農試所特刊第 173 號
Special Publication of TARI No. 173
2013 媒介昆蟲與蟲媒病害
國際研討會專刊
Proceedings of the 2013 International
Symposium on Insect Vectors and
Insect-Borne Diseases
主編 張宗仁 李啟陽 石憲宗
Edited by
Chung-Jan Chang, Chi-Yang Lee, and Hsien-Tzung Shih
行政院農業委員會
Council of Agriculture, Executive Yuan
行政院農業委員會農業試驗所
Taiwan Agricultural Research Institute, COA, Executive Yuan
行政院農業委員會動植物防疫檢疫局
Bureau of Animal and Plant Health Inspection and Quarantine, COA, Executive Yuan
中華民國一○二年八月
The 2013 International Symposium on Insect Vectors and Insect-Borne
Diseases
2013 媒介昆蟲與蟲媒病害國際研討會
Agenda 議程表
Schedule Program/ schedule Speaker
(Institution) Moderator (Institution) August 6th (Tue) 08:30-09:20 Registration 09:20-09:40 Opening Session: Opening Remarks:
Welcome Address (Dr. Junne-Jih Chen, Director-General, TARI, COA)
09:40-10:00 Tea Break and Group Photo
10:00-11:00 Keynote speech: Invasive potential of Xylella fastidiosa
Dr. Alexander H. Purcell (UC Berkeley, USA) 葉 瑩處長 Dr. Ying Yeh, Director General (Department of Science and Technology, Council of Agriculture, Executive Yuan) 11:00-12:00 Keynote speech: Fastidious
prokaryotes and plant health
Dr. Chung-Jan Chang (University of Georgia, USA)
12:00-13:20 Lunch Break
13:20-13:50 Matsumura’s collection of froghoppers and sharpshooters (Hemiptera: Cicadomorpha) of Taiwan in the Hokkaido University Insect Collection
Dr. Kazunori Yoshizawa (University of Hokkaido, Japan) 何琦琛博士 Dr. Chyi-Chen Ho (Applied Zoology Division, TARI: Retired Senior Entomologist) 13:50-14:40 Overview of the phylogeny,
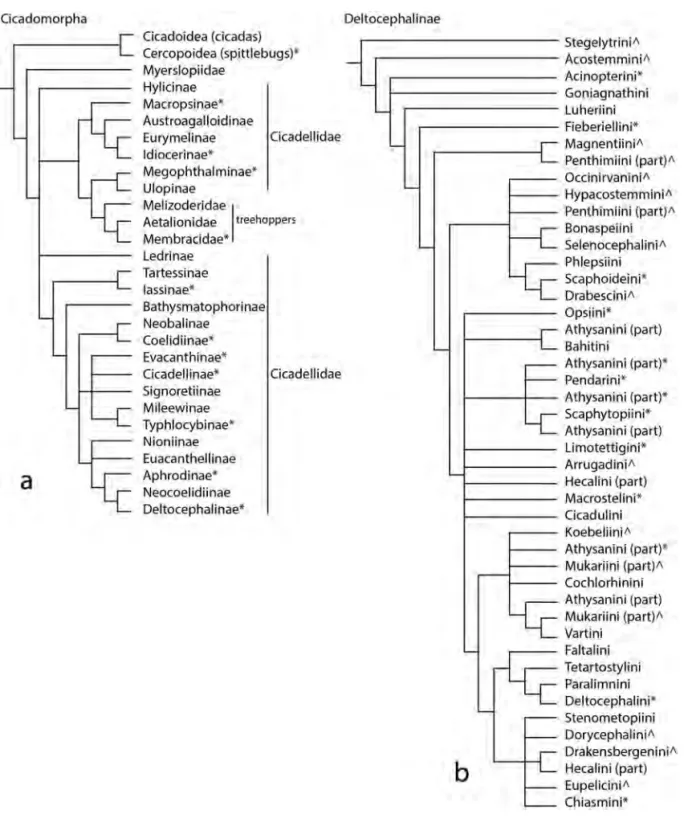
taxonomy and diversity of the leafhopper (Hemiptera: Auchenorrhyncha: Cicadomorpha:
Membracoidea:Cicadellidae) vectors of plant pathogens
Dr. Christopher H. Dietrich (INHS, Prairie Research Institute, USA)
15:00-15:50 How effective is sharpshooter control at limiting Pierce's disease spread in California vineyards? Dr. Matthew P. Daugherty (UC Riverside, USA) 蔡志偉博士 Dr. Chi-Wei Tsai (Department of Entomology, National Taiwan University (NTU)) 15:50-16:40 Habitat effects on population
density and movement of insect vectors of Xylella fastidiosa in California, USA Dr. Rodrigo Krugner (USDA-ARS in Parlier, USA) 16:40-17:30 Panel Discussion Dr. Chung-Jan Chang (University of Georgia, USA) August 7th (Wed) 08:30-09:10 Registration 09:10-10:10 Keynote speech: Xylella fastidiosa
diversity Dr. Rodrigo P. P. Almeida (UC Berkeley, USA) 高靜華組長 Cing-Hua Kao, Director (Applied Zoology Division, TARI) 10:10-10:30 Tea Break
10:30-11:10 Xylella fastidiosa-elicited leaf scorch diseases in Taiwan
Dr. Wen-Ling Deng (NCHU, Taiwan, ROC) 11:10-12:00 Taxonomy and biology of egg
parasitoids of Auchenorryncha of economic importance in Taiwan and adjacent countries, and of Proconiine sharpshooters in the New World
Dr. Serguei V. Triapitsyn (UC Riverside, USA)
12:00-13:00 Lunch Break
13:00-13:50 The occurrence of Pierce’s disease of grapevines and its control strategies in Taiwan
Dr. Chiou-Chu Su (TACTRI, Taiwan, ROC) 詹富智主任 Fuh-Jyh Jan, Director (Department of Plant Pathology, National Chung Hsing University (NCHU))
13:50-14:40 Potential vectors of Pierce’s disease in Taiwan: ecology and integrated management
Dr. Hsien-Tzung Shih (TARI, Taiwan, ROC) 14:40-15:40 Understanding bacterial virulence
genes and mechanisms of host response in insect-mediated citrus Huanglongbing Dr. Hong Lin (USDA-ARS in Parlier, USA) 15:40-16:00 Tea Break 16:00-16:50 An integrated management of citrus Huanglongbing in Taiwan
Dr. Chia-Hsin Tsai (TARI, Taiwan, ROC) 安寶貞組長 Pao-Jen Ann, Director (Plant Pathology Division, TARI) 16:50-17:30 Panel Discussion
August 8th (Thu)
08:20-08:40 Registration 08:40-09:40 Keynote speech: The new
third-generation, AC-DC EPG monitor and its usefulness for IPM research on vectors of plant pathogens Dr. Elaine A. Backus (USDA-ARS in Parlier, USA) 楊恩誠教授 Prof. En-Cheng Yang (Department of Entomology, NTU) 09:40-10:00 Tea Break
10:00-10:50 Tospoviruses and thrips- is there an evolutionary relationship? Dr. Laurence A. Mound (CSIRO Ecosystem Sciences, Australia) 王清玲博士 Dr. Chin-Ling Wang (Applied Zoology Division, TARI: : Retired Senior Entomologist and Director)
10:50-11:30 Tomato leaf curl disease in Taiwan and breeding for resistance against it Dr. Wen-Shi Tsai (AVRDC- The World Vegetable Center) 路光暉主任 Kuang-Hui Lu, Director (Department of Entomology, NCHU) 11:30-12:10 Insect transmission of tomato
yellow leaf curl viruses Dr. Chi-Wei Tsai (NTU, Taiwan, ROC)
12:10-13:10 Lunch Break
13:30-14:10 Visit to the Insect Collection at TARI
李奇峰博士 Dr. Chi-Feng Lee (Applied Zoology Division, TARI) 14:30-16:30 Group Discussion for Invited Guests Only
(1) Group 1: insect vectors and insect-borne diseases [At Seminar Room, Taiwan Soil Museum,
Agricultural Chemistry Division, TARI]
張宗仁博士; 林宏博士 Dr. Chung-Jan Chang & Dr. Hong Lin
(2) Group 2: Taxonomy workshop
[At Room 225, Applied Zoology Division, TARI] 楊正澤博士 Dr. Jeng-Tze Yang (Department of Entomology, NCHU; Department of Plant Medicine, NPUST)
Contents
Preface ... i Invasive Potential of Xylella fastidiosa ... A. Purcell 1 Fastidious Prokaryotes and Plant Health ... C. J. Chang 17 Matsumura’s Collection of Froghoppers and Sharpshooters
(Hemiptera: Cicadomorpha) of Taiwan in the Hokkaido University
Insect Collection ... K. Yoshizawa 35 Overview of the Phylogeny, Taxonomy, and Diversity of the Leafhopper
(Hemiptera: Auchenorrhyncha: Cicadomorpha: Membracoidea: Cicadellidae)
vectors of plant pathogens... C. H. Dietrich 47 How Effective is Sharpshooter Control at Limiting Pierce's Disease Spread
in California Vineyards? ... M. P. Daugherty 71 Habitat Effects on Population Density and Movement of Insect Vectors
of Xylella fastidiosa in California, USA ... R. Krugner 83
Xylella fastidiosa Diversity ... R. P. P. Almeida 107
Xylella fastidiosa-Elicited Leaf Scorch Diseases in Taiwan ... W. L. Deng 117
Taxonomy and Biology of Egg Parasitoids of Auchenorryncha of Economic Importance in Taiwan and Adjacent Countries, and of
Proconiine Sharpshooters in the New World ... S. V. Triapitsyn 123 The Occurrence of Pierce’s Disease of Grapevines and Its Control
Strategies in Taiwan ... C. C. Su 145 Potential Vectors of Pierce’s Disease in Taiwan: Ecology and Integrated
Management ... H. T. Shih 163 Understanding Bacterial Virulence Genes and Mechanisms of Host
Response in Insect-Mediated Citrus Huanglongbing... H. Lin 177 An Integrated Management of Citrus Huanglongbing in Taiwan ... C. H. Tsai 193
Plant Pathogens ... E. A. Backus 211 Tospoviruses and Thrips-is There an Evolutionary Relationship?
