@ 1996 Kluwer Academic Publishers. Printed in the Netherlands.
Disturbed ammonium assimilation is associated with growth inhibition of
roots in rice seedlings caused by NaCl
Chuan Chi Lin & Ching Huei Kao*
Department of Agronomy, National Taiwan University, Taipei, Taiwan, Republic of China (“Author for
correspondence)
Received 19 May 1995; accepted 20 July 1995
Key words: ammonium assimilation, NaCl stress, Oryza sativa L., root growth
Abstract
The effects of NaCl on changes in ammonium level and enzyme activities of ammonium assimilation in roots growth of rice (Oryza sativa L.) seedlings were investigated. NaCl was effective in inhibiting root growth and stimulated the accumulation of ammonium in roots. Accumulation of ammonium in roots preceded inhibition of root growth caused by NaCl. Both effects caused by NaCl are reversible. Exogenous ammonium chloride and methionine sulfoximine (MSO), which caused ammonium accumulation in roots, inhibited root growth of rice seedlings. NaCl decreased glutamine synthetase and glutamate synthase activities in roots, but increased glutamate dehydrogenase activity. The growth inhibition of roots by NaCl or MS0 could be reversed by the addition of L-glutamic acid or L-glutamine. The current results suggest that disturbance of ammonium assimilation in roots may be involved in regulating root growth reduction caused by NaCl.
Abbreviations: GDH = glutamate dehydrogenase; GOGAT = glutamate synthase; GS = glutamine synthetase; MS0 = methionine sulfoximine
1. Introduction 2. Materials and methods
Early work by Joshi et al. [3] underlies the hypoth- esis that ammonium assimilation and amino acid metabolism are integrally involved in plant responses to salinity. Enzyme activities of ammonium assimi- lation in plants have been shown to be affected by NaCl [8, 121. Ammonium accumulation in leaves of some subtropical grasses has been demonstrated under salinity stress [lo]. However, this is the only report on ammonium accumulation in plants exposed to salt stress. It is known that ammonium strongly inhibits the growth of many plants [ 1, 51. However, it is not known whether growth inhibition of plants in response to salinity is closely related to ammonium accumula- tion. In the present investigation, we studied the effects of NaCl on changes in ammonium level and activi- ties of enzymes involved in ammonium assimilation in roots and root growth of rice seedlings.
Rice (Oryza sativa L., cv. Taichung Native 1) seeds were sterilized with 2.5% sodium hypochlorite for 15 min and washed extensively with distilled water. These seeds were then germinated in a Petri dish (20 cm) con- taining distilled water at 37 “C under dark conditions. After 1 -day incubation, uniformly germinated seeds were selected and transferred to a Petri dish (9.0 cm) containing two sheets of Whatman No. 1 filter paper moistened with 10 mL of distilled water or test solu- tion. Each Petri dish contained 20 germinated seeds. Each treatment was replicated 4 times. The germinated seeds were allowed to growth at 27 ‘C in darkness. To avoid the lose by evaporation and taken up by the seeds, a further 3 mL of distilled water or test solutions was added to each Petri dish on day 3 of the growth.
Ammonium was measured in the crude extract by the Berthelot reaction, modified according to Weatherbum [ 131. For ammonium determination in
roots of rice seedlings, 10 roots were homogenized with a mortar and pestle using 3 mL 0.3 mkf sulphuric acid (pH 3.5). The homogenate was centrifuged for 10 min at 39000 g. Two-hundred ,uL of clear super- natant were diluted by 0.3 mM sulphuric acid to a final volume of 4 mL. For the colour reaction, 0.5 mL of solution A (5 g phenol, 25 mg nitroprusside dissolved in 100 mL water) and then 0.5 mL of solutionB (40 mL 5% sodium hypochlorite and 2.5 g NaOH were mixed, and then made up to a final volume of 100 mL with distilled water) were added. Incubation was carried out with gentle shaking in a water bath at 37 ‘C for 20 min. The absorbance was measured at 625 nm against the control without extract. Ammonium levels were calcu- lated using an extinction coefficient of 3.9982 pmol- ’ cm- ’ and expressed as pmol gg ’ fresh weight.
