Background/Purpose: This study was designed to investigate the effects of 6 months of nocturnal nasal positive pressure ventilation (NNPPV) on respiratory muscle function and exercise capacity in patients with chronic respiratory failure.
Methods: A prospective, randomized, controlled design was used. Twenty-nine patients with chronic respiratory failure were enrolled and allocated to either the NNPPV (n = 14) or control group (n = 15). Patients in the NNPPV group received bi-level positive pressure ventilation via nasal mask for 6 consecutive months. Arterial blood gas, respiratory muscle assessment and 6-minute walk test (6MWT) were performed before and after the 6-month NNPPV intervention. Respiratory muscle function was assessed using the variables of maximal inspiratory pressure (Pimax), maximal expiratory pressure (Pemax), and maximum voluntary ventilation (MVV).
Results: Subjects in the NNPPV group showed a significant improvement in blood gas exchange and increased 6-minute walk distance (6MWD) compared to baseline and the control group. The 6MWD was significantly increased from 257.1 ± 114.1 to 345.2 ± 109.9 m (34.3%) in the NNPPV group. NNPPV also significantly improved MVV and Pimax relative to baseline. MVV was significantly increased from 19.2 ± 6.5 to 22.3 ± 7.1 L/min (16.1%) in the NNPPV group (p < 0.05). Furthermore, there was a significant correlation between the magnitude of MVV improvement and 6MWD change.
Conclusion: The 6-month NNPPV treatment significantly decreased the partial pressure of carbon dioxide and improved daytime respiratory muscle function, thus contributing to exercise-capacity increase in patients with chronic respiratory failure. [J Formos Med Assoc 2006;105(6):459–467]
Key Words: chronic respiratory failure, nocturnal nasal positive pressure ventilation, respiratory muscle function, 6-minute walk test
School of Respiratory Therapy, Taipei Medical University, and1Department of Thoracic Medicine, Chang Gung Memorial Hospital, Taipei, Taiwan. Received: June 14, 2005
Revised: November 4, 2005 Accepted: December 6, 2005
Noninvasive positive pressure ventilation (NPPV) is effective for the treatment of chronic respiratory failure in patients with restrictive ventilatory orders, particularly those with neuromuscular dis-ease and kyphoscoliosis.1,2In most reports, long-term inlong-termittent NPPV was implemented during sleep to avoid interruption of daytime activities, as well as to attenuate sleep-related breathing
dis-*Correspondence to: Dr. Horng-Chyuan Lin, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, 199 Tun-Hwa North Road, Taipei 105, Taiwan.
E-mail: lin53424@ms13.hinet.net
turbances in patients with significant underlying respiratory disorders.3,4 There is limited quanti-tative data on the physiologic effects of NPPV in patients with severe hypercapnic respiratory fail-ure. In addition, conflicting results have been reported with respect to its efficacy for improving gas exchange, respiratory mechanics and exercise capability.
Six-month Nocturnal Nasal Positive Pressure
Ventilation Improves Respiratory Muscle
Capacity and Exercise Endurance in Patients
with Chronic Hypercapnic Respiratory Failure
Ling-Ling Chiang, Chih-Teng Yu,1
Chien-Ying Liu,1
Yu-Lun Lo,1
Han-Pin Kuo,1
Horng-Chyuan Lin1 *
graphy study;8 (3) medically stable; and (4) well motivated. Exclusion criteria were: (1) diagnosis of moderate to severe obstructive sleep apnea, i.e. apnea hypopnea index (AHI) > 20 events/ hour;14 (2) unable to complete the 6-minute walk test (6MWT); (3) unable to tolerate NNPPV or failure to cooperate; and (4) participation in an exercise rehabilitation program. All enrolled patients were assigned a computer-generated ran-dom number.
Subjects in the NNPPV group were asked to use bi-level positive pressure (BiPAP) ventilation via nasal mask for at least 6 hours during sleep for 6 consecutive months. The study protocol was approved by the Ethical Review Committee at Chung Gung Memorial Hospital, and informed consent was obtained from all patients prior to study enrollment.
Interventions
Nocturnal ventilation was delivered using the BiPAP system (Respironics Inc, Murrysville, PA, USA) via nasal mask (Respironics Inc). All patients were admitted to the thoracic ward to adjust the inspiratory positive airway pressure (IPAP) and the expiratory positive airway pressure (EPAP). Initially, IPAP was set at 2 cmH2O and EPAP was set at 2 cmH2O. IPAP was then increased to 8, 12, 16 and 20 cmH2O and EPAP was then increased to 4, 6 and 8 cmH2O. Inspiratory and expiratory pressures were set to achieve daytime PaCO2 < 45mmHg and PaO2 > 60 mmHg or oxygen satura-tion (O2SAT) > 88%. Supplemental oxygen was added to maintain O2SAT * 88% during NNPPV. Subjects in the NNPPV group were instructed to use nasal ventilation at night during sleep. Com-pliance with the NNPPV treatment regimen was evaluated by reviewing the subjects’ log records and the machine meter. Subjects were followed up monthly at our clinic. Five patients in the NNPPV group withdrew from the study due to intolerance of BiPAP.
