In Utero Exposure to Manganese and Psychomotor
Development at the Age of Six Months
Feng-Chiao Su1, Hua-Fang Liao2, Yaw-Huei Hwang1, Wu-Shiun Hsieh3, Hui-Chen Wu1, Suh-Fang Jeng2, Yi-Ning Su4, Pau-Chung Chen1
1 Institute of Occupational Medicine and Industrial Hygiene, National Taiwan University
College of Public Health, Taipei, Taiwan
2 School and Graduate Institute of Physical Therapy, National Taiwan University College
of Medicine, Taipei, Taiwan
3 Department of Pediatrics, and 4 Department of Medical Genetics, National Taiwan
University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan
Abstract
Objective:
We conducted the present study to evaluate associations between prenatal exposure to environmental manganese and psychomotor development in children from the general population in Taipei, Taiwan.
Methods:
The study is a part of the Taiwan Birth Panel Study. A total of 132 pairs of parents and their full-term babies were selected into this study. The Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT) was conducted at the age of six months. The CDIIT was used to assess development in the areas of cognition, language, motor (including gross motor and fine motor), social, and self-help of children. Samples of cord blood were analyzed by an Agilent 7500C ICP-MS. Regression models and adjusted means were used to evaluate the association of exposure to manganese and psychomotor development.
Results:
The fine-motor developmental quotients (DQ) of the CDIIT were significantly influenced by the level of manganese (crude β = -7.0, SE = 2.5); the reverse relationship, however, was slightly diminished after adjustment (adjusted β = -6.0, SE = 2.5). Based on logistic regression models, the results corresponded with the linear regression models (crude OR = 3.20, 95% CI = 1.31-7.85; adjusted OR = 3.02, 95% CI = 1.18-7.76). In addition, the manganese concentration was separated by every 25% and analyzed though adjusted means. The relationship between manganese levels and fine-motor DQ showed an inverted U shape.
Conclusion:
Fetuses may be vulnerable to environmental manganese exposure, particularly in terms of motor performance at the early age of six months. Some confounding factors, however, could not be excluded. Thus, further research is necessary to verify our findings. Nonetheless, to prevent the risk of poorer psychomotor development, we advise pregnant women to avoid exposure to excessive manganese in ordinary life.
Key words: In utero exposure, Manganese, Psychomotor development, Children
Accepted 25 July, 2007
*Correspondence to: Pau-Chung Chen, Institute of Occupational Medicine and Industrial Hygiene, National Taiwan University College of Public Health, Room 733, No. 17 Syujhou Road, Taipei 10055, Taiwan, Phone: +886-2-3322-8088, Fax: +886-2-2358-2402, e-mail: pchen@ntu.edu.tw
205
Introduction
Pregnancy, a period of rapid growth and cell differentiation, is very susceptible to alterations in dietary supply, especially of nutrients which are marginal under normal circumstances [1]. Manganese is both an essential element and a potent neurotoxin. It is required for normal amino acid, lipid, protein, and carbohydrate metabolism. The manganese-dependent enzyme, manganese superoxide dismutase, is involved in the defense mechanisms against free radicals, and animal studies have found that manganese deficiency was associated with impaired growth, skeletal defects, reduced reproductive function, and so on [2,3]. Fortunately, because manganese is generally present in human diets, deficiency is not common [2]. Therefore, this study focuses on the neurotoxicological side.
However, experiments with animals perinatally exposed to excess manganese showed some effects such as reduced birth weight, reduced size of testes and seminal vesicles, transient ataxia and exposure during infancy was related to adverse neurobehavior development in later life [4,5]. Furthermore, manganese, a well-known occupational hazard for people via inhalation, mainly has adverse effects on adult human respiratory tracts and nervous systems, leading to conditions such as bronchitis, pneumonitis, and manganism [6]. In addition, recently a review article highlighted published studies that have investigated associations between neurotoxicity and exposure
to manganese, not only at the workplace but also from residences, from sources like well water, for example [7]. Nevertheless, the relationship between neurologic effects and exposure to manganese from drinking water has still not been conclusively made.
Moreover, it is known that manganese blood levels are increased during pregnancy and manganese ions easily cross the placenta by active transport to result in higher manganese levels in cord blood than in maternal blood [8-10]. Compared to adults, children will absorb more and excrete less manganese [11]. In addition, manganese easily gets access to the underdeveloped brain because of an immature blood-brain barrier and the accumulation of manganese in the developing brain has a dose-response effect by oral exposure [12,13]. Researchers have suggested that the hepatic homeostatic control of manganese elimination in neonates may be underdeveloped [14,15]. Research that examines the association between manganese exposure and neurotoxicity in children is not as common as it is for adults, and most reports come from individual case studies. For example, in China, children 11-13 years of age exposed to sewage of high level manganese had lower scores on tests of short-term memory, manual dexterity, and visuo-perceptual speed than unexposed children, while another case report showed a ten-year-old boy who was exposed to elevated concentrations of manganese from a drilled well performed poorly in tests of verbal and visual memory [16,17].