For extraction of enzymes, plant tissue was homogenized with 10 mk! Tris-HCl buffer (pH 7.6, containing 1 rnk! MgC12, 1 mM EDTA and 1 mM 2-mercaptoethanol in a chilled pestle and mortar. The homogenate was centrifuged at 15000 g for 30 min and the resulting supernatant was used for determination of enzyme activities. The whole extraction procedure was carried out at 4 ‘C.
GS (EC 6.3.1.2) was assayed by the method of Oaks et al. [9]. The reaction mixture contained in a final volume of 1 mL, 80 pmol Tris-HCl buffer, 40 pmol L-glutamic acid, 8 pmol ATF’, 24 pmol MgS04, and 16 pmol NH20H; the final pH was 8.0. Reaction was started by addition of the enzyme extract and after incubation for 30 min at 30 ‘C was stopped by adding 2 mL 2.5% (w/v) FeCls and 5% (w/v) trichloroacetic acid in 1.5 M HCl. After centrifugation at 3000 g the absorbance of the supematant was read at 540 nm. One unit of GS activity is defined as 1 pmol L-glutamate y-monohydroxamate formed per min.
GOGAT (EC 1.4.7.1) activity was determined after the method of Singh & Srivastava [ 1 I]. The assay mix- ture contained 0.4 mL 20 mM L-glutamine, 0.05 mL 0.1 M 2-oxoglutarate, 0.1 mL 10 m&Z KCI, 0.2 mL 3 mk2 NADH and 0.5 mL of the enzyme extract in a final volume of 3 mL, made up with 25 mM Tris-HCl buffer (pH 7.6). The reaction was started by adding L- glutamine immediately following the enzyme prepara- tion. The decrease in absorbance was recorded for 3 min at 340 nm. One unit of enzyme activity is defined as a decrease of 1 OD340 per min.
GDH (ED 1.4.1.2) activity was assayed by the method of Kanamori et al. [4]. The assay mixture contained 0.3 mL 0.1 M 2-oxoglutarate, 0.3 mL 1 M NH4 Cl, 0.2 mL 3 mk2 NADH and 1 mL of the enzyme
20 I I I 1 -=\. Shoot \ 0 \- i I I 0 50 100 150 NaCI, mM
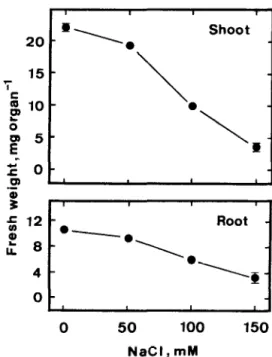
Fig. 2. Effects of NaCl on seedling growth of rice. Seedling growth was measured after 5 days of treatment. Vertical bars represent standard errors. Only those standard errors larger than the symbols are shown.
extract in a final volume of 3 mL, made up with 0.2 M Tris-HCl buffer (pH 8.0). Reaction was started by adding the enzyme extract. The decrease in absorbance was recorded for 3 min at 340 nm. One unit of enzyme activity is defined as a decrease of 1 OD340 per min.
For all measurements, each treatment was repeated four times. All experiments described here were repeated three times. Similar results and identical trends were obtained each time. The data reported here are from a single experiment.
3. Results
The growth of rice seedlings was followed by measur- ing the fresh weight of shoots or roots. Increasing con- centrations of NaCl from 50 to 150 mA4 progressively decreased shoot and root growth (Fig. 1). The growth of shoots and roots at 150 mM NaCl was reduced to 15 and 30%, respectively, of the control value.
GS and GOGAT activities in roots were found to decrease in a concentration-dependent manner with NaCl treatment (Fig. 2). In contrast to GS and GOGAT, the GDH activity in roots increased in the presence of NaCl (Fig. 2). In shoots of rice seedlings, NaCl slightly
‘i UJ (I) c c a L 5- 4-i 3- 2- ’ Q OShoot 0 Root g*5 - GDH 7.5 - - 0 50 100 150 NaCl,mM
Fig. 2. Effects of NaCl on GS, GOGAT andGDH activities in shoots and roots of rice seedlings. Enzyme activities were determined after 5 days of treatments. Vertical bars represent standard errors. Only those standard errors larger than the symbols are shown.
increased GS and GOGAT activities but did not affect GDH activities (Fig. 2). It seems that GS and GOGAT in shoots are enzymes resistant to NaCl stress.