Measurements
Patients in the NNPPV group were asked to stop using bronchodilators for 12 hours and then It has been demonstrated that short-term
NPPV markedly improves sleep efficiency and to-tal sleep time in patients with hypercapnic chro-nic obstructive pulmonary disease (COPD) with-out significantly improving gas exchange.5 The long-term nightly use of noninvasive mechani-cal ventilation (NIMV) to treat chronic respiratory failure in COPD patients, however, is not widely recommended, partly because of the lack of clear clinical results, and partly because the physiologic mechanisms by which the daily application of NIMV would be helpful in these patients have not yet been clarified.6
The exercise capacity of patients with chronic ventilatory failure is markedly reduced.7 It appears reasonable to suggest that daytime respiratory muscle dysfunction may contribute to exercise im-pairment. Accessory muscle and diaphragmatic electromyographic activity may be reduced by both negative8 and positive9 ventilation in patients with chronic respiratory failure. It could be hypo-thesized, therefore, that the reduction in energy expenditure at night could result in improved respiratory muscle function during the day. Al-though many studies have explored the effects of nocturnal nasal positive pressure ventilation (NNPPV) on respiratory muscle function, the benefits proposed for long-term NNPPV, in terms of exercise performance, remain controversial.10–12 We investigated the effects of a 6-month course of NNPPV treatment on arterial blood gas tension, respiratory muscle function, and exercise capacity in a prospective, randomized controlled trial in patients with chronic respiratory failure.
Methods
SubjectsPatients with chronic hypercapnic respiratory failure were recruited from the outpatient clinic of Chung Gung Memorial Hospital. The inclusion criteria were: (1) daytime PaCO2 > 45 mmHg, pH 7.30–7.45;13 (2) pulse oxygen saturation (SpO2) < 88% for more than 5 consecutive minutes on room air during sleep according to
polysomno-breathed room air for 4 hours before tests. Base-line data were measured before subjects began the study treatment. These included arterial blood gas tension, respiratory muscle function, and 6-minute walk distance (6MWD). All measurements were then repeated at the end of the 6-month treatment. Arterial blood samples were collected on room air. A Corning 278 blood gas analyzer (Ciba-Corning Diagnostics Co, Walpole, MA, USA) was used to perform arterial blood gas analysis. Maximum vol-untary ventilation (MVV) was measured by a port-able spirometer (Spiro Analyzer ST 250; Fukuda, Sangyo, Japan) in accordance with the recommen-dations of the American Thoracic Society.15 The 12-second MVV was also measured. Mouth occlusion pressures at maximum inspiratory pressure (Pimax) and maximum expiratory pressure (Pemax) were measured using a plethysmograph pulmonary function test system (Erich Jaeger, Hoechberg, Germany). Pimax was measured at residual vol-ume (RV) and Pemax was measured at total lung capacity (TLC) with the subject seated in a hard-backed chair. As soon as the patient commenced inhalation, the shutter closed and the pressure was measured automatically. These procedures were repeated until three measurements with less than 5% variability were recorded, with the highest of these utilized for analysis.
Exercise capacity was measured by 6MWT16 before and after 6 months of treatment. In order to exclude the learning effect, all patients were asked to complete a practice session of 6MWT 1 week before enrollment in the study. Subjects were encouraged every 30 seconds using one of the two phrases, “You are doing well” and “Keep up the good work”. Subjects were allowed to stop and rest during the test, but were instructed to resume walk-ing as soon as they felt able to do so. The longest of the two walk distances was used in the analysis. The test was performed without oxygen supple-mentation. Modified Borg scales were evaluated at rest and immediately after exercise, and expressed as RBorg and ExBorg, respectively.17 Subjects were connected to a pulse oximeter (3301; BCI Interna-tional Co, Waukesha, WI, USA) for monitoring of O2SAT. O2SAT was recorded and printed out every
6 seconds at rest and during exercise. Both resting oxygen saturation (RO2SAT) and exercise oxygen saturation (ExO2SAT) were determined by the pulse oximeter.
Statistical analysis
SPSS version 10.0 software (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. The results are presented as mean ± SD. The Wilcoxon signed rank test was used to determine within-group dif-ferences before and after treatment. The Mann-Whitney rank sum test was used to assess the dif-ference between groups, with the Spearman rank correlation coefficient used to examine the rela-tionship between the changes in walking distance and respiratory muscle function. A p value of less than 0.05 was considered to be statistically signifi-cant for all tests.
