+
MODEL Journal of the Formosan Medical Association (2012) xx, 1e7Available online at www.sciencedirect.com
journal homepage: www . jfma - online . com
ORIGINAL ARTICLE
The translation and validation of Chinese overactive
bladder symptom score for assessing overactive
bladder syndrome and response to solifenacin
treatment
Eric Chieh-Lung Chou
a
, Man-Jung Hung
b
,
c
, Ta-Wei Yen
d
, Yao-Chi Chuang
e
,
f
,
En Meng
g
, Shih-Tsung Huang
h
, Hann-Chorng Kuo
i
,
*
a Department of Urology, China Medical University Hospital, Taichung, Taiwan
b Department of Obstetrics and Gynecology, Taichung Veterans General Hospital, Taichung, Taiwan
c Department of Obstetrics and Gynecology, Chung Shan Medical University School of Medicine, Taichung, Taiwand Department of Urology, Lo-Sheng Sanatorium, Department of Health, New Taipei City, Taiwan
e Division of Urology, Chang Gung Memorial Hospital, Kaohsiung Medical Center, Kaohsiung, Taiwan f College of Medicine, Chang Gung University, Taoyuan, Taiwan
g Division of Urology, Department of Surgery, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan h Division of Urology, Department of Surgery, Chang Gung Memorial Hospital-Linkou, College of Medicine, Chang GangUniversity, Taoyuan, Taiwan
i Department of Urology, Buddhist Tzu Chi General Hospital and Tzu Chi University, Hualien, Taiwan
Received 3 March 2012; received in revised form 26 July 2012; accepted 30 July 2012
KEYWORDS Background/Purpose: Overactive bladder symptom score (OABSS) was developed by a Japanese
antimuscarinics; urologist and is widely used in Asian countries. The aim of this study was to develop and validate
detrusor overactivity; a Chinese OABSS for assessing overactive bladder (OAB) and treatment outcome after solifenacin.
incontinence; Methods: The Chinese OABSS was developed by linguistic validation of the original version. Its
overactive bladder; reliability and validity, and correlations with a three-day bladder diary were tested. Patients
symptom score; answered the Chinese OABSS at enrollment and repeated the questionnaire after a
non-voiding diary treatment period of 2 weeks, and at 4 and 12 weeks after solifenacin (5 mg/day). Patients also completed a three-day bladder diary and forms including patient perception of bladder condi-tion, International Prostatic Symptom Score and quality of life index at each study visit (for a total of four visits). An analysis was conducted to evaluate the reliability and validity of the Chinese OABSS and the correlations with a three-day bladder diary and a patient perception of bladder
Corresponding author. Department of Urology, Buddhist Tzu Chi General Hospital, 707, Section 3, Chung-Yang Road, Hualien, Taiwan. E-mail address: hck@tzuchi.com.tw (H.-C. Kuo).
0929-6646/$ - see front matter Copyright ª 2012, Elsevier Taiwan LLC & Formosan Medical Association. All rights reserved.
http://dx.doi.org/10.1016/j.jfma.2012.07.044
Please cite this article in press as: Chou EC-L, et al., The translation and validation of Chinese overactive bladder symptom score for assessing overactive bladder syndrome and response to solifenacin treatment, Journal of the Formosan Medical Association (2012), http://dx.doi.org/10.1016/j.jfma.2012.07.044
+
MODEL2 E.C.-L. Chou et al.
condition, respectively.
Results: A total of 60 patients with OAB, including 31 OAB wet and 29 OAB dry, were enrolled. The testeretest reliability of Chinese OABSS was moderate to good with weighted kappa coefficients of 0.515e0.721 for each symptom score and 0.610 for total symptom score. Forty-eight (80%) patients completed the responsiveness study and were followed-up at all time points. Patients’ OAB symptoms improved significantly from baseline to 3 months after solifenacin treatment. The changes in OABSS decreased gradually with time within the three months of solifenacin treat-ment.
Conclusion: The Chinese OABSS has been validated as a reliable instrument for assessing OAB. So-lifenacin 5 mg once daily improved urgency and other symptoms of OAB including frequency, urge incontinence, OABSS and International Prostatic Symptom Score. The adverse effects were acceptable and became less significant with time in the three months of treatment.