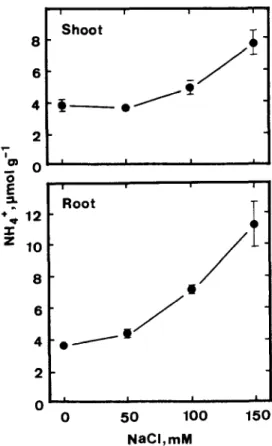
The effects of NaCl on ammonium levels in shoots and roots of rice seedlings are presented in Fig. 3. Increasing concentration of NaCl from 50 to 150 rr04 progressively increased the ammonium level in roots. However, ammonium accumulation in shoots was observed only when seedlings were treated at high concentration (100-l 50 n&f) of NaCl. It seems that root growth reduction caused by NaCl is closely linked with ammonium accumulation in roots.
If ammonium accumulation is important in regulat- ing growth reduction of roots but not shoots caused by NaCl, then exogenous application of ammonium chlo-
a
6 Shoot I 0 50 100 NaCI, mMI
150Fig. 3. Effects of NaCl on ammonium levels in shoots and roots of rice seedlings. Ammonium level was determined after 5 days of treatments. Vettical bars represent standard errors. Only those standard errors larger than the symbols are shown.
ride would be expected to reduce root growth but not shoot growth. As indicated in Fig. 4, ammonium chlo- ride significantly inhibited root growth and increased the ammonium level in roots. However, addition of ammonium chloride up to 10 mM did not inhibit shoot growth (data not shown).
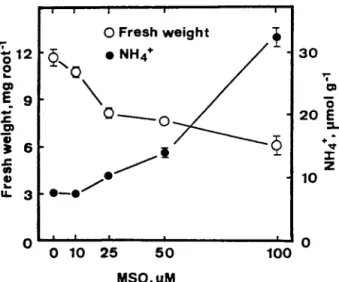
Miflin & Lea [7] suggested that an active GS could be responsible for the efficient assimilation of ammonium, and there is evidence that addition of MSO, an inhibitor of GS, results in an accumulation of ammonium [6, 73. To characterize further the role of ammonium accumulation in regulating root growth reduction under NaCl stress, rice seedlings were grown in the presence of various concentrations of MSO. As indicated in Fig. 5, MS0 increased ammonium level in roots and decreased root growth. From the shape of the curves, it is apparent that ammonium level is closely associated with root growth reduction. It was also found that MS0 did not inhibit shoot growth of rice seedlings (data not shown).
0
0 Fresh weight
l NHd+
0 2 4 6 8
NH&I, mM
Fig. 4. Effects of ammonium chloride on root growth and ammo- nium level in roots of rice seedlings. Root growth and ammonium level were measured after 5 days of treatment. Vertical bars represent standard errors. Only those standard errors larger than the symbols are shown. 21 18 3 0 i -t 7 ‘; 12 2 :9 E m 5 36 0 0 10 25 50 MSO, pM I I I I I 0 Fresh weight T -'O 100 30 5 WJ 20; m +w I 10
Fig. 5. Effects of methionine sulfoximine (MSO) on root growth and ammonium level in roots of rice seedlings. Root growth and ammonium level were determined after 5 days of treatment. Vertical bars represent standard errors. Only those standard errors larger than the symbols are shown.