Results
A total of 37 patients were enrolled and allocated randomly to either the control (standard treatment;
n = 18) or the NNPPV groups (standard treatment
plus NNPPV; n = 19) during the period from June 2001 through July 2003, inclusively. Four subjects in the NNPPV group were unable to tolerate the treatment within 3 months of commencement and were excluded from the data analysis. One patient in the NNPPV group and three controls died dur-ing the 6-month intervention period, leavdur-ing re-spective patient completion totals of 14 and 15. No significant between-group differences were found on comparing age, gender distribution, body mass and AHI, disease character, and home oxy-gen usage (Table 1).
For the NNPPV group, peak inspiratory pres-sure was 15.8 ± 0.6 cmH2O, and positive expira-tory pressure was 4.5 ± 0.4 cmH2O. Treatment com-pliance was confirmed by duration of use as re-corded by the ventilator. The mean nightly duration of use was 6.8 ± 0.6 hours. There was a strong cor-relation between patient and machine records (r = 0.88), with the former exceeding the latter by about 12%.
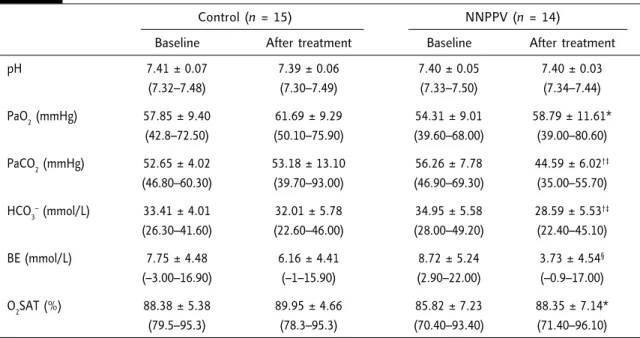
Arterial blood gas tension at baseline and after completion of the 6-month study are shown in Table 2. There were no significant between-group differences comparing the baseline data for pH, PaO2, PaCO2, and HCO3
–
. NNPPV therapy showed a significant improvement in daytime blood gas parameters, with a significant increase in PaO2 (p = 0.013) and a significant decrease in PaCO2, HCO3
–
and BE (p = 0.001, p = 0.001 and p = 0.002, respectively). Further, the magnitudes of the PaCO2 and HCO3
–
decreases were more significant for the NNPPV group than for the controls.
At the end of the 6-month treatment period, MVV was significantly improved in the NNPPV group (from 19.16 ± 6.45 to 22.33 ± 7.11 L/min; p < 0.05, n = 14), but not in the control group (from 18.07 ± 5.68 to 17.04 ± 6.17 L/min; p > 0.05, n = 15) (Figure 1). Further, Pimax was signi-ficantly increased in the NNPPV group (from 4.00 ± 1.44 to 4.83 ± 1.88 Kpa; p < 0.05, n = 14) but not in the controls (from 4.68 ± 1.79 to 4.47 ± 2.03 Kpa; p > 0.05, n = 15) (Figure 2). In this study, 6-month NNPPV therapy resulted in mean in-creases of 16.7% and 20.8% from baseline for Table 1. Demographic, clinical and respiratory related characteristics of patients in the control and
nocturnal nasal positive pressure ventilation (NNPPV) groups
Control (n = 15) NNPPV (n = 14) p Age (yr) 65.6 ± 10.0 58.9 ± 12.7 0.1904 Gender (M:F) 11:4 8:6 0.3590 Weight (kg) 54.5 ± 12.8 50.1 ± 14.1 0.3049 Height (cm) 155.2 ± 9.700 151.4 ± 12.40 0.4712 BMI (kg/m2) 22.6 ± 5.50 21.2 ± 4.30 0.6625 Parameters during sleep study
Desaturation, minimum O2SAT (%) 71.1 ± 10.9 71.1 ± 11.3 0.9478
ODI (%) 23.3 ± 28.0 17.7 ± 20.3 0.8613
AHI (%) 9.6 ± 7.0 9.0 ± 9.2 0.4321
Patients using oxygen at home, n (%) 8 (53.3) 7 (50.0) 0.5730
Diagnosis related to respiratory function, n (%)
COPD 11 (73.3)0 10 (71.4)0
Pneumoconiosis 2 (13.3) 1 (7.1)0 0.7610
Scoliosis 2 (13.3) 3 (21.4)
Baseline ABG analysis
pH 7.41 ± 0.07 7.40 ± 0.05 0.7328 PaO2(mmHg) 57.85 ± 9.400 54.31 ± 9.010 0.3114 PaCO2(mmHg) 52.65 ± 4.020 56.26 ± 7.780 0.1237 HCO3 –(mmol/L) 33.41 ± 4.010 34.95 ± 5.580 0.3974 Borg scale RBorg 2.7 ± 0.9 3.2 ± 1.5 0.2928 ExBorg 5.8 ± 1.2 5.8 ± 1.5 0.9774 Respiratory parameters Pimax (Kpa) 4.68 ± 1.79 4.00 ± 1.44 0.2738 Pemax (Kpa) 7.40 ± 2.27 6.11 ± 1.48 0.0846 MVV (L/min) 18.07 ± 5.680 19.16 ± 6.450 0.6370
BMI = body mass index; O2SAT = oxygen saturation; ODI = oxygen desaturation index (frequency of 4% < baseline per hour); AHI = apnea hypopnea index (events per hour); COPD = chronic obstructive pulmonary disease; ABG = arterial blood gas; PaO2= partial pressure of arterial oxygen; PaCO2= partial pressure of arterial carbon dioxide; HCO3–= arterial bicarbonate;
RBorg = resting Borg score; ExBorg = Borg score at the end of the 6-minute walk test; Pimax = maximal inspiratory pressure; Pemax = maximal expiratory pressure; MVV = maximum voluntary ventilation.