... L. A. Mound 231 Tomato Leaf Curl Disease in Taiwan and Breeding for Resistance
Against it ... W. S. Tsai 239 Insect Transmission of Tomato Yellow Leaf Curl Viruses ...C. W. Tsai 255
Preface
Economic losses caused by insect-borne diseases on crops are much higher than those caused solely by insects or diseases. Insect-borne diseases may influence food security (production issues) and food safety (pesticide residues issues). So far, no simple ways to deal with these diseases. The integrated management strategy has been considered a good practice for disease prevention at present, including eradication of infected plants, insect control and breeding of disease-resistant crops. Unfortunately, early symptoms of insect-borne diseases are similar to those of physiological disorders caused by lack of nutrients or water deficiency. Misdiagnosis, therefore, occurs easily. Until the insect-borne diseases are confirmed, in most cases, it is too late to initiate appropriate and timely remedies. That is why the development of the techniques for the integrated management of insect-borne diseases has become an important and urgent issue worldwide.
The effective techniques of the integrated management for insect-borne diseases can benefit both producers and consumers. To maximize the performance of these techniques it requires the consideration of the characteristics of crop growth, manure management, and climatic conditions. Additionally these methods should be appropriated for and recognized by farmers. When applied at right time, the use of suitable control methods would achieve both economic benefits and environmental safety concerns.
For this purpose and under the International Agricultural Cooperation Act of 2013, the Council of Agriculture (COA) is interested in providing funds to support this international symposium. The symposium is co-organized by COA, Taiwan Agricultural Research Institute (TARI) and Bureau of Animal and Plant Health Inspection and Quarantine (BAPHIQ). Topics discussed at the meetings include current state of important vector-borne diseases, innovation of new technologies, techniques of the integrated management and ecology and classification of insect vectors. Eleven experts from the United States, Australia and Japan and six scholars from local institutions have been invited as keynote speakers. The scientific findings and recommendation in the papers contained in this volume are contributions of all participating scientists and published as a special publication of TARI.
as all participants. I look forward to witnessing this symposium becoming a communication platform for domestic and international scholars that undertake the study of insect-borne diseases and insect vectors. Papers published in this proceedings would be a good reference for agricultural authorities to develop a plan for the integrated researches of insect-borne diseases and insect vectors. Foreign scholars are in particular encouraged to keep contact with researchers in Taiwan. Together we can build up the fundamental knowledge of insect-borne diseases that leads to reduce the economic impact of diseases and insect vectors in agricultural industry.
Junne-Jih Chen, Ph.D. Director General Taiwan Agricultural Research Institute Council of Agriculture, Taiwan ROC August, 2013
序
蟲媒病害對農作物所造成的經濟損失,遠高於昆蟲或病害單獨造成的危 害,其直接影響範圍包括糧食安全 (產量問題) 與食品安全 (農藥殘留問題) 兩大議題。由於這類病害皆不易防治,降低此類病害對產業造成經濟衝擊的 最佳策略,在於剷除病株、防治媒介昆蟲與抗病育種等。惟蟲媒病害初期受 害徵狀與植物缺乏養分之生理病徵或缺水極為相似,容易誤判,一旦確認為 蟲媒病害再予以防治,多為時已晚。基此,蟲媒病害的整合防治技術成為全 球重要的課題。 有效的蟲媒病害及其媒介昆蟲之整合管理技術,對產區與社會的經濟效 益皆有所助益,其操作過程必需考量作物生育特性、肥培管理與氣候條件, 於適當時機、運用適用防治方法,使防治過程兼具經濟效益與環境安全,才 能建立適地適用且受農友認同的整合管理技術。 農委會在 2013 年的「國際農業合作」領域,以計畫經費支持辦理本次 國際研討會,由農業試驗所和防檢局共同策劃,針對國內外重要蟲媒病害研 究現況、創新研究技術、整合防治技術與媒介昆蟲之分類與生態研究等主 題,邀請十一位來自美國、澳州與日本之國外專家和六位國內學者進行專題 報告,並將專家之書面論文編印成專刊。 本人謹代表本所同仁,熱烈歡迎參與本次研討會的國內外專家學者。也 期待本次研討會能夠作為從事蟲媒病害與媒介昆蟲國內外學者的交流平 台,建立起蟲媒病害與媒介昆蟲整合研究之知識基礎,期以有助於未來降低 此類病害與媒介昆蟲對產業的經濟衝擊。行政院農業委員會農業試驗所
所長
中華民國一
O 二 年八月
Invasive Potential of Xylella fastidiosa
Alexander Holmes Purcell 1, 21 Department of Environmental Science, Policy and Management, University of California, Berkeley, California 94720-3114 USA
2 Corresponding author, E-mail: ahpurcell@berkeley.edu
ABSTRACT
Evaluating the risks of invasion by the bacterium Xylella fastidiosa to geographic regions where this plant pathogen currently does not occur is an important challenge. Various strains of X. fastidiosa, differentiated by their plant host range, comprise a formidable variety of serious plant diseases throughout the tropical through subtropical Americas. These diseases do not seem present as significant threats outside the Western Hemisphere, except for Taiwan, which has recorded diseases in pear and grape caused by X. fastidiosa. Identifying the factors limiting or even prohibiting the spread of X. fastidiosa and their modes of action would be useful in attempts to estimate risks of new invasions by this bacterium and-more importantly-to identify the most effective phytosanitary strategies to prevent the bacterium from establishing in new regions. Although it is clear that cold severity of sub-freezing winter climates limit the geographic spread of X. fastidiosa, we lack an understanding of the underlying mechanisms of how freezing eliminates it from plants. Other aspects of climatic temperature regimes, such as limiting high temperatures or sustained cool but above freezing temperatures need to be addressed. For some regions, the lack of suitable insect vectors or suitable alternative hosts of X. fastidiosa may prevent introductions of X. fastidiosa in infected plant hosts from establishing a permanent presence. It is likely the permanent establishment of X. fastidiosa requires a suitable combination of vectors’ distribution, abundance, plant preferences, phenology, transmission efficiency, and dispersal behavior in conjunction with the abundance and distribution of plant hosts of X. fastidiosa and the characteristics of the plant communities in which they are embedded. The intriguing possibilities of interactions with other bacteria and viruses have only begun to be explored as limiting factors.
