swiatkowski and J. Choma, Active Carbon, Ellis Horwood, New York (1991). 4. Warhurst, A.M., G.D. Fowler, G.L. McConnachie and S.J.T. Pollard, “Pore Structure and Adsorption Characteristics of Steam Pyrolysis Carbons from Moringa Oleifera,” Carbon, 35(8), 1039-1045 (1997). 5. Warhurst, A.M., G.L. McConnachie and S.J.T. Pollard, “Characterisation and Applications of Activated Carbon Produced from Moringa Oleifera Seed Husks by Single-step Steam Pyrolysis,” Water Research, 31, 759-766 (1997). 6. Tsai, W.T., C.Y. Chang and S.L. Lee, “A Low Cost Adsorbent from Agricultural Waste Corn Cob by Zinc Chloride Activation,” Bioresource Tech., 64(3), 211-217 (1998). 7. Tsai, W.T., C.Y. Chang and S.L. Lee, “Preparation and Characterization of Activated Carbon from Corn Cob,” Carbon, 35(8), 1198-1200 (1997). 8. Tsai, W.T., C.Y. Chang, S.Y. Wang, C.F. Chang, S.F. Chien and H.F. Sun, “Cleaner Production of Carbon Adsorbents by Utilizing Agricultural Waste Corn Cob,” Resources Conservation and Recycling, 32, 43-53 (2001). 9. Tsai, W.T., C.Y. Chang, S.Y. Wang, C.F. Chang, S.F. Chien and H.F. Sun, “Utilization of Agricultural Waste Corn Cob for the Preparation of Carbon Adsorbent,” J. Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes, B36(5), 677-. 686 (2001). 10. Chang, C.F., C.Y. Chang, S.L. Lee, P.C. Chiang, S.K. Tseng and W. T. Tsai, “Effect of Physical Carbonization and Activation Methods on the Preparation of Activated Carbon from Corn Cob,” J. Chinese Institute Environm. Eng. (Taiwan), 8, 227-232 (1998). 11. Chang, C.F., C.Y. Chang and W. T. Tsai, “Effects of Burn-off and Activation Temperature on Preparation of Activated Carbon from Corn Cob Agrowaste by CO2 and Steam,” J. Colloid and Interface Science, 232, 45-49 (2000). 12. Bagby, M.O. and E.W. Widstrom, Biomass Uses and Conversions. In: Corn: Chemistry and Technology, S.A. Watson, and P.E. Ramstad (eds.), American Association of Cereal Chemists Inc., St. Paul, Minnesota, 575-590 (1987). 13.Walker, P.L., “Production of Activated Carbons: Use of CO2 versus H2O as Activating Agent,” Carbon, 34, 1297-1299 (1996). 14. Rodriguez, F. and M. Molina-Sabio, “Activated Carbons from Lignocellulosic Materials by Chemical and/or Physical Activation: an Overview,” Carbon, 30, 1111-1118 (1992). 15. Tsai, W.T., C.Y. Chang, S.L. Lee and S.Y. Wang, “Thermogravimetric Analysis of Corn Cob Impregnated with Zinc Chloride for Preparation of Activated Carbon,” J. of Thermal Analysis and Calorimetry, 63, 351-357 (2001). 16. Heschel, W. and E. Klose, “On the Suitability of Agricultural By-products for the Manufacture of Granular Activated Carbon,” Fuel, 12, 1786-1791 (1995). 17. Torregrosa R. and J. Martin-Martinez, “Activation of Lignocellulosic Materials: a Comparison between Chemical, Physical and Combined Activation in Terms of Porous Texture,” Fuel, 70, 1173-1180 (1991).. Discussions of this paper may appear in the discussion section of a future issue. All discussions should be submitted to the Editor-in-chief within six months. Manuscript Received: February 10, 2003.
(7) C.F. Chang et el. : Production of Activated Carbons from Agricultural Waste Corn Cob by Chemical and/or Physical Activations: an Overview. 以化學及物理活化法由玉米穗軸製備活性碳 張瓊芬 張慶源 謝哲隆 李世龍 王旭淵 林珮萱 國立台灣大學環境工程學研究所. 蔡文田 嘉南藥理科技大學環境工程衛生系. 關鍵詞:玉米穗軸、活性碳、物理活化法、化學活化法. 摘. 要. 本文探討並比較以化學活化(活化劑為:ZnCl2、KOH、K2CO3)及物理活化(氧化劑為:CO2、蒸汽-氮之 混合氣體)兩種方法由玉米穗軸備製所得一系列活性碳之主要特性。應用前述兩種方法都能製得與商用活性 碳(Calgon F400)同級之高比表面積及多微孔結構之活性碳。在兩種方法中,以後者所得活性碳之微孔度較 高。在碳化過程中,鉀鹽不僅會影響熱分解行為,而且有觸媒之功能。將使用 ZnCl2 及鉀鹽為活化劑之化學 活化結合使用 CO2 之物理活化可製備得到高產率之活性碳。 而以蒸汽-氮之混合氣體於 1173 K 進行物理活 性,不僅可製得好的多微孔性活性碳,同時也符合清潔生產及永續環境之訴求。. 141.
(8)
數據

相關文件
Transforming Graphene Moire Blisters into Geometric Nanobubbles Jiong Lu, Antonion C.. Decouple graphene and merging of
Jinhua Chen, “A Chemical ‘Explosion’ Triggered by an Encounter between Indian and Chinese Medical Sciences: Another Look at the Significances of the Sinhalese Monk
Reuse, Reduction and Recycle of Construction and Demolition Waste Construction and Demolition Waste... What is Construction Waste What is
Methods include the implementation of waste management plan, reducing the generation at source, charging on disposal of construction waste, recycling of inert hard
An alternative activated-state model was generated by substituting the single ligand-biased receptor refinement protocol with the following procedure: (1) rotation of TM VI by
• Information retrieval : Implementing and Evaluating Search Engines, by Stefan Büttcher, Charles L.A.
Augmented reality (AR) is a live direct or indirect view of a physical, real-world environment whose elements are augmented (or supplemented) by computer-generated sensory input
The study of water transferring from agricultural section to other sections was emphasized by the government and scholars form the 1985. Industrial section operationally