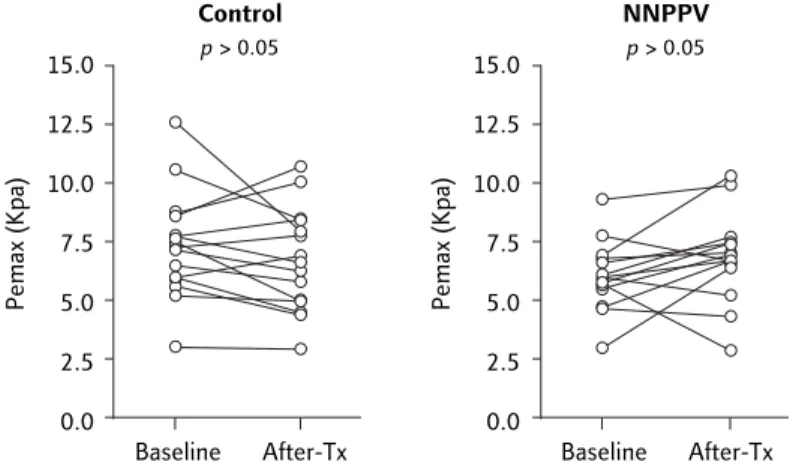
MVV and Pimax, respectively. As shown in Figure 3, however, there were no significant differences in Pemax for either group (from 6.11 ± 1.48 to 6.87 ± 1.92 Kpa and from 7.40 ± 2.27 to 6.76 ± 2.17 Kpa for the NNPPV and control groups, re-spectively; p > 0.05). As shown in Figure 4, a signi-ficant correlation was found between improved Pimax and increased MVV (rr = 0.56, p < 0.05; n =s 14). No significant differences were found between baseline data for MVV, Pimax and Pemax between the NNPPV and control groups.
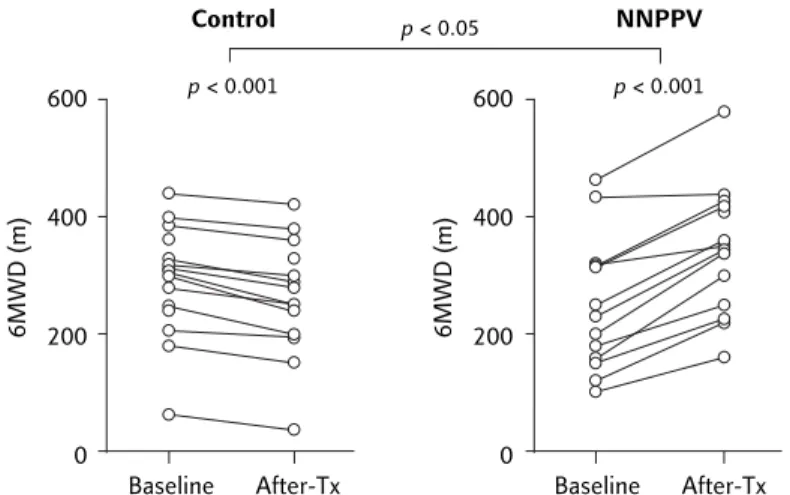
As shown in Figure 5, NNPPV treatment also resulted in a significant improvement in 6MWD compared to baseline (from 257.1 ± 114.1 to 345.2 ± 109.9 m; p < 0.001), whereas this value was significantly decreased in the control group (from 291.7 ± 96.0 to 258.8 ± 97.7; p < 0.001). The mean increase from baseline was 88.1 m (34.3%) for the NNPPV group, and the mean re-duction was 32.9 m (–11.3%) for the control group (p < 0.05; Figure 5). As shown in Figure 6, the change in 6MWD in the NNPPV group was signi-ficantly correlated with increased MVV (rr = 0.77,s p < 0.01; n = 14) for the NNPPV group.