Keywords: Xylella, Pierce’s disease, citrus variegated chlorosis, sharpshooter, quarantine, phytosanitary
INTRODUCTION
Why be concerned about the invasiveness of Xylella fastidiosa?
Our practical concerns with the bacterium Xylella fastidiosa emphasize the prevention and control of the numerous plant diseases that this plant pathogen causes. Countries without these diseases want to prevent their invasion and establishment. The large diversity of serious diseases in numerous crop and forest species that are caused by X. fastidiosa have been reviewed recently (9, 27, 45). These range from diseases affecting grape, almond, oleander and alfalfa in California and other southwestern parts of North America, to diseases of grape, peach, plum, pecan, blueberry and numerous forest tree species in southeastern North America, to diseased orange, coffee and plum crops in South America. Outside of the Americas, the only confirmed occurrences of diseases caused by X. fastidiosa are diseases of pear and grape in Taiwan (9, 30, 52). .X. fastidiosa has also been reported in grape in Serbia (now Bosnia) (4) and a brief report in almond in Turkey (21), but it unclear if the diseases concerned are spreading in Europe.
The theoretically huge list of plant species that support the multiplication of X.
fastidiosa (considerably more than 50% of those species tested so far) (16, 25, 45, 47)
should facilitate the spread of the bacterium through the commercial movements of live plants. Yet there is no convincing evidence that this has contributed to transoceanic of X. fastidiosa. The introduction of grapevines unknowingly infected with X. fastidiosa may have introduced Pierce’s disease (PD) into commercial vineyards in France, where new plantings with PD were removed and insecticides applied to prevent the establishment of PD (7). Without untreated controls, the subsequent disappearance of PD could not prove the effectiveness of removing suspect plants in conjunction with insecticide applications. Classically, most introductions of exotic organisms fail to establish a permanent presence, but eventually many exotic invaders establish permanently (31). Plant quarantines by practical necessity are based on logic instead of scientific proof.
Why hasn’t X. fastidiosa invaded more regions outside of the Americas? Numerous fungal and viral pathogens of grape that are native to North America have
become widespread throughout much of the world, beginning in the late 1700s with of the importation of grape species to European botanical collections. Yet PD, another disease of grape indigenous in southern North America, never became established. This was despite the massive importation from the southern United States to Europe of wild grapevines for use as rootstocks against the grape phylloxera(18). It is inconceivable that X. fastidiosa was not present in some of these vines. Species of wild grape native to the southern United States are tolerant of X. fastidiosa (22). We can only speculate about possible explanations to answer the question of why any invasive organism has not established where it is continually introduced.
For X. fastidiosa, we have evidence for some limiting factors such as winter climate. For regions where freezing temperatures are rare, we must search for explanations, realizing that key limitations may be completely different from one location to another. Finding explanations of factors that limit invasions of X. fastidiosa into new locations may not only provide new ideas or improved quarantine measures or other phytosanitary strategies, but may also provide new ideas for control of Xylella-caused diseases in areas where this pathogen is native.
POSSIBLE LIMITING FACTORS Climate
Winter climate is an important feature of the epidemiology of PD in northern California (27, 40) and probably elsewhere (27). Subfreezing winter temperatures are important for recovery of grapevines with PD (29, 36, 40). Almond leaf scorch disease has similar results in California (8). Vector-borne new infections of X. fastidiosa in grapevines after the first two months of the growing season in northern California had recovered and were free of X. fastidiosa after the next winter (13, 41). Northern California vines that did not recover overwinter had populations of X. fastidiosa that were sufficient for vector acquisition only after June (51). The overwinter recovery phenomenon, in conjunction with the seasonal changes in X. fastidiosa populations, explains why the early growing season is critical for establishing chronic infections (no recovery) of X. fastidiosa (27, 43, 45). They also offer a hypothesis to explain the failures of X. fastidiosa to permanently establish in Europe. Europe lacks known or potential vectors of X. fastidiosa that overwinter as adults, thus avoiding having flying vectors during the critical early growing season that is so important to establishing chronic
infections (42). Chronically infected plants eventually die of disease, age or other factors. For X. fastidiosa to persist in a location indefinitely, the rate of spread of X. fastidiosa to new host plants must exceed the rate at which the bacterium disappears from colonized hosts (either diseased or symptomless).
Overwinter recovery has been modeled with regression-based mathematical models based on experimental data (33). Despite some promising clues (36), an understanding of how freezing temperatures induce recovery is still a mystery. Understanding the mechanism of cold therapy might provide insights in how to develop climate-based models to predict the geographic range of X. fastidiosa or provide new ideas for disease control or therapies.
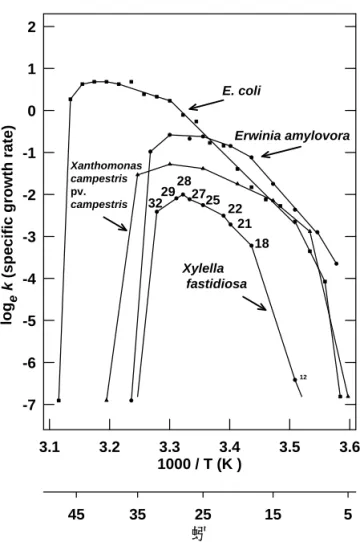
Another way that climatic temperature patterns can influence the potential for the spread of diseases caused by X. fastidiosa is the pattern of growing season temperatures. Some temperate regions may lack the subfreezing temperatures needed for environmental therapy of diseased vines, but not have sufficient degree-days to support population growth of X. fastidiosa to sustain the development of severe symptoms or to promote rapid plant-to-plant spread. Temperature also may affect vector transmission (11). It is surprising that a pathogen that is most virulent in tropical climates has a maximum temperature for sustained growth under 34oC (14) and is susceptible to temperatures below 15oC (Fig. 1). Is it possible some climates are too hot to sustain PD? Both of thee possibilities are unexplored.
Diseased Plant Hosts
Hybrids of grapevines that are tolerant or resistant to X. fastidiosa nonetheless can harbor populations of X. fastidiosa (17) that are adequate for vector acquisition of the bacterium (24). Illegal importations of new PD-resistant grape varieties could thus introduce plants with persistent populations of X. fastidiosa if the winter climate is suitable for survival of the bacterium. This is also true of resistant cultivars of other perennial plants that may harbor populations of X. fastidiosa but with mild or no symptoms. Nursery plants of citrus with CVC represent a proven threat for the movement of CVC to new locations, as occurred in Brazil (19). The use of nursery plants free of X. fastidiosa is a fundamental part of the current control methods for CVC in Brazil (19).
The importance of having diseased crop plants as inoculum is quite clear for CVC in Brazil (19, 20, 29, 49) (reviewed in (43)), despite the CVC strains being able to infect
many weed species (35). This is not so apparent for PD in California or Florida, where it has been most extensively studied. The genetic regulation of growth and movement of X. fastidiosa in European grape (Vitis vinifera) apparently is not adequate to prevent severe symptoms in grape. This may make commercial vineyards with PD especially important as acquisition sources in late summer for adult vectors that inoculate the bacterium into grapevines the following spring.
Fig. 1. The response of growth rates of Xylella fastidiosa and 3 other bacteria to temperature (Arrhenius plot). Note the narrower range of temperatures suitable for X. fastidiosa compared to two other plant pathogenic bacteria (Erwinia and Xanthamonas). Adapted from (14).