Copyright ª 2012, Elsevier Taiwan LLC & Formosan Medical Association. All rights reserved.
Introduction
Overactive bladder (OAB) is defined as a symptom syndrome of urinary urgency, with or without urgency incontinence, usually with urinary frequency and nocturia, in the absence of infection or other obvious pathological features.1 OAB is highly prevalent and affects physical and mental health, activities of daily life and the quality of life (QoL) of patients, interfering with daily activities, travel and sleep/ vitality.2 Epidemiological studies have demonstrated that OAB is found at frequencies of 12.4e53.1%, depending on the target population and definition of OAB.3,4 OAB is highly prevalent in Asia too. The prevalence ranges from 8.0% in China to 28.4% in Korea.5,6
Homma et al published the OAB symptom score (OABSS) in 2006.7 This is a single symptom score that employs a self-report questionnaire to quantify OAB symptoms. The authors selected four symptomsddaytime frequency, nighttime frequency, urgency and urgency incon-tinencedfor the questionnaire.7 The OABSS is highly sensitive to treatment-related changes in OAB symptoms. Its simplicity and dependability mean that the OABSS can be an alternative to a bladder diary for symptom and effi-cacy assessment in daily clinical practice.8
OABSS evaluates symptoms from the patient’s viewpoint and does not require diary recording. Its responsiveness to treatment was confirmed in Japanese patients.8 OABSS has an advantage over other scales for OAB symptoms, but it has also one concern in that it was developed using Japanese language and validated based on Japanese patient data only. In order to use the OABSS outside Japan, it needs to be translated into the local language and validated based on local patient data.
The present study (the Reproducibility Study of OABSS and Its Response to Treatment or RESORT) consisted two parts in order validate a traditional Chinese version of OABSS with Taiwanese patients:
₃ The reproducibility study that evaluated the reliability and validity of the Chinese OABSS and correlations with other measures of OAB including a three-day bladder diary and a patient perception of bladder condition measure (PPBC).
₃ The responsiveness study compared the Chinese OABSS scores before and after pharmacotherapy with an antimuscarinic agent, Solifenacin (5 mg/day).
The aim of this study was to demonstrate the thera-peutic efficacy of solifenacin assessed by this newly-validated Chinese OABSS.
Materials and methods
Translation of the Chinese OABSS
A standard linguistic validation process was conducted to ensure conceptual equivalence between the Chinese translations and the original OABSS using the methods described by Acquadro et al.9 The Taiwan Continence Society commenced linguistic validation and other elements of the production of a Chinese version of the OABSS. The process involved forward- and back-translation, and review by urologists and gynecologists in expert meetings.
Part one: The reproducibility study
A total of 60 consecutive patients with OAB were enrolled from urological or urogynecological outpatient clinics in this prospective, multicenter study in Taiwan. A brief protocol including the study calendar and inclusion/exclu-sion criteria for enrolling patients is given in Supplement 1. Patients answered the Chinese OABSS at enrollment and repeated the questionnaire after a non-treatment period of two weeks.
Part two: The responsiveness study
Patients answered the questionnaire again four and 12 weeks after pharmacological treatment with the anti-muscarinic agent solifenacin (5 mg/day). Patients also completed a three-day bladder diary and a PPBC at each study visit (for a total of four visits). Patients were also requested to report their International Prostatic Symptom Score (IPSS) and QoL index at each visit. An analysis was conducted to evaluate the reliability and validity of the Chinese OABSS and the correlations with a three-day bladder diary and a PPBC, respectively.
Adverse events were recorded and the severity and possible causal relationship to study medication were analyzed. Safety assessments were performed at weeks
Please cite this article in press as: Chou EC-L, et al., The translation and validation of Chinese overactive bladder symptom score for assessing overactive bladder syndrome and response to solifenacin treatment, Journal of the Formosan Medical Association (2012), http://dx.doi.org/10.1016/j.jfma.2012.07.044
+
MODELfour and 12 after treatment. If the patient discontinued before completion of the full course of study, the reasons (e.g. adverse event, insufficient therapeutic response, failure to return for follow-up, etc.) were recorded. All patients gave informed consent at enrollment and the study protocol was approved by the Joint Institutional Review Board in Taiwan as well as by the institutional review board at each hospital.