Compared to baseline, NNPPV patients also achieved significant reductions in resting dyspnea
sensation (RBorg) and improved RO2SAT; how-ever, no such significance was achieved for the con-trol group (Table 3).
Discussion
This study demonstrated that 6 months of NNPPV treatment was effective in improving arterial blood gas tension and 6MWD in patients with chronic respiratory failure. NNPPV was also associated with Table 2. Arterial blood gases at baseline and after 6 months in the control and nocturnal nasal
positive pressure ventilation (NNPPV) groups
Control (n = 15) NNPPV (n = 14)
Baseline After treatment Baseline After treatment
pH 7.41 ± 0.07 7.39 ± 0.06 7.40 ± 0.05 7.40 ± 0.03 (7.32–7.48) (7.30–7.49) (7.33–7.50) (7.34–7.44) PaO2(mmHg) 57.85 ± 9.40 61.69 ± 9.29 54.31 ± 9.01 58.79 ± 11.61* (42.8–72.50) (50.10–75.90) (39.60–68.00) (39.00–80.60) PaCO2(mmHg) 52.65 ± 4.02 53.18 ± 13.10 56.26 ± 7.78 44.59 ± 6.02†‡ (46.80–60.30) (39.70–93.00) (46.90–69.30) (35.00–55.70) HCO3–(mmol/L) 33.41 ± 4.01 32.01 ± 5.78 34.95 ± 5.58 28.59 ± 5.53†‡ (26.30–41.60) (22.60–46.00) (28.00–49.20) (22.40–45.10) BE (mmol/L) 7.75 ± 4.48 6.16 ± 4.41 8.72 ± 5.24 3.73 ± 4.54§ (–3.00–16.90) (–1–15.90) (2.90–22.00) (–0.9–17.00) O2SAT (%) 88.38 ± 5.38 89.95 ± 4.66 85.82 ± 7.23 88.35 ± 7.14* (79.5–95.3) (78.3–95.3) (70.40–93.40) (71.40–96.10) *p < 0.05 vs. baseline; †p < 0.001 vs. baseline; ‡p < 0.05 vs. control group;§p < 0.01 vs. baseline. PaO
2= partial pressure of arterial
oxygen; PaCO2= partial pressure of arterial carbon dioxide; HCO3–= arterial bicarbonate; BE = base excess; O
2SAT = oxygen saturation.
Figure 1. Maximum voluntary ventilation (MVV) was significantly improved for the
nocturnal nasal positive pressure ventilation (NNPPV) group (n = 14), but not for the control group (n = 15). 40 30 20 10 0 Baseline After-Tx NNPPV p < 0.05 MVV (L/min ) 40 30 20 10 0 Baseline After-Tx Control p > 0.05 MVV (L/min )
improvements in respiratory muscle capacity (including Pimax and MVV), which may have also contributed to the increase in daytime exercise endurance.
While several previous studies suggested that NNPPV may improve arterial blood gas tension in chronic respiratory failure patients, these studies lacked a randomized controlled design and the treatment period ranged from 3 to 6 months.3,4,18,19 Conversely, Krachman et al concluded that, despite improved sleep efficiency and total sleep time, short-term NPPV does not alter gas exchange in patients with hypercapnic COPD.5 Our study dem-onstrated that 6-month NNPPV is effective for im-proving alveolar ventilation in chronic respiratory failure patients. The increased level of ventilation may result in a resetting of the central respiratory chemoreceptors that lead to a reduction in day-time PaCO2.
20,21
The benefits of NNPPV with respect to respira-tory muscle function remain controversial. Some studies of respiratory muscle function have failed to confirm improvement in Pimax and Pemax after NNPPV treatment in chronic respiratory fail-ure patients,3,17 especially for specific diagnostic subgroups such as severe COPD22 and severe cys-tic fibrosis.23 However, Piper et al found that NNPPV improved Pimax in patients with cystic fibrosis and hypercapnic respiratory failure.24 Moreover, Schonhofer et al11 and Goldstein et al12 reported beneficial effects of NNPPV on inspira-tory threshold-loading test in patients with restric-tive lung diseases. Similarly, Cropp and Dimarco found that nocturnal ventilation increased day-time respiratory muscle endurance in patients with severe COPD based on the measurements of the maximum duration of 12-second MVV.10 It could be speculated, therefore, that the major benefits of NNPPV are enhancement of rest in chronically fatigued inspiratory muscles and improved alveo-lar ventilation and exercise tolerance. This study demonstrated that NNPPV treatment is not only associated with a significant increase in Pimax, but that it also improves MVV, which influences muscle strength and endurance. We also found that the Pimax increase was associated with improve-ment in MVV, suggesting that the major effects of NNPPV on maximum voluntary alveolar ventila-tion may be through influence on respiratory mus-cle capacity.