Arrhenius plot of growth rates of Xylella fastidiosa compared to three other bacteria
1000 / T (K ) 3.1 3.2 3.3 3.4 3.5 3.6 log e k (s pe ci fi c gr owth r a te ) -7 -6 -5 -4 -3 -2 -1 0 1 2 蚓 45 35 25 15 5 32 18 22 28 12 21 25 29 27 E. coli Erwinia amylovora Xanthomonas campestris pv. campestris Xylella fastidiosa
Symptomless Plant Hosts
Current evidence suggests that the cell signaling system of X. fastidiosa directs gene expression in ways that minimize host injury by slowing its population growth and increasing adhesion (immobility) to retard systemic movements and spread within the host plant (10, 38). In the experiments (46) to identify the host status of plants that were favorable for an important vector for PD, recoveries of X. fastidiosa were attempted only after an incubation period of about 2 to 3 months. In later repetitions of these experiments for plants in which no growth of X. fastidiosa had been detected, populations of inoculated X. fastidiosa increased for several weeks, then rapidly decreased to undetectable levels (46). It appears that xylem elements in which X.
fastidiosa completely fills the vessel, most of the bacterial cells are dead (10). This
emphasizes the importance to X. fastidiosa of systemic movement within a host plant. The bacterium dies out in the plant if it completely packs the host cell and cannot move to new xylem cells (46).
The most common disease syndrome caused by infections of X. fastidiosa begins with the progressive decline and eventual death of foliar tissue, usually beginning at the leaf margins and progressing toward the leaf base before spreading further to woody tissues and fruit. PD of grapevine, which is the first described disease now attributed to X. fastidiosa, exemplifies this “leaf scorch” syndrome (Fig. 2). However, we can describe two other categories of syndromes. A second symptom type includes the dwarfing or stunting diseases such as alfalfa dwarf (54), phony peach (55), and citrus variegated chlorosis (CVC) (32). These three diseases have a slower decline than the leaf scorch diseases and without leaf “burn” symptoms (Figs. 3-4). For example, the distinctive foliar lesions (Fig. 4) on citrus with CVC do not resemble the marginal decline and death of leaf tissues characteristic of PD. Like phony peach disease, the most damaging aspect of CVC is the dwarfing of fruits and new stem growth (Fig. 4). These diseases typically take longer to appear following inoculation via insect vector transmission. A third symptom group includes the “symptomless infections”, where X. fastidiosa multiplies within the host plant and may or not may not move systemically
(16, 25, 46). This third group may differ from the dwarfing syndrome group only in the
rapidity and degree to which stunted growth results from colonization of the plant by X. fastidiosa. Most experimental evaluations of symptoms are made within a year of inoculation or even shorter, so chronic stunting that causes substantial reductions in
growth may not be seen for many months or years. The most difficult potential sources of X. fastidiosa to detect for plant quarantines are plants that have small and scattered populations of the bacterium and have a long incubation period before they develop symptoms, although these may be the least important for introducing X. fastidiosa because the bacterium is most likely to die out in such plants and they are poor sources for vector acquisition. The persistence of X. fastidiosa in and symptomless hosts varies with plant species (3) and probably with winter climate.
Fig. 2. Leaf scorch symptoms of Pierce’s disease (PD) in grape in a Florida experimental vineyard. Note the young and missing vines in the background in this resistance screening planting – the result of high rates of infections with X. fastidiosa and favorable conditions for severe symptom development. Photo by A. H. Purcell.
Fig. 3. Citrus variegated chlorosis disease (CVC) symptoms in orange fruit (dwarfing) and leaves (stunting, chlorosis). Photo by A. H. Purcell.
Fig. 4. Symptoms of alfalfa dwarf (left)-stunting of leaves and stems, darker leaf color-after 9 months in greenhouse conditions. Photo by A. H. Purcell.
Vector abundance and distribution
Vector transmission is an essential part of the disease cycle for X. fastidiosa. It is clear that the types and numbers of vectors are important requirements to sustain the presence of PD. For example, the invasion of a new vector species can cause major changes in the incidence of PD. The successful invasion of southern California in the 1990s by the sharpshooter leafhopper Homalodisca vitripennis (formerly coagulata) caused major outbreaks of PD where PD previously had not been a major problem (6). In California, PD occurs primarily near vector breeding habitats (23). In Napa Valley, California, the sharpshooter Graphocephala atropunctata is the major vector (23, 39). The spatial pattern of populations of G. atropunctata during the early growing season is very similar to spatial gradients of the incidence of PD in vineyards, with the proportion of vines with PD decreasing with distance from the sharpshooter’s overwintering habitat.
Other vector characteristics are also influential. The following comments address characteristics of vectors that are known to be important for the spread of Xylella diseases.
Transmission efficiency
Not every individual vector that has acquired X. fastidiosa transmits it to all plants on which the insect feeds. Vector transmission efficiency is the proportion of plants infected per insect access period. Vector species vary greatly in transmission
efficiency, depending on the combination of vector species and plant species (reviewed
in (48)). For example, the grass-feeding sharpshooter Draeculacephala minerva is more
efficient in transmitting X. fastidiosa to alfalfa than G. atropunctata, which prefers woody plants, whereas the reverse is true for transmission to grape (50). The differences in distribution and anatomy between grasses and dicotyledonous plants may require differences in feeding behavior to respond to the different cues found among various plant species.
Although it has been long hypothesized that all xylem sap-feeding, sucking insects are potentially vectors of X. fastidiosa (15), there are strains of this bacterium that differ in transmission efficiency depending on the vector and plant species (34). This makes it difficult to predict how a certain strain of X. fastidiosa will be transmitted in a new location with different vectors and plant communities.
Plant and habitat preferences
Xylem feeders often have wide plant host ranges (reviewed in (48)), but all species demonstrate strong preferences when they have a choice of hosts. The condition of the plant can also be as important as the species in feeding preferences. Xylem feeders generally prefer more succulent, fast-growing plant tissues (37). The most important native vectors (Draeculacephala minerva and Xyphon fulgida) in central California are two grass-feeding sharpshooters (23, 44). Because they prefer succulent plants, they reach their highest populations during California’s dry summers where grasses are irrigated
(23, 44) Both of these two species are rarely found feeding on grape; their importance as
vectors is deduced from their consistent association with PD outbreaks in vineyards next to habitats harboring these sharpshooters (23, 44).
In contrast, grape is a highly preferred host of the major vector (G. atropunctata) in coastal California vineyards (23, 44). Surprisingly the incidence of PD in central California vineyards compared to Napa Valley vineyards can be very similar, despite only rarely observing vectors on grape in central California vineyards (45). In addition, the central California vectors are much less efficient transmitters of X. fastidiosa to grape. The feeding of D. minerva and X. fulgida on grape is hypothesized to occur in the evenings, as these two species fly mostly in early evening, when they may drift or wander into nearby vineyards from their normal habitats (44).
Vector dispersal
Vector mobility can compensate for inefficient transmission, as exemplified by the sharpshooter H. vitripennis. The transmission efficiency of X. fastidiosa to grape by H. vitripennis is relatively low, about 5% to 15% per day per insect, (1), but the longer range dispersal and frequent daily movements or over longer time periods by H.
vitripennis compared to more traditional vectors created epidemic spread of PD (5, 6).
It is the combination of vector traits that determines its overall effectiveness and importance as a vector, not just a single trait. This can be illustrated with a simple mathematical model. The probability of transmission by n vectors per plant per time interval (Pnt) depends on how many vectors (n) and the number of time intervals (t)
they are present on a plant, as well as the fraction of vectors that are infective with the pathogen (i) and that transmit per time interval (transmission efficiency E). The relationship is
Pnt = 1-P-niEt (41)
In this equation, all four determinants (n, i, E, t) are mathematically equivalent. Thus an abundant (high n) but inefficient (low E) vector can be very damaging as a vector if it has frequent plant to plant movements (high t) or a high rate of infectivity (high i). Host preferences can affect the frequency of vector movements. As already discussed, transmission efficiency (E) by a single vector species can vary with host plant. Thus the mix of plant host species can affect how important any vector species will be. Phenology
Recall that in temperate climates with almost all grapevines inoculated with X. fastidiosa after the early growing season recover completely. Thus overwintering adult vectors that are infective with X. fastidiosa establish most of the infections destined to be chronically diseased. This is one possible explanation as to why PD never established in Europe, where most xylem sap-feeders found in vineyards overwinter in the egg stage(42). Another explanation for the increase in PD accompanying the establishment of H. vitripennis in southern California is that this sharpshooter is able to transmit X. fastidiosa to dormant vines during winter (2). H. vitripennis overwinters in California as an adult, so this characteristic could be important.
Antagonists of Xylella fastidiosa?