Statistical analysis
The testeretest reliability of the Chinese OABSS was eval-uated by comparison of the variations between the baseline and the two-week assessment with weighted kappa anal-ysis. The internal consistency of the questionnaire was analyzed by Cronbach’s alpha. For interpreting correlation coefficients, the guidelines proposed by Cohen were used, with a coefficient of 0.1 being low, 0.3 being moderate and 0.5 being high.10 Kappa coefficients were interpreted using the guidelines provided by Altman as follows: kappa values of less than 0.2 represent poor agreement; values of 0.21e0.40, fair agreement; values of 0.41e0.60, moderate agreement; values of 0.61e0.8, good agreement; and values of 0.81e1.00, very good agreement.11 All statistical tests were two-tailed and conducted with a significance level of 0.05. SAS software version 9.1.3 (SAS Institute, Cary, NC) was used for statistical computations.
Results
Development of the Chinese OABSS
The Chinese OABSS is shown in Supplement 2. A pre-test was conducted on 15 OAB patients who confirmed that the questionnaire had clear wording, was relevant to patients’ complaints and did not neglect any important symptoms.
Validation of the Chinese OABSS
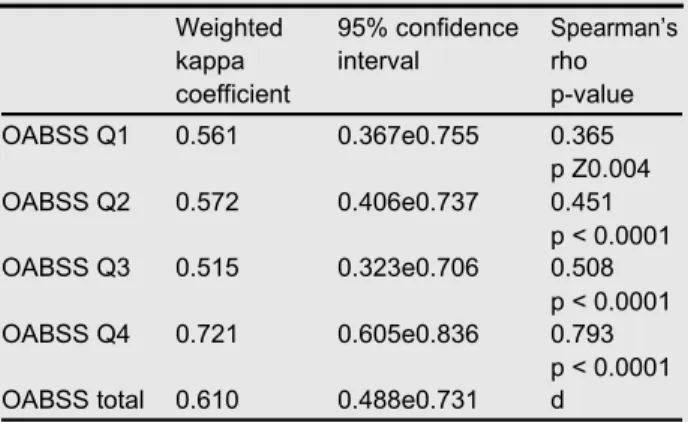
A total of 60 patients with OAB, either incontinent (OAB wet; n Z31) or continent (OAB dry; n Z29), were consec-utively enrolled in the study. Each patient answered the questionnaire at the time of enrollment and repeated it after a two-week non-treatment period. The characteristics and the baseline OABSS of patients are shown in Table 1.The Chinese OABSS had moderate to good testeretest reliability, with the weighted kappa coefficients ranging between 0.515 and 0.721 for each symptom score and 0.610 (95% confi-dence interval 0.488e0.731) for the total symptom score. In addition, the questionnaire was internally consistent with a Cronbach’s alpha of 0.674, see Table 2.
The responsiveness study
Forty-eight patients, including 36 women and 12 men, completed the responsiveness study and were followed-up at all time points. Twelve patients were not enrolled after part 1 of the reproducibility study, including four patients who did not meet inclusion criteria, two patients who could
not tolerate adverse effects and six patients who did not return for follow-up for personal reasons.