Recently, another study of school-age children residing near a hazardous waste site observed a significant relationship between hair manganese levels and worse general intelligence scores, particularly on verbal IQ tests and tests of memory for stories and word lists [18].
However, there are few studies that show evidence linking low manganese levels to adverse neurodevelopment in children. To investigate the adverse effect of prenatal low level exposure to manganese on child psychomotor development, Takser and Tasker’s colleagues undertook a study of health pregnant women and their babies [19]. The study reported reverse associations between cord blood manganese levels and several psychomotor subscales at the age of three years, including attention, non-verbal memory, and hand skills. It suggests that low level manganese exposure from the environment in utero may influence early psychomotor development. In addition, another cross-sectional study investigated intellectual function in the general population at the age of ten years in Bangladesh, with those children having consumed tube-well water with a higher manganese concentration than the U.S. Environmental Protection Agency lifetime health advisory level [20]. It found that higher manganese level was associated with poor intellectual function- Full-Scale, Performance, and Verbal raw scores, and exhibited a dose-response effect.
For li m ited i n for mat ion about t he possible effects of manganese exposure on
neurodevelopment in children, we conducted the present study to evaluate the associations between prenatal exposure to environmental manganese and psychomotor development in children from the general population in Taipei, Taiwan.
Materials and Methods
Study population
The study is a part of the Taiwan Birth Panel Study (TBPS). A total of 335 sets of subjects including parents and their live births were recruited from a medical center in Taipei City from April 2004 to January 2005. The protocols used in our study were approved by the Ethical Committee of National Taiwan University Hospital. Informed consent was obtained from study subjects, and we asked all of them to supply specimens of maternal blood, umbilical cord blood, and placenta. We excluded the subjects with insufficient biological samples and incomplete interview data. To prevent confounding, we only included singleton and full-term infants and eliminated the few active smoking mothers during pregnancy or those who had a history of workplace exposure to manganese. There were a total of 308 sets selected into our study and 132 of them implemented the early childhood Home Observation for Measurement of the Environment (HOME) inventory and Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT) [21,22]. There
207 were no significant differences between the original group and the participating population for sociodemographic variables and birth outcomes.
Data collection
All mothers were interviewed after delivery by trained interviewers using a structured questionnaire to obtain information on parental life style, sociodemographic characteristics, personal and familial medical history, and habits of tobacco smoking and alcohol consumption.
The HOME inventory is designed to measure the quality and quantity of stimulation and support available to a child (birth to age three) in the home environment, and is composed of 45 items clustered into six subscales: parental responsivity, acceptance of child, organization of the environment, learning materials, parental involvement, and variety of experience. Subjects were observed and their main caregivers were interviewed with the HOME inventory by a well-trained physiotherapist in the subjects’ home when children were six months old [21, 23].
Child developmental status was evaluated by the CDIIT. The CDIIT was designed and standardized to assess development in the areas of cognition (attention, perception, memory, reasoning, and concept), language (comprehensive and expressive), motor (including gross motor and fine motor), gross motor (antigravity control, locomotion, and
body movement coordination), fine motor (basic hand use and visual-motor coordination), social (inter-personal, affection, self-responsibility, and adaptation), self-help (feeding, dressing, and hygiene), and behavioral characteristics of youngsters with ages ranged from 3 to 71 months or those who would have developmental delays within the range.
The standardization sample of 3,703 infants (1,055 boys and 1,068 girls), aged 3-71 months, was randomly selected according to age, sex, and geographic regions in Taiwan. Urban versus rural residence was also considered [24]. The CDIIT has acceptable test-retest reliability (r = 0.89-0.99, p value < .001), internal consistency (Cronbach α = 0.75-0.99), content validity, and concurrent and construct validity [22,24,25]. One recent study showed that the overall diagnostic accuracy of the CDIIT motor subtest was high, with an area under the receiver operating characteristic curve of 0.97 for children with motor disabilities [26]. These results indicated that clinicians could diagnose motor disabilities correctly 98% of the time with the test results of the CDIIT motor subtest [27].
Items on the CDIIT are scored 0 or 1, indicating failure or success, respectively, during the test or observation at home by the caregivers. In this study, all items of the cognitive and motor subtests, and part of the language subtest were individually and directly elicited by the tester. The social and self-help subtests were scored from a questionnaire completed by the main caregivers. From the results of the CDIIT
tested, developmental quotients (DQ) of the whole test (whole DQ), five subtests (cognitive DQ, language DQ, motor DQ, social DQ, and self-help DQ), and two subdomains (gross motor DQ and fine motor DQ) of the CDIIT of each child were obtained. In the norm, the mean DQ (standard deviation) is 100 [15]. Six pediatric physical therapists with pediatric assessment experience (including CDIIT and HOME) for 2-20 years were responsible for the HOME and CDIIT testing in this study. To increase the inter-rater reliabilities of the test results, two-day workshop was held. After lecture and demonstration, two video tapes were used for scoring practice. And before independent test, every tester should have 95% agreement for three infants with the senior physical therapist (HF Liao) who is the instructor for the CDIIT workshop.