There is not yet much to be said about microbial antagonists of X. fastidiosa because of a lack of research in this area. Bacteriophages that attack X. fastidiosa have been identified (53), as well as a number of prophages in the genome of X. fastidiosa identified from genome sequencing (12) (reviewed in (43)).
Strains of X. fastidiosa that do not cause disease in grape but protect against PD strains of X. fastidiosa have been reported in Florida (26). Other xylem inhabiting bacteria have shown some antagonism but are not yet been proven to be effective as protective agents against X. fastidiosa (28).
It would be unexpected to find that antagonistic interactions with other microbes would completely inhibit the invasion of X. fastidiosa into a new geographic region, but relatively few studies of microbial antagonism to X. fastidiosa have been made. This is an area that may have potential for control.
CONCLUSION
The persistence of X. fastidiosa in a given environment depends on a combination of suitable conditions at the proper times, not just the introduction of the bacterium in a plant or insect. Climate, host plants, suitable vectors and the vegetation and habitats needed to support them are all essential requirements to sustain the spread X. fastidiosa from plant to plant.
Xylella fastidiosa may be present in some geographic regions but unrecognized because it is causes no or very subtle symptoms or if any severely affected plants are not common. PD was not recognized in the southeastern USA until the 1950s, yet is one of the limiting factors for growing grapes there (22).
Taiwan is unique in being the only location outside the western hemisphere with documented established diseases caused by X. fastidiosa. If we can discover the explanation for the success of X. fastidiosa in Taiwan, it might provide new ideas on how to prevent invasions by X. fastidiosa from occurring in other countries.
LITERATURE CITED
1. Almeida, R. P., P and Purcell, A. H. 2003. Transmission of Xylella fastidiosa to grapevines by Homalodisca coagulata (Hemiptera: Cicadellidae). J. Econ. Entomol. 96:264-271.
2. Almeida, R. P. P., Wistrom, C., Hill, B. L., Hashim, J., and Purcell, A. H. 2005. Vector transmission of Xylella fastidiosa to dormant grape. Plant Dis. 89:419-424. 3. Baumgartner, K., and Warren, J. G. 2005. Persistence of Xylella fastidiosa in
riparian hosts near northern California vineyards. Plant Dis. 89:1097-1102.
4. Berisha, B., Chen, Y. D., Zhang, G. Y., Xu, B. Y., and Chen, T. A. 1998. Isolation of Pierce’s disease bacteria from grapevines in Europe. Euro. J. Plant Pathol. 104:427–433.
5. Blua, M. J., and Morgan J. W. 2003. Dispersion of Homalodisca coagulata (Hemiptera: Cicadellidae), a vector of Xylella fastidiosa, into vineyards in southern California. J. Econ. Entomol. 96(5):1369-1374.
6. Blua M. J., Phillips P. A., and Redak, R. A. 1999. A new sharpshooter threatens both crops and ornamentals. Calif. Agr. 53(2):22-25.
7. Boubals, D. 1989. Pierce's disease reaches the European vineyards (La maladie de Pierce arrive dans les vignobles d'Europe). Bull. de l'OIV 62 (699-700):309-314. 8. Cao, T., Connell J. H., Wilhem, N., and Kirkpatrick, B. C. 2011. Influence of
inoculation date on the colonization of Xylella fastidiosa and the persistence of almond leaf scorch disease among almond cultivars. Plant Dis. 95:158–65.
9. Chang, C. J., Shih, H. T., Su, C. C., and Jan, F. J. 2012. Diseases of important crops, a review of the causal fastidious prokaryotes and their insect vectors. Plant Pathol. Bull. 21: 1-10.
10. Chatterjee, S., Almeida, R. P. P., and Lindow, S. E. 2008. Living in two worlds: The plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 46:243-271.
11. Daugherty, M. P., Bosco, D., and Almeida, R. P. P. 2009. Temperature mediates vector transmission efficiency: inoculum supply and plant infection dynamics. Ann. Appl. Biol. 155:361-369.
12. de Mello Varani, A., Souza, R. C., Nakaya, H. I., de Lima, W. C., Paula de Almeida, L. G., Kitajima, E. W., Chen, J., Civerolo, Vasconcelos, A. T. R., and Van Sluys, M. A. 2008. Origins of the Xylella fastidiosa prophage-like regions and their impact in genome differentiation. PLoS ONE 3(12): e4059.
13. Feil, H., Feil W. S., and Purcell AH. 2003. Effects of date of inoculation on the within-plant movement of Xylella fastidiosa and persistence of Pierce's disease within field grapevines. Phytopathology 93:244-251.
14. Feil, H., and Purcell, A. H. 2001. Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Dis. 85:1230-1234. 15. Frazier, N. W. 1944. Phylogenetic relationship of the nine known leafhopper
vectors of Pierce’s disease of grape. Phytopathology 34:1000-1.
16. Freitag, J. H. 1951. Host range of Pierce's disease virus of grapes as determined by insect transmission. Phytopathology 41 920-934.
17. Fritschi, F. B., Lin, H., and Walker, M. A. 2007. Xylella fastidiosa population dynamics in grapevine genotypes differing in susceptibility to Pierce’s disease. Am. J. Enol. Vitic., 58(3):326-332.
18. Galet, P. 1982. "Les maladies et le parasites de la vigne, Tome 2" Paris, Lavoisier, pp. 1059-1313.
19. Gonçalves, F. P., Stuchib, E., da Silva, S. R., Reiff, E.T., and Amorima, L. 2011. Role of healthy nursery plants in orange yield during eight years of citrus variegated chlorosis epidemics. Scientia Hortic. 129:343-345.
20. Gottwald, T. R., Gidtti, F. B., Santos, J. M., and Carvalho, AC. 1993. Preliminary spatial and temporal Analysis of citrus variegated chlorosis (CVC) in São Paulo, Brazil. Proc. Twelth. Int. Org. Citrus Virologists Conf., 12th. Riverside, CA, p. 323-335. Gainsville, FL: Univ. Fla. Press.
21. Güldr, M. E., Çaglar, B. K., Castellano, M. A., Ünlü, L., Güran, S., Yılmaz, M. A., and Martelli, G. P. 2005. First report of almond leaf scorch in Turkey. J. Plant Pathol. 87(3).
22. Hewitt, W. B. 1958. The probable home of Pierce's disease virus. Plant Dis. Rep. 42: 211-215.
23. Hewitt W. B., Frazier, N.W., and Freitag, J. H. 1949. Pierce's disease investigations. Hilgardia 19: 207-64.
24. Hill, B. L., and Purcell, A. H. 1997. Populations of Xylella fastidiosa in plants required for transmission by an efficient vector. Phytopathology 87: 1197-201. 25. Hill, B. L., and Purcell, A. H. 1997. Multiplication and movement of Xylella
fastidiosa within grape and four other plants. Phytopathology 87:1376-1382. 26. Hopkins, D. L., 2005. Biological control of Pierce’s disease in the vineyard with
strains of Xylella fastidiosa benign to grapevine. Plant Dis. 89:1348-1352.
27. Hopkins, D. L., and Purcell, A. H. 2002. Xylella fastidiosa: cause of Pierce's disease of grapevine and other emergent diseases. Plant Dis. 86: 1056-1066.
28. Lacava, P. T., Araujo, W. L., Marcon, J., Maccheroni, W., and Azevedo, J. L. 2004. Interaction between endophytic bacteria from citrus plants and the phytopathogenic bacteria Xylella fastidiosa, causal agent of citrus variegated chlorosis. Ltrs Appl. Microbiol. 39(1): 55-59.
29. Laranjeira F. F., Bergamin F. A., and Amorim L. 1998. Dynamics and structure of citrus variegated chlorosis (CVC) foci. Fitopatol. Bras. 23:36-41 (In Portuguese). 30. Leu, L. S., and Su, C. C. 1993. Isolation, cultivation, and pathogenicity of Xylella
fastidiosa , the causal bacterium of pear leaf scorch disease in Taiwan. Plant Dis. 77:642-646.