If rice seedlings maintained for 2 days on NaCl (150 m&Q are transferred to distilled water, they then resume root growth (Fig. 6). However, only slight growth was observed if seedlings remained in NaCl (Fig. 6). Figure 6 also shows that the ammonium level in roots of seedlings remaining in the NaCl medium
-i g 10 L O8 E, E UJ 6 .- $4 c u) t! 2 IA 0 15 T m z 13 E a 11 +- g 9 7 0 ONaCl-tH20 0 NaCI-NaCI 2 3 4 5 Time,days
Fig. 6. Changes in fresh weight of and ammonium level in roots of NaGtreated rice seedlings grown in the presence or absence of NaCl (150 mM). Rice seeds were germinated for 2 days in NaCl (150 mkf) and then seedlings were transferred to distilled water (0) and NaCl (0). respectively. Vertical bars represent standard errors.
was higher than that of seedlings transferred to distilled water.
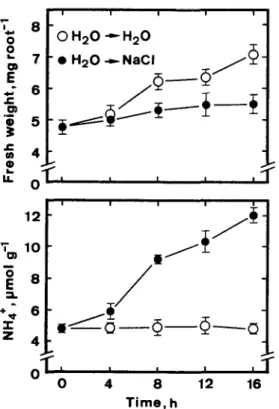
To test the causal relationship between ammonium accumulation and root growth reduction caused by NaCl, 2-day-old seedlings were transferred to distilled water and NaCl, respectively, for a short time. Changes in ammonium levels in roots and root growth were then monitored. As shown in Fig. 7, ammonium accumu- lation was observed at 4 h after treatment, whereas a reduction in root growth was visible 8 h after treatment started.
Table 1 shows the effect of L-glutamic acid and L- glutamine on NaCl- and MSO-inhibited root growth. Addition of L-glutamine or ‘L-glutamic acid alone resulted in a slight reduction of root growth when compared with the controls. However, addition of L- glutamic acid or L-glutamine to the medium with NaCI, caused partial recovery of root growth when compared with root growth of rice seedlings treated only with NaCl alone. Table 1 also shows that MSO-inhibited
4. Discussion
L
88
GI 7
E
- E 8 0) .- P 5 f 4 !?! IL 0 I I I I I 12 - Tm 10 -l ’
+‘I-z
E 8
a
.
+t 6- / s 4- *d-Q--Q--o / / ON 0 4 8 12 16 Time, hFig. 7. Changes in fresh weight of and ammonium level in roots of 2-day-old-rice seedlings grown in the presence or absence of NaCl (150 mM). Rice seeds were germinated for 2 days in distilled water and then seedlings were transferred to NaCl (0) or remained in distilled water (0 ). Vertical bars represent standard errors.
Table I. Effect of L-glutamic acid and L-glutamine on NaCl- and methionine sulfoximine (MSO)- inhibited root growth of rice seedlings. The concentrations for NaCl, MSO, L-glutamic acid and L-glutamine were 150 mkf, 50 PM, 1 mM and 1 mA4, respectively. Root growth was determined after 5 days of treat- ment. Values are means with standard errors
Treatment Fresh weight (mg root-‘)
Control 12.0 It 0.3
NaCl 4.3 f 0.3
MS0 6.7 f 0.1
L-Glutamic acid 10.4 f 0.4
L-Glutamine 10.2 f 0.6
NaCl + L-Glutamic acid 5.5 f 0.2 NaCl + L-Glutamine 6.0 f 0.3 MS0 + L-Glutamic acid 8.5 f 0.2
MS0 + L-Glutamine 8.2 f 0.2
root growth could be partially reversed by the addition of L-glutamine or L-glutamic acid.
In the present study, we show that ammonium accumulates in both shoots and roots of seedlings exposed to NaCl. However, ammonium accumulation in shoots was observed only at high concentrations of NaCl. Ammonium ion is a central intermediate in the metabolism of nitrogen in plants. Ammonium is produced during nitrate assimilation, deamination of amino acids from storage proteins and photores- piration [6]. The assimilation of ammonium requires carbon skeletons, energy, GS and GOGAT. Since our experiments were conducted in darkness, ammonium is unlikely to have been produced from photorespira- tion. In the present investigation, we have observed that NaCl treatment results in inhibitionof GS and GOGAT activities in roots. This inhibitionmay result, at least in part, in the accumulation of ammonium accumulation in roots of rice seedlings in response to NaCl.