Laboratory analysis
Umbilical cord blood of the subjects were collected at birth in ethylenediaminetertraacetic acid disodium salt dehydrate (EDTA) tubes and separated into two tubes of whole blood and four tubes of serum. They were placed under -80℃ frozen conditions until laboratory analysis was carried out. Whole blood of the subjects were analyzed by Agilent 7500C Inductively Coupled Plasma Mass Spectrometry (ICP-MS) [28]. To make sure measurements were reliable we used a spike every ten samples. In our study, the detection limit of manganese concentration
was 1.2 μg/L. If the manganese concentration in whole blood was lower than the detection limit, a value of detection limit × 1/2 would be regarded as the manganese levels in the whole blood. However, all the manganese concentrations of our subjects were higher than the detection limit.
Statistical analysis
According to the manual of CDIIT, the CDIIT DQ of children who were classified as delayed and borderline were below 70 and within 70-84, respectively [25]. In this study, subjects were from general population, no infants were classified as delay and 11 infants borderline. Most infants were within normal limits with whole DQ ≥ 85. Thus, we decided on the cut-off point of CDIIT DQ by using sensitivity analysis. The cut-off points of DQ of the whole test and 7 subtests and 2 subdomains of the CDIIT and raw scores of the HOME inventory were dichotomized at the first quartile of these samples distribution. Because there was no definite value of normal range for manganese concentration in cord blood of children, high exposure was defined as the fourth quartile of the manganese concentration and low exposure was defined as all other quartiles (≥ 61.9 μg/L vs. <61.9 μg/L).
We used linear reg ression models, logistic regression models, and adjusted means to evaluate the association of exposure to manganese and psychomotor development. According to the statistics (10% change in
209
Characteristic ≥ 61.9 µg/L (n = 33)Mn level in cord blood< 61.9 µg/L (n = 99) p value
Maternal characteristics
Age (years) 31.4 ± 3.7 31.9 ± 3.8 0.5748
Education (%) 0.5214
High school and below 15.2 20.2
University and above 84.8 79.8
Infant characteristics
Gender (%) 0.5397
Male 63.6 57.6
Female 36.4 42.4
Birth weight (g) 3249.8 ± 452.8 3288.2 ± 354.7 0.6171
Gestational age (week) 38.9 ± 1.1 39.0 ± 1.1 0.7192
Apgar score (score)
1 minute 8.8 ± 0.9 8.9 ± 0.5 0.5811 5 minute 9.0 ± 0.0 9.0 ± 0.2 0.5664 HOME a (point) 37.1 ± 3.8 39.1 ± 3.0 0.0023 CDIIT b (DQ c) Whole test 95.3 ± 9.3 99.5 ± 9.3 0.0278 Cognitive 95.3 ± 7.5 97.2 ± 8.8 0.2683 Language 105.6 ± 11.7 105.1 ± 11.4 0.8336 Motor 90.4 ± 9.5 94.3 ± 10.1 0.0541 Gross-motor 96.1 ± 8.8 97.6 ± 10.1 0.4411 Fine-motor 84.5 ± 13.5 91.5 ± 11.9 0.0054 Social 104.2 ± 12.2 107.2 ± 10.7 0.1802 Self-help 94.1 ± 12.1 96.9 ± 12.6 0.2593 Exposure characteristics
Prenatal and postnatal ETS d (%) 18.2 16.2 0.7874
Manganese levels e (µg/L) 77.2 ± 14.5 43.5 ± 10.6 <.0001
Lead levels f (µg/L) 15.4 ± 6.8 12.0 ± 6.9 0.0165
Values are mean ± standard deviation or percent.
a HOME, Home Observation for Measurement of the Environment; b CDIIT, Comprehensive Developmental Inventory for Infants
and Toddlers; c DQ, developmental quotients; d ETS, environmental tobacco smoke; e the range of manganese concentration
21.4-122.8 μg/L in whole blood; f the range of lead concentration 1.0-41.4 μg/L in whole blood (3 missing data).
Table 1 Characteristics of the study population by Mn exposure level (N=132)
estimate) and literature review, the covariate variables were maternal age, infant gender, exposure to environmental tobacco smoke during pregnancy, and the HOME inventory for adjusted regression models. We performed all statistical analyses using SPSS 11.0 and SAS 9.1 for Windows.