31. Leung, B., Lodge, D. M., Finnoff, D., Shogren, J. F., Lewis, M. A., and Lamberti, G. 2002. An ounce of prevention or a pound of cure: Bioeconomic risk analysis of invasive species. P roc. R. Soc. Lond. B 269:2407-2413.
32. Li, W. B., Zreik, L., Fernandes, N. G., Miranda, V. S., Teixeira, D. C., Ayres, A. J., and Bové, J. M. 1999. A triply cloned strain of Xylella fastidiosa multiplies and induces symptoms of citrus variegated chlorosis in sweet orange. Curr. Microbiol. 39(2):106-108.
33. Lieth, J. H., Meyer, M. M., Yeo, K. H., and Kirkpatrick, B. C. 2011. Modeling cold curing of Pierce’s disease in Vitis vinifera ‘Pinot Noir’ and ‘Cabernet Sauvignon’ grapevines in California. Phytopathology 101:1492-1500.
34. Lopes, J. R. S., Daugherty, M. P., and Almeida, R. P. P. 2009. Context-dependent transmission of a generalist plant pathogen: host species and pathogen strain mediate insect vector competence Entomo. Exp. et Appl. 131:216-224.
35. Lopes, S A., Marcussi, S, Torres, S. C. Z, Souza, V., Fagan, C., França S. C., Fernandes, N. G., and Lopes, J. R. S. 2003. Weeds as alternative hosts of the citrus, coffee, and plum strains of Xylella fastidiosa in Brazil. Plant Dis. 87:544-549. 36. Meyer, M. M., and Kirkpatrick, B. C. 2011. Exogenous applications of abscisic
acid increase curing of Pierce’s disease-affected grapevines growing in pots. Plant Dis. 95:173-177.
37. Mizell, R. F. and French, W. J.. 1987. Leafhopper vectors of phony peach disease: feeding site preference and survival on infected and uninfected peach, and seasonal response to selected host plants. J. Entomol. Sci. 22:11-22.
38. Newman,K. L., Almeida, R. P. P., Purcell, A. H., and Lindow, S. E. 2004. Cell–cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. 101:1737-1742.
39. Purcell, A. H. 1975. Role of the blue-green sharpshooter, Hordnia circellata, in the epidemiology of Pierce’s disease of grapevines. Environ. Entomol. 4:745-752. 40. Purcell, A. H. 1980. Environmental therapy for Pierce's disease of grapevines. Plant
Dis. 64(4): 388-390.
41. Purcell, A. H. 1981. Vector preference and inoculation efficiency as components of varietal resistance to Pierce’s disease in European grapes. Phytopathology 71: 429-435.
42. Purcell, A. H. 1997. Xylella fastidiosa, a regional problem or global threat? J Plant Pathol. 79:99-105.
43. Purcell, A. H. 2013. Paradigms: Examples from the bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. in press.
44. Purcell, A. H. and Frazier, N. W. 1985. Habitats and dispersal of the leafhopper vectors of Pierce’s disease in the San Joaquin Valley USA. Hilgardia 53:1-32. 45. Purcell, A. H. and Hopkins, D.L. 1996. Fastidious xylem-limited bacterial plant
pathogens. Annual Review of Phytopathology 34: 131-151.
46. Purcell, A. H., and Saunders, S.R. 1999. Fate of Pierce's disease strains of Xylella fastidiosa in common riparian plants in California. Plant Dis. 83:825-830.
47. Rathé, A. A., Pilkington, L. J., Gurr, G. M., and Daugherty, M. P. 2012. Potential for persistence and within-plant movement of Xylella fastidiosa in Australian native plants. M. P. Australasian Plant Pathol. 41(4):405-412.
48. Redak, R. A., Purcell, A. H., Lopes, J. R. S., Blua, M. J., Mizell, R. F., and Andersen, P. C. 2004. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 49:243-70.
49. Rodas V. 1994. Convivencia com a clorose variegada dos citros (living with citrus variegated chlorosis). Laranja 15:129-34.
50. Severin, H. H. P. 1949. Transmission of the virus of Pierce’s disease of grapevines by leafhoppers. Hilgardia 19:190-206.
51. Smart, C. D., Hendson, M., Guilhabert, M. R., Saunders, S., Friebertshauser, G., Purcell, A. H., and Kirkpatrick B. C. 1998. Seasonal detection of Xylella fastidiosa in grapevines with culture, ELISA and PCR. Phytopathology 88 Suppl.: S83 (abstract).
52. Su, C. C., Chang, C. J., Chang, C. M., Shih, H. T., Tzeng, K. C., Jan, F. J., Kao, C. W., Deng, W. L. 2013. Pierce’s disease of grapevines in Taiwan: Isolation,
cultivation and pathogenicity of Xylella fastidiosa. J. Phytopathol. 161:389-396. 53. Summer, E. J., Enderle, C. J., Ahern, S. J., Gill, J. J., Torres, C. P., Appel, D. N.,
and Gonzalez, C. F. 2010. Genomic and biological analysis of phage Xfas53 and related prophages of Xylella fastidiosa. J. Bacteriol. 192(1):179-190.
54. Weimar, J. R. 1931. Alfalfa dwarf, a hitherto unreported disease. Phytopathology 21:71–75.
55. Wells, J. M., Raju, B. C., Thompson, J. M., and Lowe, S. K. 1981. Etiology of phony peach and plum leaf scald diseases. Phytopathology 71(11):1156-1161.
Fastidious Prokaryotes and Plant Health
Chung-Jan Chang 1, 2, Hsien-Tzung Shih 3, Chiou-Chu Su 4, and Fuh-Jyh Jan 2, 5
1 Department of Plant Pathology, University of Georgia, Griffin, GA, USA
2 Department of Plant Pathology, National Chung Hsing University, Taichung 402, Taiwan
3 Applied Zoology Division, Taiwan Agricultural Research Institute, Council of Agriculture, Taichung 413, Taiwan
4 Pesticide Application Division, Taiwan Agricultural Chemicals and Toxic Substances Research Institute, Council of Agriculture, Taichung 413, Taiwan
* To be published in Plant Pathology Bulletin 22: xxx-xxx (2013)
5 Corresponding author, E-mail: fjjan@nchu.edu.tw; Fax: +886-4-22854145
ABSTRACT
The prokaryotes are almost everywhere or we can phrase like this “prokaryotes are wherever there is life”. They were the earliest organisms on earth. Today, they still dominant the biosphere for the following two facts: 1) their collective biomass outweighs all eukaryotes combined at least tenfold, and 2) more prokaryotes inhabit a handful of fertile soil or the mouth or skin of a human than the total number of people who have ever lived. They thrive in habitats that are too cold, too hot, too salty, too acidic, or too alkaline for any eukaryote because they display diverse adaptations that allow them to inhabit many environments and they have great genetic diversity. Phytopathogenic fastidious prokaryotes are plant pathogens that either resist to grow in any available bacterial culture media or require specific or enriched media to grow. They include Xylella fastidiosa, Leifsonia xyli subsp. xyli, L. xyli subsp. cynodontis and Clavibacter michiganensis subsp. sepedonicus and C. michiganensis subsp. michiganensis that reside in xylem and spiroplasmas, phytoplasmas and Candidatus Liberibacter spp. that reside in phloem. X. fastidiosa is the causal agent of more than 19 diseases; among them Pierce’s disease of grape and citrus variegated chlorosis are two major maladies that cause serious economic loss on wine and citrus juice industry. L. xyli subsp. xyli, and L. xyli subsp. cynodontis are associated with ratoon stunting disease of sugarcane and Bermuda grass stunting respectively and C. michiganensis subsp. sepedonicus with bacterial ring rot in potato and C. michiganensis subsp.
michiganensis with bacterial tomato canker. Spiroplasmas are the causal agents of citrus stubborn, corn stunt and periwinkle diseases. Phytoplasmas are associated with more than 500 diseases worldwide. Ca. Liberibacter spp., are the causal agents of citrus Huanglongbing or citrus greening, zebra chip disease of potato and others. Pierce’s disease is the limiting factor for the establishment of wine industry for the entire southeastern United States from Texas to the Carolinas along the gulf coast of Mexico. Recent introduction of the glassy-winged sharpshooter leafhoppers in California has threatened the winery industry of California. The successful isolation of X. fastidiosa from the tissues with citrus variegated chlorosis (CVC) symptoms followed by the identification of the major insect vectors provided crucial information for citrus growers and citrus juice industry to deal with the CVC crisis in Brazil. The successful isolation of X. fastidiosa from blueberry tissues with leaf scorch symptoms followed by the identification of the susceptibility/resistance of various blueberry cultivars provided significant information for the blueberry industry which has recently become the number one fruit commodity in Georgia. The biological characteristics of the three phloem-limited prokaryotes, namely spiroplasmas, phytoplasmas and Ca. Liberibacter spp., and the diseases they induce and their vectors will be discussed. Most plant pathogenic prokaryotes do not require an active insect vector to spread them from plants to plants, while X. fastidiosa, Ralstonia syzygii, Ca. Liberibacter spp., phytoplasmas, and spiroplasmas do. To date among all known vectors, the single most successful insects vectoring the diseases belong to the Order of Hemiptera.