Our results indicate that ammonium accumula- tion is likely to participate in the regulation of root growth reduction of rice seedlings under NaCl con- dition. This conclusion is based on the observations that (a) ammonium accumulation in roots preceded root growth reduction caused by NaCl; (b) ammo- nium levels in roots were inversely associated with root growth; (c) rice seedlings fed with ammonium chloride or MSO, which resulted in an accumulation of ammonium in roots, reduced root growth in the same way that NaCl did; and (d) when rice seedlings pre- treated with NaCl were transferred to distilled water, root growth was restored and ammonium levels in the roots declined.
High levels of ammonium are known to have toxic effects in plant cells [5]. To inhibit root growth, ammonium must be blocking cell division or expan- sion. Perhaps, ammonium accumulation in root cells is sufficient to disturb metabolic pathways, or ion and pH balances [2, 51. Whatever the mechanism, the inhibition is reversible, because root growth could be restored in NaCl-treated seedlings if they were trans- ferred to distilled water.
Plant growth is generally considered to be an energy-requiring process. Thus, an elevated ammo- nium levels in root cells caused by NaCl are most likely acting as a way to save energy by inhibiting root growth and as a readily utilizable source of nitrogen once NaCl stress is relieved.
Inhibition of GS and GOGAT activities in roots by NaCl and the effect of MS0 on roots may also result in lowering the levels of L-glutamine and L-
glutamic acid. Since addition of L-glutamine or L- glutamic acid causes partial recovery of root growth inhibition caused by NaCl or MSO, it seems that the lack of L-glutamine and L-glutamic acid is probably another reason for the growth inhibition of rice roots under NaCl stress or treated with MSO. In conclu- sion, disturbance of ammonium assimilation in roots by NaCl, which results in an increase of ammonium and a decrease of L-glutamine and/or L-glutamic acid, seems to be associated with growth reduction of roots. 3. 4. 5. 6. 8. 9. Acknowledgements 10.
This work was supported by a grant from the National Science Council of the Republic of China (NSC 84- 2321-B002-097).
References
1. Cao Y, Glass ADM and Crawford NM (1993) Ammonium inhi- bition ofArabidopsisroot growth can be reversed by potassium and by auxin resistance mutation aux I, uxrl, and axr.2. Plant Physiol 102: 983-989
2. Haynes RJ (1986) Uptake and assimilation of mineral nitrogen by plants. In: Haynes RJ (eds) Mineral Nitrogen in the Plant- Soil System, pp 303-378. San Diego: Academic Press
11.
12.
13.
Joshi G, Dolan T, Gee Rand Saltman P ( 1962) Sodium chloride effect on dark fixation of CO2 by marine and terrestrial plants. Plant Physio137: 446449
Kanamori T, Konishi S and Takahashi E (1972) Inducible formation of glutamate dehydrogenase in rice plant roots by the addition of ammonia to the media. Physiol Plant 26: 1-6 Marschner H (1986) Mineral Nutrition of Higher Plants. San Diego: Academic Press
Miflin BJ and Lea PJ (1976) The pathway of nitrogen assimi- lation in plants. Phytochemistry 15: 873-885
Miflin BJ and Lea PJ (1977) Amino acid metabolism. Annu Rev Plant Physio128: 199-329
Misra N and Dwivedi UN (1990) Nitrogen assimilation in germinating Phase&s ourens seeds under saline stress. J Plant Physiol 135: 719-724
Oaks A, Stulen J, Jones K, Winspear MJ and Boosel IL (1980) Enzymes of nitrogen assimilation in maize roots. Planta 148: 477-484
Pulich WM (1986) Variation in leaf soluble amino acids and ammoniumcontent in subtropical seagrassesrclated to salinity. Plant Physiol80: 283-286
Singh RD and Srivastava HS (1986) Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol Plant 66: 413-416 Stewart GR and Rhodes D (1978) Nitrogen metabolism of halophytesII1. Bnzymesof ammonia assimilation. New Phytol 80: 307-3 16
Weatherbum MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39: 97 l-974