Results
Information on the characteristics of the study population is shown in Table 1. We
divided the infants into two groups according to manganese levels, and the demographics and birth outcomes of these two groups were not significantly different when tested by t test or chi-square test. The birth outcomes including birth weight, gestational age, and Apgar score of these two groups were all within normal limits (birth weight ≥ 2500 g; gestational age ≥ 37 week). The arithmetic means of manganese and lead in cord blood were 51.9 and 12.9 μg/L, respectively.
a CDIIT, Comprehensive Developmental Inventory for Infants and Toddlers; b DQ, developmental quotients; c SE,
standard error; d adjusted for maternal age, infant gender, environmental tobacco smoke during pregnancy, and Home
Observation for Measurement of the Environment. *<0.05, **<0.01
Table 2 Linear regression models of the CDIIT a DQ b and the manganese exposure (N = 132)
Mn level (≥ 61.9 µg/L vs. < 61.9 µg/L)
Crude β (SE c) p value Adjusted d β (SE) p value
Whole DQ -4.2 (1.9) 0.0278* -3.6 (1.9) 0.0591 Cognitive DQ -1.9 (1.7) 0.2683 -1.6 (1.8) 0.3754 Language DQ 0.5 (2.3) 0.8336 0.6 (2.3) 0.8106 Motor DQ -3.9 (2.0) 0.0541 -3.5 (2.1) 0.0912 Gross-motor DQ -1.5 (2.0) 0.4411 -1.6 (2.0) 0.4416 Fine-motor DQ -7.0 (2.5) 0.0054** -6.0 (2.5) 0.0181* Social DQ -3.0 (2.2) 0.1802 -2.7 (2.3) 0.2395 Self-help DQ -2.8 (2.5) 0.2593 -2.4 (2.5) 0.3355
a CDIIT, Comprehensive Developmental Inventory for Infants and Toddlers; b DQ, developmental quotients; c
adjusted for maternal age, infant gender, environmental tobacco smoke during pregnancy, and Home Observation for Measurement of the Environment.
*<0.05, **<0.01
Table 3 Logistic regression models of the CDIIT a DQ b and the manganese exposure (N = 132)
Mn level (≥ 61.9 µg/L vs. < 61.9 µg/L)
Crude OR (95% CI) p value Adjusted c OR (95% CI) p value
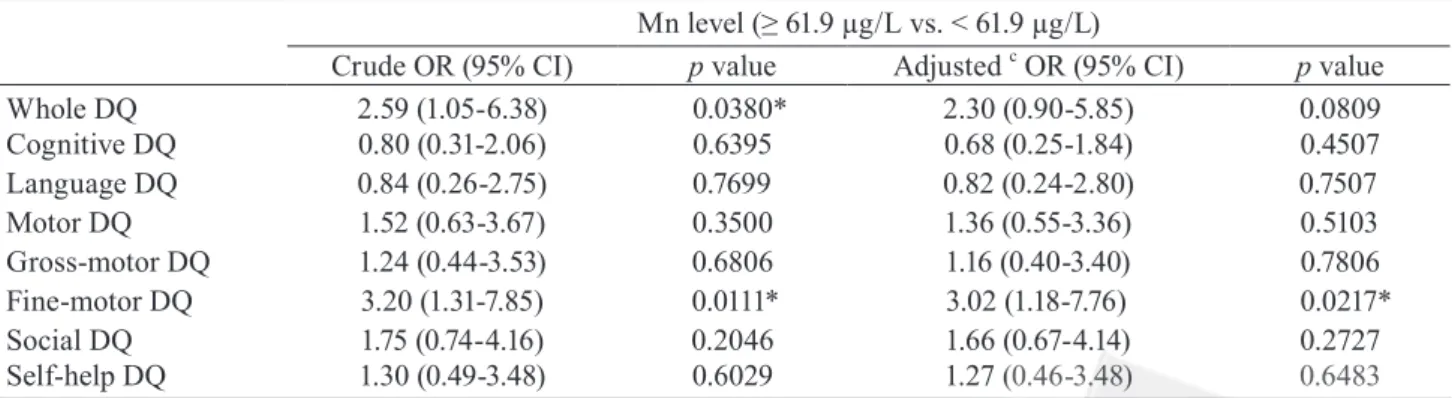
Whole DQ 2.59 (1.05-6.38) 0.0380* 2.30 (0.90-5.85) 0.0809 Cognitive DQ 0.80 (0.31-2.06) 0.6395 0.68 (0.25-1.84) 0.4507 Language DQ 0.84 (0.26-2.75) 0.7699 0.82 (0.24-2.80) 0.7507 Motor DQ 1.52 (0.63-3.67) 0.3500 1.36 (0.55-3.36) 0.5103 Gross-motor DQ 1.24 (0.44-3.53) 0.6806 1.16 (0.40-3.40) 0.7806 Fine-motor DQ 3.20 (1.31-7.85) 0.0111* 3.02 (1.18-7.76) 0.0217* Social DQ 1.75 (0.74-4.16) 0.2046 1.66 (0.67-4.14) 0.2727 Self-help DQ 1.30 (0.49-3.48) 0.6029 1.27 (0.46-3.48) 0.6483
In Table 2, we investigated the associations b e t we e n ex p o s u r e t o m a ng a n e s e a n d psychomotor development by linear regression models. We found that the whole DQ and fine-motor DQ of the CDIIT were significantly changed by the level of categorized manganese (crude β = -4.2, SE = 1.9, p value = 0.0278; crude β = -7.0, SE = 2.5, p value = 0.0054), however, the reverse relationship of the fine-motor DQ was slightly diminished after being adjusted for covariates including maternal age, infant gender, environmental tobacco smoke, and the HOME inventory (adjusted β = -6.0, SE = 2.5, p value =
0.0181), and the reverse relationship with whole DQ was in the significant borderline (adjusted β= -3.6, SE = 1.9, p value = 0.0591).