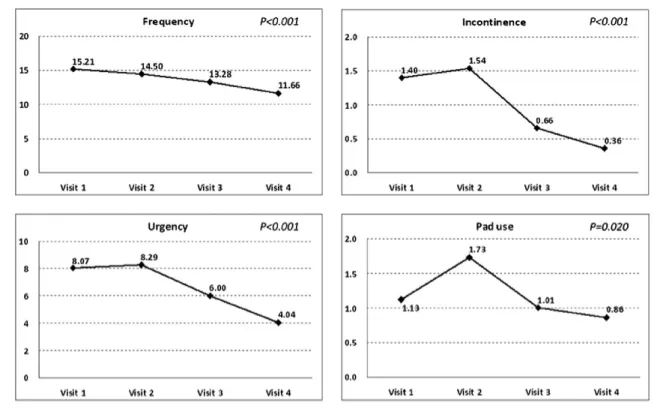
The patients’ OAB symptoms improved significantly from baseline to 3 months after solifenacin treatment. In terms of bladder diaries: the number of episodes of micturition per day decreased from 15.2 to 11.7 (p < 0.001); the number of incontinence episodes per day decreased from 1.4 to 0.4 (p < 0.001); and the number of pads used due to urinary incontinence per day decreased from 1.1 to 0.9 (p Z 0.02), see Fig. 1. All scores used to evaluate symptoms showed improvement (the numbers decreased):
₃ the OABSS decreased from 10.2 to 6.0 (p < 0.001); ₃ IPSS empty score dropped from 6.9 to 3.0 (p < 0.001); ₃ IPSS storage score decreased from 8.9 to 5.4
(p < 0.001);
₃ IPSS total score dropped from 15.8 to 8.4 (p < 0.001, see Fig. 2);
₃ The PPBC decreased from 4.7 to 2.6 (p < 0.001); and ₃ QoL index dropped from 4.6 to 2.4 (p < 0.001).
We observed more obvious improvement of OAB symp-toms in the period of 3 months’ treatment with solifenacin.
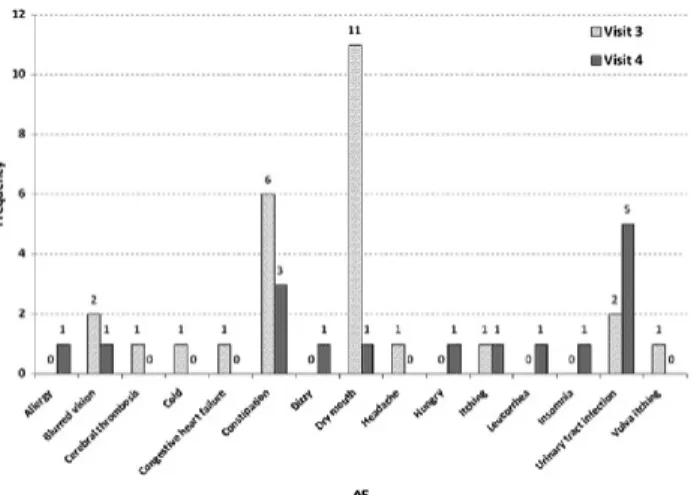
Common adverse effects included dry mouth in 12 (25%) patients and constipation in nine (18.8%). We also observed that the patients experienced more obvious side effects 2 weeks after treatment but these gradually became less significant at 6 weeks and 12 weeks after treatment (see
Fig. 3).
Discussion
OAB is highly prevalent in the population. A population-based survey of OAB was carried out in USA and Europe showing that OAB is estimated to occur in 12e17% of the population, and the prevalence of OAB tends to increase with increasing age.12,13 Recent epidemiological studies have shown that the prevalence of OAB is similar across the Eastern and Western worlds,6,14 but there is a lower level of public awareness and healthcare-seeking behavior in the East. A questionnaire-based survey performed in 11 coun-tries in Asia showed that OAB in Asian females was signifi-cant but also highlighted the low treatment-seeking rate among its sufferers.15 In 2006, a population-based survey in Taiwan showed that the age-adjusted prevalence of OAB was 16.9%, including 4.5% with urge incontinence, but only 13% of sufferers had consulted doctors for this problem.16 We need an appropriate tool to screen and assess Taiwa-nese patients with OAB.
The diagnosis of OAB is based on symptoms without physiological markers of disease activity. However, the consensus of which symptoms or evaluations should be used to define OAB is still lacking.3 To capture the patient’s perspective, i.e. symptoms and their impact on QoL, several patient-reported outcome instruments have been created, including the overactive bladder questionnaire, patient perception of bladder condition (PPBC), urgency questionnaire, and the primary OAB symptom question-naire (POSQ).17 However, most of the available measures evaluate OAB symptoms, rather than their effects in daily life.
