行政院國家科學委員會專題研究計畫 期中進度報告
在幽門螺旋桿菌高感染區域以血清學方式偵測萎縮性胃炎
並在根除幽門螺旋桿菌之後追蹤其血清學變化(1/3)
計畫類別: 個別型計畫
計畫編號: NSC93-2314-B-002-299-
執行期間: 93 年 08 月 01 日至 94 年 07 月 31 日
執行單位: 國立臺灣大學醫學院內科
計畫主持人: 林肇堂
共同主持人: 吳明賢
報告類型: 精簡報告
處理方式: 本計畫可公開查詢
中 華 民 國 94 年 5 月 3 日
INTERMEDIARY REPORT
ON THE RESEARCH PROJECT
Serological detection of atrophic gastritis and its
evolution following H.pylori eradication therapy in
areas with high prevalence of H.pylori infection.
BY
Jaw-Town Lin, National Taiwan University Hospital, Taiwan
Marcis Leja, Faculty of Medicine, University of Latvia , Latvia
Limas Kupcinskas, Kaunas Medical University, Lithuania
Malignant diseases are major killers worldwide; in difference from the Western Europe and US, gastric cancer is among the most frequent malignancies in Taiwan, Latvia and Lithuania.
H.pylori infection is directly related to gastric cancer, and the progression to cancer mainly
occurs through atrophic gastritis, caused by this bacteria. Atrophic gastritis is diagnosed by upper endoscopy and consecutive histological evaluation of multiple biopsy samples. New serological tests have been proposed for evaluation of atrophic gastritis, still there is little data available on the improvement of atrophic gastritis as diagnosed by these serological markers after successful eradication of H.pylori infection.
Considering the common scope of the problem in all the participating countries, the necessity to grow the research resources in each of them as well as the potential of transferring the expertise between the participating centres, the objective of the project is to facilitate the collaboration and exchange the expertise between the scientists from Taiwan, Latvia and Lithuania with the goal to prevent and combat H.pylori and atrophic gastritis associated gastric cancer disease in these highly prevalent areas for the disease. The activities within the project will be directed in two key areas: 1) to acquire new research data and 2) to improve the competences of research staff, with distinct emphasis for the involvement of new researchers. The following 6 tasks of the project are set: 1) To validate the method of serological testing for atrophic gastritis (Gastro Panel) vs. gastric biopsy histopathology; 2) To standardize the histopathological reporting of gastritis biopsy specimens over the boarders; 3) To determine the functional and anatomical reconvalescence of atrophic gastritis after the eradication of H.pylori; 4) To acquire the clinical material and competences for future molecular biology research; 5) To facilitate young specialist involvement in medical research; 6) To increase the significance of the obtained research results by stimulating international co-operation.
To achieve the set goals 100 H.pylori infected patients over the age of 55 presenting with dyspeptic symptoms, but without gastric cancer and ulcer disease will be examined in each of the study centres, i.e. in Taiwan, Latvia and Lithuania. Each of the patients will undergo serological GastroPanel testing (pepsinogen I and II, gastrin-17, H.pylori IgG antibodies) and upper endoscopy with standardized biopsy sampling and histological evaluation. Biopsy material for H.pylori culture and full-blood sample for further testing of host response factors to H.pylori infection will be obtained for further research. Patients with atrophic gastritis will be included to the follow-up group; these patients will be offered H.pylori eradication therapy and followed-up. Blood sampling for the GastroPanel follow-up testing will be performed on annual basis during the study period, and the patients will be invited to undergo a follow-up upper endoscopy 2 years after the initial sampling (baseline endoscopy).
The serological appearance of atrophic gastritis will be compared to the histopathology results, the evolution of atrophic gastritis will take place either serologically or histologically.
Important attention will be paid to the exchange of expertise and experience between the participants; industry supported assistance from the reference institutions is planned. Training of new research staff by practical involvement in the project is considered of outstanding importance. The material gathered within the current project is planned to be used in joint research activities of the participants also after the completion of the current project. The project is considered to be a step towards serological population screening in high prevalence
H.pylori infection for early detection of gastric cancer and prevention of this cancer disease
by eradicating H.pylori in gastritis patients.