In Table 3, we then used the first quartile of the CDIIT DQ as the cut-off point of the every CDIIT subtest and undertook the logistic regression models to examine the associations between exposure and psychomotor outcomes. The results corresponded with the linear regression models. The crude odds ratios of whole DQ and fine-motor DQ of the CDIIT were significant (crude OR = 2.59, 95% CI =
211
M anganese levels(ug/L)
Fi ne -m ot or su bd om ai n( D /Q ) 21.4-38.3 38.4-50.6 50.7-61.8 61.9-122.8 80 85 90 95 100 105
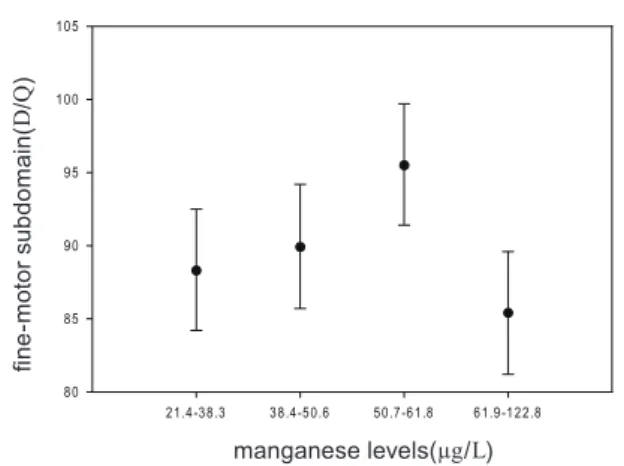
Figure 1 Adjusted a means of fine-motor DQ b classified
by manganese levels in cord blood (N = 132)a
Adjusted for maternal age, infant gender, e nv i ron me nt al t oba cco smoke du r i ng preg nancy, and Home Obser vation for Measurement of the Environment; b DQ,
developmental quotients. manganese levels(µg/L) fin e-m ot or s ub do m ai n( D /Q ) 1.05-6.38, p value = 0.0380; crude OR = 3.20, 95% CI = 1.31-7.85, p value = 0.0111), but the inverse relationship between whole DQ and manganese level was not significant (adjusted OR = 2.30, 95% CI = 0.90-5.85, p value = 0.0809), and the association between fine-motor DQ and manganese level was weaken, although still significant (adjusted OR = 3.02, 95% CI = 1.18-7.76, p value = 0.0217) after adjustment for covariates. The other subtest DQ of the CDIIT did not correlated with manganese level significantly in the present study.
To further estimate the associations between manganese levels and the fine-motor subdomain, we separated the manganese concentration by every 25% and analyzed it by adjusted means (Figure 1). It revealed that the group with the highest manganese level had the lowest fine-motor DQ. However, the highest fine-motor DQ was not the group of lowest manganese level.
Discussion
In the present study, we examined the effect of exposure to manganese in utero on psychomotor development in children at six months of age and found that the higher the manganese levels the lower the DQ in the fine-motor subdomain, including basic hand use and visual-motor coordination. It is suggested that the adverse effects of exposure to excessive manganese levels on vulnerable children could detect at the early age of six months.
In previous studies other researchers also observed that manganese exposure was associated with poorer motor performance. For example, He et al.’s study reported that the Chinese children at age of 11-13 years exposed to manganese contaminated drinking water showed worse performance in the subscales of manual dexterity and visuo-perceptual speed than unexposed ones [16]. In a community-based study, researchers found an inverse relationship between exposure to higher levels of manganese from a variety of environmental sources and poorer DQ on tasks requiring rapid coordinated movement [29]. Another normal population-based study also showed that low levels of cord blood manganese were inversely associated with hand skill performance in boys at three years old [19].