Keywords: fastidious prokaryotes, Xylella fastidiosa, Ca. Liberibacter spp., spiroplasmas, phytoplasmas, Huanglongbing, Hemiptera, glassy-winged sharpshooter, Pierce’s disease of grape, citrus variegated chlorosis, bacterial leaf scorch of blueberry
INTRODUCTION
In the Kingdom Prokaryotae, there are two domains, Archaea and Bacteria which differ in structure, physiology, and biochemistry(33). Archaea like bacteria but are thought to be more closely related to eukaryotes than to bacteria. The prokaryotes are almost everywhere or we can phrase like this “prokaryotes are wherever there is life”. They were the earliest organisms on earth. Today, they still dominant the biosphere for the following two facts: 1) their collective biomass outweighs all eukaryotes combined at least tenfold, and 2) more prokaryotes inhabit a handful of fertile soil or the mouth
or skin of a human than the total number of people who have ever lived. They thrive in habitats that are too cold, too hot, too salty, too acidic, or too alkaline for any eukaryote because they display diverse adaptations that allow them to inhabit many environments and they have great genetic diversity. [http://www.course-notes.org/Biology/Outlines/Chapter_27_Prokaryotes].
The prokaryotes are small and most are unicellular with the cell sizes ranging from 1 µm to 10 µm, but they can vary in size from 0.2µm to 750µm. Being so small, they have both harmful and beneficial impacts on humans and plants. Human life is only possible due to the action of prokaryotic microbes, both those in the environment and those species that call us home. Internally, they help us digest our food, produce crucial nutrients for us, protect us from pathogenic microbes, and help train our immune systems to function correctly. However on the harmful side, though pathogenic prokaryotes represent only a small fraction of prokaryotes species, yet they cause about half of human diseases. For example, there are between 2 and 3 million people a year die of the lung disease tuberculosis, caused by the bacillus Mycobacterium tuberculosis.
The prokaryotes that cause plant diseases belong in the Bacteria Domain. In the Division Gracilicutes, the Gram-negative bacteria, under the Class Proteobacteria, prokaryotes that cause plant diseases belong in three known Families and one unnamed Family. In Family Enterobacteriaceae, there are four Genera: Erwinia, Pantoea, Serratia, and Sphingomonas. In Family Pseudomonadaceae, there are seven Genera: Acidovorax, Pseudomonas, Ralstonia, Rhizobacter, Rhizomons, Xanthomonas, and Xylophilus. In Family Rhizobiaceae, there are two Genera: Agrobacterium and Rhizobium. In a still unnamed Family, there are two Genera: Xylella and Candidatus Liberibacter. In the Division Firmicutes, the Gram-positive bacteria, under the Class Firmibacteria, there are two Genera: Bacillus and Clostridium whereas under the Class Thallobacteria, there are six Genera: Arthrobacter, Clavibacter, Curtobacterium, Leifsonia, Rhodococcus, and Streptomyces. In the Division Tenericutes, under the Class Mollicutes, prokaryotes that cause plant diseases belong in two Families. In the Family Spiroplasmataceae, there is one Genus, Spiroplasma and in the Family Acholeplasmataceae, there is one Genus, Candidatus Phytoplasma (1).
Fastidious prokaryotes are those that either resist to grow in any available media, such as phytoplasmas, Ca. Liberibacter spp., and Ca. Phlomobacter fragariae or those that require specific and enriched media, such as spiroplasmas, X. fastidiosa, Leifsonia xyli subsp. xyli, L. xyli subsp. cynodontis and Clavibacter michiganensis subsp.
sepedonicus. Based on the inhabitant, X. fastidiosa, Leifsonia spp., and C. michiganensis subsp. sepedonicus are xylem-inhabiting while spiroplasmas, phytoplasmas, Ca. Liberibacter spp., and Ca. Phlomobacter fragariae are phloem- inhabiting prokaryotes. Xylem-limited bacterial plant pathogens
According to Wells et al. (32), X. fastidiosa possesses the following characteristics: predominately single, straight rods with a cell size ranges from 0.25-0.35 μm in width and 0.9-3.5 μm in length; two types of colonies: convex to pulvinate smooth opalescent with entire margins or umbonate rough with finely undulated margins; Gram-negative, nonmotile, aflagellate, oxidase negative, catalase positive, and strict aerobic; nonfermentative, nonhalophilic, nonpigmented; and require a specific and enriched medium such as CS20, PD2, PW, or BCYE for growth. The optimal temperature for growth is 26-28 ℃, whereas the optimal pH is 6.5-6.9. The habitat is the xylem of plant tissue. The G+C content of the DNA is 51.0 to 52.5 mol% determined by thermal denaturation or 52.0 to 53.1 mol% determined by bouyant density.
Ever since Wells et al. (32) named then xylem-limited bacterium as X. fastidiosa in 1987, X. fastidiosa has been reclassified into five subspecies according to their differences in genetic makeup, host range, physiology, and biochemistry. They are X. fastidiosa subsp. fastidiosa for strains of grape, almond, alfalfa, and maple, X. fastidiosa subsp. multiplex for strains of peach, plum, almond, elm, sycamore, and pigeon grape, X. fastidiosa subsp. pauca for strains of citrus (25), X. fastidiosa subsp.
sandyi for strains of oleander, daylily, jacaranda, and magnolia (26), and X. fastidiosa
subsp. tashke for strains of Chitalpa tashkentensis, a common ornamental landscape plant (23). However, the last two subspecies have not been officially recognized by the researchers in the community of systematic bacteriology.
X. fastidiosa requires specific and enriched media to grow as compared to other bacteria (8). There are seven complex components that are used in the listed four media: soy peptone (Scott Laboratories), Bacto tryptone (Difco), phytone peptone (BBL), trypticase peptone (BBL), soytone (Difco) or phytone (BBL), and yeast extract; either one or two complex components for each medium; two iron sources for the medium either hemin chloride (Sigma) or soluble ferric pyrophosphate; four inorganic salts: ammonium phosphate, potassium phosphate (monobasic or dibasic) or magnesium sulfate; three amino acids and two Krebs cycle intermediates: citrate or succinate; and three detoxifying components: potato starch (J. T. Baker), activated charcoal (Norit
SG), or bovine serum albumin (Sigma). Rippled cell walls seemed to be unique for all X. fastidiosa cells regardless of the origin of its host plants. That was one of the reasons why they were first described as “rickettsia-like bacteria”. However, a thorough study of Pierce’s disease (PD) strain by Huang et al. (19) disclosed that in addition to the predominated rippled cell walls there are intermediate cell walls and smooth cell walls.
Based on the diseases reported around the world, X. fastidiosa causes diseases in the America Continent including North and South America. In the US, they occur in the whole southeastern States along the Gulf coast of Mexico, and California. In the southern hemisphere, the diseases occur in Brazil, Argentina, and Paraguay. In Asia, the pear leaf scorch (21) and PD of grapes (29) were reported in Taiwan. In Europe there was a report describing PD of grapes in Kosovo (3), former Yugoslavia which sits in southern Europe. The X. fastidiosa-induced diseases seemed to occur in the region between 15-45 degrees latitude of both north and south of Equator. It is interesting to note that Taiwan sits at the Tropic of Cancer where the pear leaf scorch disease and PD occur and that Sao Paulo in Brazil sits at the Tropic of Capricorn where the severe citrus variegated chlorosis (CVC) (10,16) and coffee leaf scorch occur. Kosovo sits at about 45 degree North of Equator.