Recruitment of the patients and initial sampling procedure
Recruitment of the patients has been started in accordance with the updated protocol conditions. Each of the participants has the responsibility for following the protocol technical conditions in detail.Considering the extended work-up of the patients the recruitment was started with some delay if compared to the initial plan, still an extended patient material is being obtained following this protocol version, so the value of the obtained data is increased substantially.
The numbers of screened patients are corresponding to the initial plans, but the data acquired in the real life were showing differences from the figures been used in the initial calculations. The number of drop-outs in the screened population was significantly higher than expected from the beginning, mostly due to the history of peptic ulcer disease and the previous intake of medications (PPIs, antibiotics) limiting the inclusion as to the updated protocol.
On the other hand the proportion of patients with atrophic gastritis has been significantly higher in the group of enrolled patients if to compare to the initial estimates. So according to the data from Latvia, the real prevalence of atrophic gastritis in the enrolled population was 66% instead of the initially estimated 15%. This has been requiring additional workload on the researchers, and increased the number of patients requiring eradication medication.
In general the shift in the real figures has been generating new research information (e.g. the prevalence of atrophic gastritis in the population of dyspeptic patients over the age of 55); although causing increased workload to the researchers and causing delay from the initial recruitment schedule, this is further increasing the research value of the collected material.
To illustrate the performed field work and the recruitment status from each of the
particular countries, detailed analysis is given below:
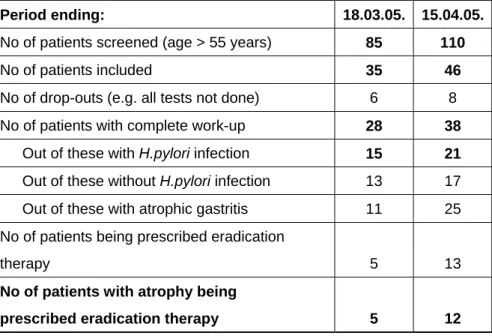
Table 1. Number of patients investigated in Taiwan until April 18, 2005
To illustrate the recruitment status, data from Taiwan are given below:
Period ending: 18.04.05.
No of patients screened 858 No of patients older than 55 357 No of patients included 8 No of drop-outs (e.g. all tests not done) 2 No of patients with complete work-up 6 Out of these with H.pylori infection 4 Out of these without H.pylori infection 2 Out of these with atrophic gastritis 4 No of patients being prescribed eradication
therapy 4
No of patients with atrophy being
prescribed eradication therapy 4
Among the 858 screened subjects, 357 subjects met the age criteria(greater than 55
years old). The low inclusion rate in Taiwan is mainly due to our routine health
check-ups based screening and most of the subjects are asymptomatic and unwilling
to receive second session of endoscopic examination. The second point is that more
than 70 % of the subjects were sedated when performing screening endoscopy at
health check-ups and the cost of conscious sedation during another session of
endoscopy for this study became extra financial load to the screened subjects.
Table 2. Reasons for screen failures in Taiwan until April 18, 2005 Reason for screen - failures: 18.04.05.
Total No of screen –failures out of these due to: 349
Previous peptic ulcer 83
Previous (1 month) antibiotic therapy 4
Eradication therapy before 42
Table 3. Number of patients investigated in Latvia until April 15, 2005
Period ending: 18.03.05. 15.04.05.
No of patients screened (age > 55 years) 85 110
No of patients included 35 46
No of drop-outs (e.g. all tests not done) 6 8
No of patients with complete work-up 28 38
Out of these with H.pylori infection 15 21
Out of these without H.pylori infection 13 17
Out of these with atrophic gastritis 11 25
No of patients being prescribed eradication
therapy 5 13
No of patients with atrophy being
prescribed eradication therapy 5 12
Table 4. Reasons for screen failures in Latvia until April 15, 2005
Reason for screen - failures: 18.03.05. 15.04.05.
Total No of screen -failures
out of these due to: 50 62 Previous peptic ulcer 22 26 Previous (1 month) PPI therapy 16 20 Previous (1 month) antibiotic therapy 1 1 Eradication therapy before 7
There have been also other minor reasons for screening failures, but fewer patients failed the screening because of these.