Please cite this article in press as: Chou EC-L, et al., The translation and validation of Chinese overactive bladder symptom score for assessing overactive bladder syndrome and response to solifenacin treatment, Journal of the Formosan Medical Association (2012), http://dx.doi.org/10.1016/j.jfma.2012.07.044
+
MODEL4 E.C.-L. Chou et al.
Table 1 Patient data and the symptom scores of three patient groups.
Score OAB OAB wet OAB dry
Patients (M/F) d 60 (14/46) 31(7/24) 29 (7/22)
Age (yr) Mean ₃ SD 55.3 ₃ 13.8 61.5 ₃ 14.0 48.6 ₃ 10.2
p value OAB wet vs. OAB dry p < 0.0001
BMI (kg/m2) Mean ₃ SD 24.1 ₃ 4.44 25.3 ₃ 4.75 22.8 ₃ 3.71
p value d OAB wet vs. OAB dry p Z0.026
Question 1 0 1 1 0
Daytime frequency 1 38 19 19
2 21 11 10
p value d OAB wet vs. OAB dry p Z0.868
Mean 1.33 1.32 1.35
Question 2 0 5 3 2
Nighttime frequency 1 9 6 3
2 9 3 6
3 37 19 18
p value OAB wet vs. OAB dry p Z0.562
Mean 2.30 2.23 2.38 Question 3 0 0 0 0 Urgency 1 0 0 0 2 2 1 1 3 8 3 5 4 18 9 9 5 32 18 14
P value d OAB wet vs. OAB dry p Z0.415
Mean 4.33 4.42 4.24 Question 4 0 29 0 29 Urgency incontinence 1 2 2 0 2 4 4 0 3 7 7 0 4 15 15 0 5 3 3 0
p value d OAB wet vs. OAB dry p < 0.0001
Mean 1.77 3.42 0
OABSS Range 4 to 15 7 to 15 4 to 10
p value d OAB wet vs. OAB dry p < 0.0001
Mean ₃ SD 9.73 ₃ 2.60 11.4 ₃ 2.28 7.97 ₃ 1.57 Key: BMI: body mass index; OAB: overactive bladder; OABSS: overactive bladder symptom score.
The validated Chinese OABSS is the first foreign language version outside Japan. The first part of our study showed the correlations of the Chinese OABSS with the three-day bladder diary were high throughout the study visits. The three-day bladder diary records the status of the micturi-tion, but it does not reflect the status of urgency. There-fore, we cannot evaluate the severity of OAB using the three-day bladder diary alone. Furthermore, patients must prepare containers and record the amount of urine produced each time to complete the diary, which is inconvenient in daily life and may interrupt sleep at night. Compared with the three-day bladder diary, it is simpler and more convenient to fill out the OABSS in a few minutes. If the OABSS can reflect the symptoms of OAB, it can replace the three-day bladder diary and be of great help to patients.
The IPSS includes the symptoms of urgency and frequency, but the IPSS was originally designed to assess patients with benign prostatic hypertrophy, and not those
Table 2 Test-retest reliability and internal consistency of the Chinese OABSS.
Weighted 95% confidence Spearman’s kappa interval rho coefficient p-value OABSS Q1 0.561 0.367e0.755 0.365 p Z0.004 OABSS Q2 0.572 0.406e0.737 0.451 p < 0.0001 OABSS Q3 0.515 0.323e0.706 0.508 p < 0.0001 OABSS Q4 0.721 0.605e0.836 0.793 p < 0.0001 OABSS total 0.610 0.488e0.731 d
Please cite this article in press as: Chou EC-L, et al., The translation and validation of Chinese overactive bladder symptom score for assessing overactive bladder syndrome and response to solifenacin treatment, Journal of the Formosan Medical Association (2012), http://dx.doi.org/10.1016/j.jfma.2012.07.044
+
MODELChinese overactive bladder assessment and treatment 5
with OAB. The IPSS has been widely used and accepted by urologists, but general practitioners and gynecologists are not so familiar with it.
The PPBC is a single-item measure that assesses subjective impressions of a current urinary problem.18 The advantages of the PPBC are its simplicity and usefulness.