Figure 2. Maximal inspiratory pressure (Pimax) was significantly improved for
the nocturnal nasal positive pressure ventilation (NNPPV) group (n = 14), but not for the control group (n = 15).
10.0 7.5 5.0 2.5 0.0 Baseline After-Tx NNPPV p < 0.05 Pimax (Kpa) 10.0 7.5 5.0 2.5 0.0 Baseline After-Tx Control p > 0.05 Pimax (Kpa)
Figure 3. There were no significant differences in maximal expiratory pressure
(Pemax) in either group.
15.0 12.5 10.0 7.5 5.0 2.5 0.0 Baseline After-Tx NNPPV p > 0.05 Pemax (Kpa) 15.0 12.5 10.0 7.5 5.0 2.5 0.0 Baseline After-Tx Control p > 0.05 Pemax (Kpa) –1 0 1 2 3 4 5
Change in Pimax (Kpa) 15 10 5 0 –5 Change in MVV (L/min)
Figure 4. Correlation between changes in maximum voluntary
ventilation (MVV) and maximal inspiratory pressure (Pimax) (rs =
Patients with severe COPD often have intrinsic positive end-expiratory pressure (PEEPi), which requires that inspiratory muscles overcome an op-posing positive-recoil pressure before inspiratory airflow begins.25 The expiratory positive airway pressure settings used in NNPPV treatment in our patients may have partially compensated for the PEEPi, thus reducing the inspiratory threshold load needed to trigger the IPAP boost. This may decrease inspiratory muscle work and central drive. A decrease in inspiratory threshold load also al-lows a triggered IPAP-related increase in tidal vol-ume (VT). This may partially explain the observed NNPPV-related improvements in Pimax and MVV in our chronic respiratory failure patients. How-ever, the lack of use of an endoesophageal balloon to measure the effects of NPPV on inspiratory mus-cle effort, which would have also enabled us to measure PEEPi and the effects of NPPV on EELV, is an important study limitation.
In recent years, there has been increasing inte-rest in the use of NPPV to increase exercise capa-city in chronic respiratory failure patients. Patho-physiologic factors known to contribute to exer-cise limitations in chronic respiratory failure pa-tients include increased intrinsic mechanical load-ing of inspiratory muscles (i.e. PEEPi), inspiratory threshold load,26 increased mechanical restriction of the thorax, inspiratory muscle weakness, in-creased ventilatory demand relative to capacity, gas-exchange abnormalities, dynamic airway compres-sion, cardiovascular factors, or any combination of the above.27 Gains in muscle mass and strength have been associated with improved exercise tol-erance and survival.28 In this study, we found that NNPPV therapy for 6 months was associated with significantly increased 6MWD. We also demon-strated that NNPPV improved gas exchange, Pimax and MVV, which may have also contributed to im-provements in 6MWD. These findings suggest that the mechanisms responsible for the effects of NNPPV on exercise endurance are complex and their delineation will require further investigation. A sensation of breathlessness is the most com-mon symptom limiting exercise, with alleviation of breathlessness seemingly important for
improv-Figure 5. Relative to baseline, nocturnal nasal positive pressure ventilation (NNPPV)
resulted in a significant improvement in 6-minute walk distance (6MWD), whereas this value was significantly decreased in the control group.
600 400 200 0 Baseline After-Tx NNPPV p < 0.001 6MWD (m) 600 400 200 0 Baseline After-Tx Control p < 0.001 6MWD (m) p < 0.05
ing exercise endurance.29,30
This study showed that NNPPV not only significantly decreased the sensation of breathlessness (RBorg; Table 3), but it also increased RO2Sat (Table 3) and Pimax (Figure 2). Thus, the reduction in breathlessness in our study may have been due to an improve-ment in gas exchange.