The decision to participate in the study was entirely up to the patients referred to
endoscopy; still only few patients have refused their participation. At the same time
there were some individuals, who have accepted their participation, but did not appear
for the other day testing; therefore were excluded from the group of complete
work-up.
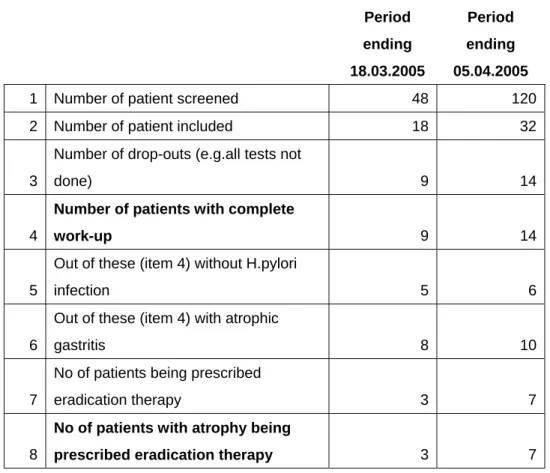
Table 5. Number of patients investigated in Lithuania until April 5, 2005
Period ending 18.03.2005 Period ending 05.04.20051 Number of patient screened 48 120
2 Number of patient included 18 32
3
Number of drop-outs (e.g.all tests not
done) 9 14
4
Number of patients with complete
work-up 9 14
5
Out of these (item 4) without H.pylori
infection 5 6
6
Out of these (item 4) with atrophic
gastritis 8 10
7
No of patients being prescribed
eradication therapy 3 7
8
No of patients with atrophy being
prescribed eradication therapy 3 7
Table 6. Reasons for screen failures in Lithuania until April 5, 2005
Total number of screen- failures 30 88
out of these due to:
previous peptic ulcer 19 34
previous (1 month) PPI therapy 6 35
previous (1 month) antibiotic therapy 5 19
The reasons of screening failure in Lithuania were presented in Table 5. There have
been also other minor reasons for screening failures, but fewer patients failed the
screening because of these. The relatively low inclusion rate for H.pylori eradication
was related with late supplying of drugs necessary for H.pylori eradication (we
received drugs only in the beginning of March, 2005), however in case every
month we will eradicate 10 patients with atrophic gastritis, we will collect necessary
number of eradicated patients (50) till beginning of August, 2005 according to
updated our working schedule.
Histopathology testing and the related quality assurance
Histopathology testing is performed by each of the individual centres by following the standards set by the Updated Sydney classification. Details have been discussed by the leading experts in the field Prof. Pentti Sipponen (Finland) and Prof. Manfred Stolte (Germany).
Kaunas University hospital has been appointed as the reference centre for the histopathology testing to be performed within the project.
The involved pathologist from Latvia has been visiting the pathology department in the Kaunas University with the objective to agree on common standards. Part of the samples from Latvia will be followed-up at the Kaunas University Pathology department.
The initial diagnosis of the mucosal atrophy is important for prescribing H.pylori eradication therapy to these patients. Nevertheless if this will be agreed at a later stage, all the slides will be read by the second expert pathologists.
The paraffin blocks are stored by each of the participants, and will be available for further research testing (e.g. molecular biology testing), if agreed so by the participants at later stage and if the funding will be available for the purpose.
H.pylori eradication and its efficacy control
Since patients having received previous H.pylori eradication therapy have been excluded at the screening phase, all the individuals eligible for the eradication are prescribed first-line standard therapy for 7 days, consisting of Esomeprasole 20 mg BID; Clarythromycine 500 mg BID, and Amoxicilline 1000 mg BID.
All those showing positive UBT results during the screening phase are invited to undergo another UBT for the efficacy control of the therapy in 30 days after completing the therapy. If any of the patients are for any reasons taking PPIs or antibiotics during this period, the test is recommended 30 days after completion of this therapy.