Although our study found the similar results to others, there were some different results between our study and others. In Figure 1, we observed that the association between
manganese levels and fine-motor DQ showed an inverted U shape. It may indicate that too high or too low manganese level leads to poorer fine-motor performance, and fits the manganese dichotomous effect on the human body. Furthermore, there were no significant differences in the whole DQ or cognitive DQ between two manganese groups. However, other studies found worse cognitive function caused by elevated manganese level [16,19]. Several reasons may be able to explain this. First, our subjects are the youngest ones among these similar studies. One previous study showed that the fine-motor DQ of the CDIIT was significantly correlated with Bayley Scales of Infant Development Mental Development Index (BSID-II MDI) for preterm children aged 6-40 months [25]. The fine-motor DQ at early age can predict the later MDI of BSID-II in children with developmental delay [30]. From the Piagetian theory point of view, motor experience is the basis of cognitive development. Therefore, the child with fine-motor development delay may have high risk for later cognitive delay. Second, the manganese level in this study was only slightly elevated (arithmetic mean: 51.9 μg/L; geometric mean: 48.8 μg/L). Even though the manganese concentration of cord blood in our study is higher than in Takser et al.’s study (geometric mean: 38.5 μg/L), it still below the upper limit of the normal manganese range, 15-56 μg/L according to the Aschner et al.’ s review study [2]. Third, the test instruments for developmental status of the children were
different among different studies, such as the McCarthy scales of children’s abilities performed by Takser et al. and the CDIIT inventory used by us.
Manganese, a well-known occupational hazard, is infamous for its neurotoxicity of motor dysfunction and triggering memory loss resembling Parkinson disease, however, the neurotoxic mechanisms in cells and molecules have not been clarified [31]. Nevertheless, a hypothesis inferred from Parkinson’s diseases suggested that manganese deposits in and affects mainly the cells of the striatum and globus pallidus in the basal ganglia of the brain, and leads to a depletion of dopamine in the caudate nucleus, norepinephrine in the hypothalamus, and neuromelanin in the substantia nigra [7]. Because manganese can increase the dopamine oxidation associated with the formation of free radicals, too high manganese levels in susceptible mitochondrial DNA may induce higher oxidative injury and oxidant damage [32, 33]. Such mechanism might be able to explain why infants with higher manganese exposure had lower fine-motor DQ.
Our study’s subjects were from the general population, and exposed to manganese from the environment. In general, environmental sources of manganese are water, air, pesticides, and diet such as grain, nuts, and tea [2]. Ingested manganese was approximately 1-3% absorbed, and rapidly cleared from the blood by the liver, and then about 98% excreted in the bile [7]. Concerns have been raised about exposure to
213 environmental manganese from a new fuel additive, methylcyclopentadienyl manganese tricarbonyl (MMT), used in unleaded gasoline [34]. Oxide forms of manganese are released from combustion of MMT by automobile engi nes, and this ai rbor ne manganese would increase the likelihood of exposure to manganese in children via chronic inhalation [35]. In South Africa, a study observed higher manganese levels of school soil and dust in a city that introduced MMT as gasoline additive compared to another city that didn’t [36]. It then found that study subjects who were first-grade schoolchildren living in the former city had more elevated manganese concentration in their blood than subjects in the latter city.
Lead, the most infamous environmental toxicant in Taiwan, has adverse effects on the hematologic and neurologic systems [37]. However, since the Taiwan government ceased using leaded gasoline in 2000, it is obvious that air lead levels lowered and that cord blood lead levels followed this decreasing trend [38]. Moreover, both cord blood lead levels in Hwang et al.’s and our studies (arithmetic mean: 23.5 μg/L and 12.9 μg/L, respectively) were lower than the 100 μg/L that is associated with poorer neurobehavioral and cognitive development [39]. Thus, we suppose that this low level of exposure to lead may not interfere with our inference.
Meanwhile, there are few active smoking mothers in our study, and most of them didn’t have high-exposure occupations, and not many infants were preterm delivery. Therefore, we
reduced those potential confounding factors associated with neurodevelopment to make our inference more precise. The strength of our study is that we took the population-based study design and gathered both biological samples and comprehensive lifestyle factors to assess the associations between exposure to manganese and psychomotor development. However, there still are some potential limitations in our study. First, it is a cross-sectional measurement of exposure at delivery, so it could not represent the exposure of the entire prenatal and postnatal periods. Furthermore, because our study subjects were all live births, there may be a selection bias that underestimates our findings. Hence, we could categorize our continued variables into category ones and evaluate the association more conser vatively. Finally, although several interviewers in the study may cause an information bias, there are no significant differences in their measurements. Thus, we could assume that the subjects interviewed by every interviewer were randomized.