The changes in exercise endurance may also be associated with the various support modalities and/or treatment periods. In a recent study, it was found that 2-month NNPPV significantly improved 6MWD in patients with chronic respiratory fail-ure (by approximately 18%), but did not improve quadriceps strength.31 In our investigation, 6 months of NNPPV therapy was associated with a significant increase in 6MWD (88.1 m, 34.3%), a finding superior to that reported by Schonhofer et al.31 This suggests that long-term treatment may
0 25 50 75 100 125 150 Change in 6MWD (m) 20 10 0 –10 Change in MVV (L/min)
Figure 6. Correlation between changes in maximum voluntary
ventilation (MVV) and 6-minute walking distance (6MWD) (rs =
be important for improved exercise endurance. The effects of the 6-month therapy on quadriceps strength remain unclear. Further, the mean level of improvement in 6MWD in our study was com-parable to that achieved after a 6-week outpatient exercise training program for COPD patients,32and superior to an inpatient rehabilitation program for severe but stable COPD patients,33 suggesting that improvements in exercise tolerance and quality of life can be achieved and sustained for 6 months in patients undergoing NNPPV compared with those receiving conventional care.
Four of the NNPPV patients withdrew from the study within 3 months because they could not tole-rate BiPAP during sleep. The dropout tole-rate in our study (21.1%; 4/19) was similar to that reported by Gay et al (19%),3but less than that described by Criner et al (28%).18The major causes for drop-out in our study included mask leak, discomfort arising from the headwear, nasal bridge pres-sure and eye irritation. The comfort and compli-ance of patients may have been improved through recruitment of a home care company to apply tech-nological readjustment, personal reassurance and education.
Other limitations of our study also need to be mentioned. Ideally, the effects of NNPPV on res-piratory muscle endurance performance should be examined using the pressure threshold-loading test. As clinical facilities for such testing were not
available, however, the MVV was measured as an alternative. Further, patients with neuromuscular disorders were excluded from this investigation because they were not able to complete the 6MWT. We conclude that, for our sample of chronic respiratory failure patients, 6-month NNPPV ther-apy improved arterial blood gas and exercise ca-pacity compared to the control group. Further, NNPPV therapy was also associated with increased Pimax and MVV, which may have contributed to the increased exercise capacity in these patients.
Acknowledgments
This study was supported by a grant from Taipei Medical University (TMU 90-Y05-A148).
References
1. Ellis ER, Bye PT, Bruderer JW, et al. Treatment of respiratory failure during sleep in patients with neuromuscular disease: positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987;135:148–52.
2. Ellis ER, Grunstein RR, Chan S, et al. Noninvasive ventilatory support during sleep improves respiratory failure in kypho-scoliosis. Chest 1988;94:811–5.
3. Gay PC, Patel AM, Viggiano RW, et al. Nocturnal nasal ventilation for treatment of patients with hypercapnic res-piratory failure. Mayo Clinic Proc 1991;66:695–703. 4. Carroll N, Branthwaite MA. Control of nocturnal hypo-Table 3. Parameters of dyspnea sensation and oxygen saturation at baseline and after 6 months in
the control and nocturnal nasal positive pressure ventilation (NNPPV) groups
Control (n = 15) NNPPV (n = 14)
Baseline After treatment Baseline After treatment
RBorg 2.7 ± 0.9 2.7 ± 0.7 3.2 ± 1.5 2.4 ± 1.2* (1.0–4.0) (2.0–4.0) (0.0–5.0) (0.0–4.0) ExBorg 5.8 ± 1.2 5.9 ± 1.6 5.8 ± 1.5 5.7 ± 1.1 (4.0–7.0) (4.0–9.0) (4.0–8.0) (4.0–7.0) RO2SAT (%) 90.2 ± 3.1 91.3 ± 2.7 87.4 ± 8.2 89.4 ± 7.6* (84.0–95.0) (88.0–97.0) (66.0–95.0) (71.0–96.0) ExO2SAT (%) 78.4 ± 7.4 79.4 ± 7.4 75.6 ± 11.2 74.9 ± 10.6 (66.0–92.0) (69.0–93.0) (54.0–88.0) (51.0–91.0) *p < 0.05 vs. baseline. RBorg = resting Borg score; ExBorg = Borg score at the end of the 6-minute walk test; RO2SAT = resting oxygen saturation; ExO2SAT = oxygen saturation at the end of the 6-minute walk test.
ventilation by nasal intermittent positive pressure ventilation.
Thorax 1988;43:349–53.
5. Krachman SL, Quaranta AJ, Berger TJ, et al. Effects of non-invasive positive pressure ventilation on gas exchange and sleep in COPD patients. Chest 1997;112:623–8. 6. Nava S, Fanfulla F, Frigerio P, et al. Physiologic evaluation
of 4 weeks of nocturnal nasal positive pressure ventilation in stable hypercapnic patients with chronic obstructive pul-monary disease. Respiration 2001;68:573–83.