There have been several H.pylori positive patient cases showing negative UBT results at the initial screen (being positive at other tests). Follow-up UBT is not recommended to these patients, but the efficacy evaluation will be based on the serological or histological report result at the later stage.
In some cases from Lithuania there have been some discrepancies noticed with a
negative UBT possibly linked to some logistics issues of the breath-bags. More data
are collected, and the details will be analyzed.
Consortium management activities
Regular co-ordination of the research activities has taken place throughout the project realization process.
Followed by the initial planning process as started during 2003 well before the submission of the initial application, joint planning activities were performed throughout 2004. A meeting of all the participants was held in Riga May 31st, 2004 by involving also invited specialists and industry representatives.
The fist participant meeting after the official start of the project by inviting other collaborating institutions has taken place in Prague, September 2005. Before that (also September, 2004) a meeting in Vienna during the European Helicobacter Study Group has taken place, where the agreement on H.pylori bacteriology testing was achieved with Prof. Lars Engstrand from the Swedish Institute for Infectious Disease Control.
Additional meeting between the pathologists from Latvia and Lithuania has taken place in Kaunas, February, 2005.
Regular communication between consortium members was ensured throughout the project realization process predominantly by e-mails. On a monthly basis the overview of the situation in the countries has been circulated between the participants.
The next participant meeting is planned October 1-2, 2005 in Taipei and will be
funded by the Taiwan research funding sources additional to the current project.
During the meeting a presentation on the status of the current project is scheduled.
Discussions of the participants on the course of the research will take place.
Additional details on molecular biology testing (extension to the current project) will
be agreed between the participants.
Already the initial workplan was expecting that the protocol details will be adjusted during the initial phase of the work - the Essential preliminary work; such modifications have taken place.
To increase the value of the obtained results and reach higher proportion of atrophic gastritis in the study population, the age of the patients was increased from 50 to 55 years. As the result the medium age of the patients has been close to 70 years, and correspondingly the prevalence of atrophic gastritis has been well above the initially expected 15%. It should be also mentioned that the tight inclusion criteria (finalized during the initial phase) have resulted in significant number of screen-failures, mostly due to past ulcer disease and medication in these patients. As the result this was requiring larger workload and time for screening and including patients as well as additional resources, e.g. for the eradication medication.
To achieve more homogeneous group of patients also those, who have been receiving prior
H.pylori eradication therapies were excluded from the target group (in difference from the
previous intention to give second-line therapy to these patients). This has resulted in better selection of the patients, but additionally increased number of screen-failures.
Additional involvement of collaboration partners and expertise from these collaborative centres made this possible to include new tests and methods to the protocol. Samples for later bacteriology testing are gathered and being frozen, the same as full-blood samples for host-factor response testing.
Due to ethical considerations and with the aim to have a larger study group it has been decided during the initial meeting of the participants that all the H.pylori infected individuals should receive eradication therapy (instead of random selection in two groups as intended from the beginning). This will give a larger eradication group for follow-up, and therefore statistically better results from the follow-up testing. On the other hand, this is additionally increasing the workload as well as the costs for the medication and follow-up testing.
Due to the prolonged recruitment period the new control timing has been determined in 12 (10-12) months and 24 (22-24) months from the recruitment (initial testing). From the discussions with the recruited patients this was clarified that more frequent blood sampling for the research purpose (without any changes in the management strategy) would be considered unacceptable for them, and a large proportion of them would not appear for the pre-scheduled appointments.
There have been certain modifications made also in the testing approaches of H.pylori. Additionally to the initially planned tests with the purpose to increase the research value of
the project results a 13C-urea breath-test (UBT) was included to the number of tests for
H.pylori assessment during the initial work-up. This has been increasing the costs
considerably (50-60 USD per a recruited patient as to the market prices), but also allowing better testing for the microorganism; following the current protocol at least 2 H.pylori tests out of 4 altogether (rapid urease test, histology, IgG group antibodies, UBT) should be positive for considering the presence of the microorganism. By considering that part of the initial UBTs have been false negatives, this has been decided not to perform a follow-up UBT for the eradication efficacy control in the patients showing negative UBT results during the recruitment.