In the present study, we found that fetuses may be vulnerable to low level exposure of environmental manganese, particularly in motor performance. However, there could be some residual confounding we did not exclude. Thus, our findings still need further research to be verified. Nonetheless, to prevent the risk of poorer psychomotor development, we advise pregnant women to avoid exposure to excessive manganese in ordinary life during pregnancy.
Acknowledgements
This study was partly supported by the grants (BHP-PHRC-92-4 and DOH93-HP-1702) from the Bureau of Health Promotion, Department of Health, Taiwan.
References
[1] McArdle HJ, Ashworth CJ. Micronutrients in fetal growth and development. Br Med Bull 1999; 55: 499-510.
[2] Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol 2005; 35: 1-32.
[3] Freeland-Graves J LC. Models to study manganese deficiency: In: Klimis-Tavantzis DL, ed. Manganese in health and disease. Boca Raton: CRC Press, Inc; 1994.
[4] Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for manganese. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2000.
[5] Tran TT, Chowanadisai W, Crinella FM, Chicz-DeMet A, Lonnerdal B. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology 2002; 23: 635-43. [6] Roels H, Lauwerys R, Buchet JP, Genet P,
Sarhan MJ, Hanotiau I, et al. Epidemiological s u r vey a mong wor ke r s ex p o s e d t o
manganese: effects on lung, central nervous system, and some biological indices. Am J Ind Med 1987; 11: 307-27.
[7] Levy BS, Nassetta WJ. Neurologic effects of manganese in humans: a review. Int J Occup Environ Health 2003; 9: 153-63.
[8] Krachler M, Rossipal E, Micetic-Turk D. Trace element transfer from the mother to the newborn--investigations on triplets of colostrum, maternal and umbilical cord sera. Eur J Clin Nutr 1999; 53: 486-94.
[9] Smargiassi A, Takser L, Masse A, Sergerie M, Mergler D, St-Amour G, et al. A comparative study of manganese and lead levels in human umbilical cords and maternal blood from two urban centers exposed to different gasoline additives. Sci Total Environ 2002; 290: 157-64.
[10] Tholin K, Palm R, Hallmans G, Sandstrom B. Manganese status during pregnancy. Ann N Y Acad Sci 1993; 678: 359-60. [11] Dorner K, Dziadzka S, Hohn A, Sievers
E, Oldigs HD, Schulz-Lell G, et al. Longitudinal manganese and copper balances in young infants and preterm infants fed on breast-milk and adapted cow's milk formulas. Br J Nutr 1989; 61: 559-72.
[12] Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. Neurotoxicology 1999; 20: 433-44. [13] Schettler T. Toxic threats to neurologic
development of children. Environ Health Perspect 2001; 109 Suppl 6: 813-6.
215 [14] Hatano S, Nishi Y, Usui T. Erythrocyte
manganese concentration in healthy Japanese children, adults, and the elderly, and in cord blood. Am J Clin Nutr 1983; 37: 457-60.
[15] Miller ST, Cotzias GC, Evert HA. Control of tissue manganese: initial absence and sudden emergence of excretion in the neonatal mouse. Am J Physiol 1975; 229: 1080-4.
[16] He P, Liu DH, Zhang GQ. Effects of high-level-manganese sewage irrigation on children's neurobehavior. Chung-Hua Yu Fang i Hsueh Tsa Chih 1994; 28: 216-8. [17] Woolf A, Wright R, Amarasiriwardena
C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ Health Perspect 2002; 110: 613-6. [18] Wright RO, Amarasiriwardena C, Woolf AD,
Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology 2006; 27: 210-6.
[19] Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology 2003; 24: 667-74.
[20] Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, et al. Water manganese exposure and children's intellect ual f u nction in A raihazar, Bangladesh. Environ Health Perspect 2006;
114: 124-9.
[21] Cadwell BM BR. Home i nventor y administration manual, comprehensive edition: Little Rock, AR: University of Arkansas for Medical Sciences and University of Arkansas at Little Rock; 2003.
[22] Liao HF PY. Test-retest and inter-rater reliability for the Comprehensive Developmental Inventory for Infants and Toddlers diagnostic and screening tests. Early Hum Dev 2005; 81: 927-37.
[23] Bradley RH, Caldwell BM. The relation of infants' home environments to achievement test performance in first grade: a follow-up study. Child Dev 1984; 55: 803-9.
[24] Wang TM SC, Liao HF, Lin LY, Chou KS, Lin SH. The standardization of the Comprehensive Developmental Inventory for Infants and Toddlers. Psychological Testing 1998; 45: 19-46.
[25] Liao HF WT, Yao G, Lee WT. Concurrent validity of the Comprehensive Developmental Inventory for Infant and Toddlers with the Bayley Scales of Infant Development-II in preterm infants. J Formos Med Assoc 2005; 104: 731-7.