However, a single-item, global measure cannot provide the depth or breadth of information that can be obtained from multi-item measures. The PPBC does not sufficiently address the core symptoms of OAB and urgency for an assessment. In contrast, the OABSS is apparently more specific in the screening and evaluation of OAB.
Figure 2 The changes in OABSS and IPSS scores after solifenacin treatment.
Please cite this article in press as: Chou EC-L, et al., The translation and validation of Chinese overactive bladder symptom score for assessing overactive bladder syndrome and response to solifenacin treatment, Journal of the Formosan Medical Association (2012), http://dx.doi.org/10.1016/j.jfma.2012.07.044
+
MODEL6 E.C.-L. Chou et al.
Figure 3 The occurrence of adverse events after solifenacin treatment at visits 3 and 4.
Solifenacin is a once-daily oral antimuscarinic treatment for OAB. A previous study showed that solifenacin 5 mg once daily was highly effective in reducing the symptoms of OAB.19 Most reports on the therapeutic effects of sol-ifenacin are from Western countries or Japan. This is the first multi-center, large-scale study using the Chinese version of the OABSS as an assessment tool to evaluate the effectiveness of solifenacin in Taiwanese patients. Our results showed that solifenacin successfully reduced the symptoms of OAB. We observed not only a IPSS empty score, but the IPSS storage score also showed a marked improvement after treatment. Antimuscarinics have been shown to reduce bladder tone during storage and to increase cystometric bladder capacity.20 The detrusor contractility increases when the bladder wall is stretched, so the increased bladder capacity might have positive effect on the voiding phase. Furthermore, these results reflect that antimuscarinics at the current dose for the treatment of storage symptoms in OAB patients does not suppress detrusor contractility during the voiding phase.21 In terms of adverse events, 25% of our patients experi-enced dry mouth and 18.8% experienced constipation. These rates are similar to those found during previous studies.22 We did, however, observe that the rate of adverse events decreased with the continuation of treat-ment. One possible explanation is that patients adapted to the adverse effects or changed their lifestyle, such as consuming more dietary fiber to treat constipation. On the other hand, this study also revealed that the change in OABSS decreased gradually with time over the 3 months of treatment. This is because the therapeutic effect of anti-muscarinics is not only attributed to direct receptor blocking effects but is also influenced by the drug concentration increasing to a maximal steady state. Sol-ifenacin needs at least 2 to 3 months to reach a maximal stable therapeutic effect, as is the case for other types of antimuscarinics.23 Our previous single-institute study showed that the improvement of urinary urgency severity score reached the plateau 3 months after the administra-tion of solifenacin and remained steady at 6 months.24 We suggest that 3 months might be a reasonable treatment period for solifenacin in OAB patients. The study does,
however, have a limitation in that there was a lack of a control arm for comparison.
Conclusion
The Chinese OABSS has been developed and validated as a reliable instrument to assess patients with OAB. This study demonstrated that solifenacin was an effective antimuscarinic for the treatment of OAB in Taiwanese individuals, in terms of improvement in frequency, urge incontinence, OABSS, IPSS, PPBC and QoL. The adverse effects are minor and became less significant over the 3 months of treatment.
Appendix A. Supplementary data
Supplementary data related to this article can be found at
http://dx.doi.org/10.1016/j.jfma.2012.07.044.
References
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Van Kerrebroeck P, et al. The standardization of terminology of lower urinary tract function: report from the Standardization Sub-Committee of the International Continence Society. Neu-rourol Urodynam 2002;21:167e78.
2. Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care 2000;6: S580e90.
3. Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urology 2002;60(5 Suppl. 1):7e12.
4. Homma Y, Yamaguchi O, Hayashi KNeurogenic Bladder Society Committee. Epidemiologic survey of lower urinary tract symptoms in Japan. Urology 2006;68:560e4.
5. Zhang W, Song Y, He X, Huang H, Xu B, Song J. Prevalence and risk factors of overactive bladder syndrome in Fuzhou Chinese women. Neurourol Urodynam 2008;25:717e21. 6. Lee YS, Lee KS, Jung JH, Han DH, Oh SJ, Seo JT, et al.