7. Wehgartner-Winkler S, Bullemer F, Marx U, et al. 6 minute walking test as stress test for patients treated by intermittent self-ventilation. Medizinische Klinik 1997;92(Suppl 1): 22–4. [In German]
8. Rochester DF, Braun NM, Laine S. Diaphragmatic energy expenditure in chronic respiratory failure. Am J Med 1977; 63:223–31.
9. Carrey Z, Gottfried SB, Levy RD. Ventilatory muscle support in respiratory failure with nasal positive pressure ventilation.
Chest 1990;97:150–8.
10. Cropp A, Dimarco AF. Effects of intermittent negative pres-sure ventilation on respiratory muscle function in patients with severe chronic obstructive pulmonary disease. Am Rev
Respir Dis 1987;135:1056–61.
11. Schonhofer B, Wallstein S, Wiese C, et al. Noninvasive mechanical ventilation improves endurance performance in patients with chronic respiratory failure due to thoracic restriction. Chest 2001;119:1371–8.
12. Goldstein RS, De Rosie JA, Avendano MA, et al. Influence of noninvasive positive pressure ventilation on inspiratory muscles. Chest 1991;99:408–15.
13. Aida A, Miyamoto K, Nishimura M, et al. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care
Med 1998;158:188–93.
14. Clinical indications for noninvasive positive pressure venti-lation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation – a consensus conference report. Chest 1999;116:521–34.
15. American Thoracic Society. Standardization of Spirometry. 1994 Update. Am Rev Respir Dis 1995;152:1107–36. 16. Steele B. Timed walking tests of exercise capacity in chronic
cardiopulmonary illness. J Cardiopulm Rehabil 1996;16: 25–33.
17. Borg GA. Psychophysical bases of perceived exertion. Med
Sci Sports Exerc 1982;14:377–81.
18. Criner GJ, Brennan K, Travaline JM, et al. Efficacy and com-pliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999;116: 667–75.
19. Waldhorn RE. Nocturnal nasal intermittent positive pres-sure ventilation with Bi-level positive airway prespres-sure
(BiPAP) in respiratory failure. Chest 1992;101:516–21. 20. Elliot MW, Mulvey DA, Moxham J, et al. Domiciliary
noc-turnal nasal intermittent positive pressure ventilation in COPD: mechanisms underlying changes in arterial blood gas tensions. Eur Respir J 1991;4:1044–52.
21. Annane D, Quera-Salva MA, Lofaso F, et al. Mechanisms underlying effects of nocturnal ventilation on daytime blood gases in neuromuscular diseases. Eur Respir J 1999;13: 157–62.
22. Renston JP, DiMarco AF, Supinski GS. Respiratory muscle rest using nasal BiPAP ventilation in patients with stable severe COPD. Chest 1994;105:1053–60.
23. Granton JT, Shapiro C, Kesten S. Noninvasive nocturnal ventilatory support in advanced lung disease from cystic fibrosis. Respir Care 2002;47:657–81.
24. Piper AJ, Parker S, Torzillo PJ, et al. Nocturnal nasal IPPV stabilizes patients with cystic fibrosis and hypercapnic respiratory failure. Chest 1992;102:846–50.
25. Petrof BJ, Kimoff RJ, Levy RD. Nasal continuous positive airway pressure facilitates respiratory muscle function during sleep in severe chronic obstructive pulmonary disease. Am
Rev Respir Dis 1991;143:928–35.
26. Rossi A, Polese G, Brandi G, et al. Intrinsic positive end-expiratory pressure (PEEPi). Intensive Care Med 1995;21: 522–36.
27. O’Donnell DE. Exertional breathlessness in chronic res-piratory disease. In: Mahler DA, ed. Dyspnea. New York: Dekker, 1998;97–147.
28. Schols AM, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157: 1791–7.
29. van Stel HF, Bogaard JM, Rijssenbeek-Nouwens LH, et al. Multivariable assessment of the 6-minute walking test in patients with chronic obstructive pulmonary disease. Am J
Respir Crit Care Med 2001;163:1567–71.
30. Redelmeier DA, Bayoumi AM, Goldstein RS, et al. Inter-preting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit
Care Med 1997;155:1278–82.
31. Schonhofer B, Zimmermann C, Abramek P, et al. Non-invasive mechanical ventilation improves walking distance but not quadriceps strength in chronic respiratory failure.
Respir Med 2003;97:818–24.
32. Strijbos JH, Postma DS, Van Altena R, et al. A comparison between an outpatient hospital-based pulmonary rehabi-litation program and a home-care pulmonary rehabirehabi-litation program in patients with COPD. Chest 1996;109:366–72. 33. Goldstein RS, Gort EH, Stubbing D, et al. Randomised con-trolled trial of respiratory rehabilitation. Lancet 1994;344: 1394–7.