[26] Wu HY LH, Yao Ge, Lee WC, Wang TM, Hsieh JY. Diagnostic accuracy of the Motor Subtest of Comprehensive Developmental Inventory for Infants and Toddlers and the Peabody Developmental Motor Scales - Second Edition. Formosan Journal of Medicine 2005; 9: 312-22.
[27] Portney LG WM. Foundations of Clinical Research: Applications to Practice: New Jersey: Prentice-Hall, Inc; 2000.
[28] Barany E, Bergdahl IA, Schütz A, Skerfving S, Oskarsson A. Inductively Coupled Plasma Mass Spectrometry for direct multi-element analysis of diluted human blood and serum. J Anal At Spectrom 1997; 12: 1005-9.
[29] Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology 1999; 20: 327-42.
[30] Liao HF LS, Soong WT, Tseng CC, Su SC. Mental and neuromotor outcome of children with psychomotor retardation. Formos J of Physical Therapy Eng 1996; 22: 121-30. [31] McMillan DE. A brief history of the
neurobehavioral toxicity of manganese: some unanswered questions. Neurotoxicology 1999; 20: 499-507.
[32] Donaldson J, LaBella FS, Gesser D. Enhanced autoxidation of dopamine as a possible basis of manganese neurotoxicity. Neurotoxicology 1981; 2: 53-64.
[33] Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JB. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J Neurochem 1994; 62:
205-16.
[34] Frumkin H, Solomon G. Manganese in the U.S. gasoline supply. Am J Ind Med 1997; 31: 107-15.
[35] Lynam DR, Roos JW, Pfeifer GD, Fort BF, Pullin TG. Environmental effects and exposures to manganese from use of methylcyclopentadienyl manganese t r i c a r b o n y l ( M M T ) i n g a s o l i n e . Neurotoxicology 1999; 20: 145-50.
[36] Rollin H, Mathee A, Levin J, Theodorou P, Wewers F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ Res 2005; 97: 93-9.
[37] Environmental Health Criteria 3: Lead. Geneva: World Health Organization (WHO); 1977.
[38] Hwang Y-H, Ko Y, Chiang C-D, Hsu S-P, Lee Y-H, Yu C-H, et al. Transition of cord blood lead level, 1985-2002, in the Taipei area and its determinants after the cease of leaded gasoline use. Environ Res 2004; 96: 274-82.
[39] Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med 1987; 316: 1037-43.
217
出生前錳暴露與六個月大嬰兒之精神運動發展
蘇楓喬1 廖華芳2 黃耀輝1 謝武勳3 吳惠琤1 鄭素芳2 蘇怡寧4 陳保中1 1台灣大學職業醫學與工業衛生研究所 2台灣大學物理治療學系暨研究所 3台灣大學醫學院附設醫院小兒部 4台灣大學醫學院附設醫院基因醫學部摘
要
目的:評估在一般族群中,胎兒於出生前暴露到來自環境中的錳,會對其日後精神運動發展的 影響。方法:本研究為「Taiwan Birth Panel Study」的一部份,共有132對包含父母及其足月新生兒
的研究對象,並在嬰兒6個月時至家中進行「嬰幼兒綜合發展測驗(Comprehensive Developmental
Inventory for Infants and Toddlers, CDIIT)」的評量。此測驗是用來評估兒童在認知、語言、動作
(含粗大動作及精細動作)、社會,與自助各方面的發展。臍帶血的錳濃度是以Agilent 7500C
ICP-MS來分析。最後,利用複迴歸與校正平均數來探討錳暴露與精神運動發展間的關係。
結果:在CDIIT精細動作的部分,我們發現到錳濃度較高的組別其發展商數(developmental
quotients)會較差(crude β = -7.0, SE = 2.5)。但在校正後,此負相關性會略微下降(adjusted β = -6.0, SE = 2.5)。再者,利用logistic regression分析後,也發現到與linear regression相同的結果(crude OR = 3.20, 95% CI = 1.31-7.85; adjusted OR = 3.02, 95% CI = 1.18-7.76)。此外,我們將錳濃度平均分 為四組,進行校正平均數的分析,並發現到錳濃度與精細動作間呈現倒U字形的相關。 結論:研究中發現,即使是來自環境中的錳暴露,仍可能對胎兒造成精神運動發展上的不良影 響,特別是在動作方面。然而,本研究尚有一些無法排除的干擾因子,為此,仍有待日後更進一步 的研究來證實。不過,為避免胎兒有精神運動發展上的風險,仍建議懷孕婦女盡量避免暴露於過高 的錳濃度之下。 關鍵字:子宮內暴露、錳、精神運動發展、兒童 民國95年5月25日收稿,96年6月21日修稿,96年7月25日接受。 通訊作者:陳保中,國立台灣大學公共衛生學院 職業醫學與工業衛生研究所,10055 台北市中正區徐州路17號733 室,電話:02-3322-8088;傳真:02-2358-2402;電子郵件:pchen@ntu.edu.tw。