Preva-lence of overactive bladder, urinary incontinence, and lower urinary tract symptoms: results of Korean EPIC study. World J Urol 2011;29:185e90.
7. Homma Y, Yoshida M, Seki N, Yokoyama O, Kakizaki H, Gotoh M, et al. Symptom assessment tool for overactive bladder syndrome-overactive bladder symptom score. Urology 2006;68:318e23.
8. Homma Y, Kakizaki H, Yamaguchi O, Yamanishi T, Nishizawa O, Yokoyama O, et al. Assessment of overactive bladder symp-toms: comparison of 3-day bladder diary and the overactive bladder symptoms score. Urology 2011;77:60e4.
9. Acquardo C, Kopp Z, Coyne KS, Corcos J, Tubaro A, Choo MS,
et al. Translating overactive bladder questionnaires in 14 languages. Urology 2006;67:536e40.
10. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Philadelphia: Lawrence Erlbaum Associates; 1988. 11. Altman DG. Practical statistics for medical research. 2nd ed.
London: Chapman & Hall; 1992.
12. Stewart EF, Van Rooyen JB, Cundiff GW, Abrams P, Herzoq AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327e36. 13. Irwin DE, Milsom I, HunsKaar S, Reilly K, Kopp Z, Herschorn
S, et al. Population-based survey of urinary incontinence, over-active bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50: 1306e14.
Please cite this article in press as: Chou EC-L, et al., The translation and validation of Chinese overactive bladder symptom score for assessing overactive bladder syndrome and response to solifenacin treatment, Journal of the Formosan Medical Association (2012), http://dx.doi.org/10.1016/j.jfma.2012.07.044
+
MODEL14. Homma Y, Yamaguchi O, Hayashi K. Neurogenic Bladder Society Committee. An epidemiological survey of overactive bladder symptoms in Japan. BJU Int 2005;96:1314e8.
15. Lapitan MC, Chye PL. Asia-Pacific Continence Advisory Board. The epidemiology of overactive bladder among females in Asia: a questionnaire survey. Int Urogynecol J Pelvic Floor Dysfunc
2001;12:226e31.
16. Yu HJ, Liu CY, Lee KL, Lee WC, Chen TH. Overactive bladder syndrome among community-dwelling adults in Taiwan: prev-alence, correlates, perception, and treatment seeking. Urol Int 2006;77:327e33.
17. Matza LS, Thompson CL, Krasnow J, Brewster-Jordan J, Zyczynsji T, Coyne KS. Test-retest reliability of four question-naires for patients with overactive bladder: the overactive bladder questionnaire (OAB-q), patient perception of bladder condition (PPBC), urgency questionnaire (UQ), and the primary OAB symptom questionnaire (POSQ). Neurourol Urodynam 2005;24:215e25.
18. Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol 2006;49:1079e86.
19. Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int 2004;93:303e10.
20. Andersson K-E, Yoshida M. Antimuscarinics and the overactive detrusor-which is the main mechanism of action? Eur Urol 2003;43:1e5.
21. Hsiao SM, Chang TC, Wu WY, Chen CH, Yu HJ, Lin HH. Comparisons of urodynamic effects, therapeutic efficacy and safety of solifenacin versus tolterodine for female overactive bladder syndrome. J Obstet Gynaecol Res 2011; 37:1084e91. 22. Garely AD, Kaufman JM, Sand PK, Andoh M. Symptom bother
and health-related quality of life outcomes following sol-ifenacin treatment for overactive bladder: the VESIcare Open-Label Trial (VOLT). Clin Ther 2006;28:1935e46.
23. Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int 2007;100:987e1006.
24. Chen YC, Chen CY, Kuo HC. Efficacy and adverse effects of solifenacin in the treatment of lower urinary tract symptoms in patients with overactive bladder. Urol Sci 2010;21:38e43.
Please cite this article in press as: Chou EC-L, et al., The translation and validation of Chinese overactive bladder symptom score for assessing overactive bladder syndrome and response to solifenacin treatment, Journal of the Formosan Medical Association (2012), http://dx.doi.org/10.1016/j.jfma.2012.07.044