There are 19 diseases that were confirmed to be caused by X. fastidiosa. They are Pierce’s disease of grape, alfalfa dwarf, phony peach (PP), plum leaf scald, CVC, periwinkle wilt, ragweed stunt, and leaf scorch of almond, elm, mulberry, oak, sycamore, pecan, maple, oleander, blueberry, coffee, pear, and Chitalpa (8,10,16,17,21,
23,25,26,27). The common symptoms induced by X. fastidiosa include marginal leaf
necrosis, scorching or scalding of leaves, early leaf fall, dieback of branches, and wilting to death. The specific symptoms vary among different hosts. Symptoms of Pierce’s disease of grapes usually start with marginal leaf necrosis to chlorosis; normally a yellow band would form between the green and necrotic tissues for white wine grapes and a purple band for red wine grapes. The following unique symptoms will follow: petioles remain attached to the canes, green island formation due to irregular maturing process of barks, dried up raisins, and eventual dying and dead vines occurs in 2-4 years after initial infection in GA (Fig. 1). The specific symptoms on peach of phony disease include darker green leaves and extremely shortened terminal growth which resulted in a shape of an umbrella canopy. In the Order Hemiptera, four main sharpshooters in the Family Cicadellidae, e. g., glassy-winged
sharpshooter (GWSS), blue-green sharpshooter, red-headed sharpshooter, and green sharpshooter were the important vectors for PD X. fastidiosa and GWSS vectoring phony peach and plum leaf scald diseases as well.
CVC was first observed in 1987 on sweet orange trees in the southwestern part of Minas Gerais, Brazil. Since then, the disease has been observed in the neighboring State of San Paulo and other citrus producing states (10). Rossetti et al. (24) were the first to show by electron microscopy that a xylem-limited bacterium, probably a strain of X. fastidiosa, was present in all symptomatic leaves and fruits tested but not in similar tissues from symptomless trees.
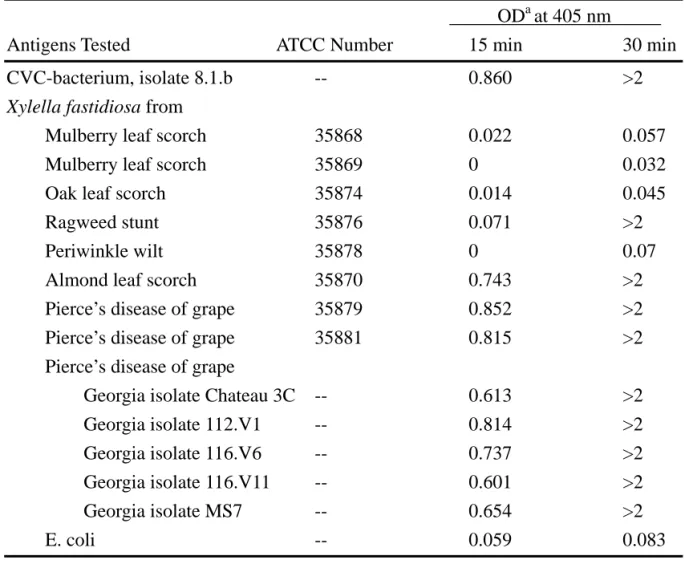
CVC causes severe leaf chlorosis between veins when young trees are infected. Symptomatic leaves exhibit brown gummy lesions on the lower side in corresponding to the chlorotic yellow areas on the upper leaf surface. Reduced growth vigor and abnormal flowering and fruit set occur in infected trees. Fruits from affected trees are often small and hard with high acidity which is not fitting for juice making and no fresh market value (10,16). A bacterium was consistently cultured from plant tissues from CVC twigs of sweet orange trees but not from tissues of healthy trees on several cell-free media known to support the growth of X. fastidiosa. Bacterial colonies typical of X. fastidiosa became visible on PW (Fig. 2), CS20 and PD2 agar media after 5 and 7-10 days of incubation, respectively. The cells of the CVC bacterium were rod-shaped, 1.4-3 µm in length, and 0.2-0.4 µm in diameter, with rippled walls. An antiserum against an isolate (8.1.b) of the bacterium gave strong positive reactions to double-antibody-sandwich (DAS), enzyme-linked immunosorbent assay (ELISA) with other cultured isolates from CVC citrus, as well as with several type strains of X.
fastidiosa (Table 1) (15). Sweet orange seedlings inoculated with a pure culture of the
CVC bacterium supported multiplication of the bacterium, which became systemic within 6 months after inoculation and could be re-isolated from the inoculated seedlings. Symptoms characteristic of CVC developed 9 months post inoculation. X. fastidiosa can infect most of the citrus cultivars, species and hybrids, yet the severity of symptoms varies. Sweet oranges are the most susceptible. Grapefruit, mandarins, mandarin hybrids, lemons, limes, kumquat and trifoliate orange are moderately susceptible, showing less severe symptoms. Rangpur lime, citron, and pummelo are less susceptible. The major vectors for citrus variegated chlorosis in Brazil are Acrogonia terminalis, Dilobopterus costalimai, Oncometopia fascialis, and Oncometopia nigricans.
Relative to total sales, blueberries are the number one fruit commodity in the state of Georgia, surpassing even peaches. Production is concentrated in the southern coastal flatwoods. Rabbiteye blueberry (Vaccinium virgatum Aiton), a native species, has long been the predominant blueberry species cultivated in Georgia. More recently, however, growers have increased the production of the southern highbush cultivars (V. corymbosum interspecific hybrids) as a result of a very favorable market window. Growers and scientists started to observe a new disorder affecting the southern highbush selection FL 86-19 in the Georgia blueberry production region. An initial symptom was marginal leaf scorch (burn) of the older leaves which is very distinct and is surrounded by a dark line of demarcation between green and dead tissue (Fig. 3A), similar to that observed with extreme drought or fertilizer salt burn. New developing shoots were usually abnormally thin with a reduced number of flower buds. Leaf drop eventually occurred with young twigs or stems of the southern highbush selection FL 86-19 developing a yellow, “skeleton-like” appearance (Fig. 3B) which was why “yellow stem” or “yellow twig” was often used to describe the disorder. At this stage, the root system still appeared healthy, except for the possible loss of fine new roots. Whole plants or individual canes showed symptoms. The plant eventually died after leaf drop, typically during the second year of observation (11).
This prompted the enzyme-linked immunosorbent assay (ELISA) tests and isolations of X. fastidiosa. A single diseased blueberry bush of the selection FL 89-16 was excavated from a blueberry farm in South Georgia on 2 Feb. 2006. The bush was subsequently stored under cold room conditions (5 °C)-in a plastic trash bag to prevent moisture loss—until attempted detection of X. fastidiosa using direct isolation and ELISA tests (Agdia, Inc., Elkhart, IN). From this initial plant, two leaf and two root tissue samples were collected for isolation and ELISA testing on 2 Mar. 2006. The diseased blueberry bush was then moved to a greenhouse and planted in a 30.5-cm diameter pot. This original diseased plant was used to monitor the survival of the bacterium and symptom development on new growth after being stored for 48 d at 5 °C. ELISA results indicated all four tissues tested positive for the bacterial pathogen, X. fastidiosa, whereas only the two root tissues provided positive isolations. One leaf and one root tissue sample were later collected from each of five additional diseased plants for isolation and ELISA testing. Both isolation and ELISA testing methods obtained positive results. Cultures were multiplied to inoculate seedlings of three cultivars: ‘Southern Belle’ (eight plants), ‘Premier’ (six), and ‘Powderblue’ (six) on 23 May 2006
and one selection, FL 86-19 (eight), on 31 May 2006. Two FL 86-19 plants started to show symptoms of marginal necrosis 54 days postinoculation , whereas one plant each of ‘Southern Belle’ and ‘Powderblue’ started to show symptoms of marginal necrosis 63 days postinoculation and ‘Premier’ stayed symptomless. All eight culture-inoculated FL 86-19 plants (100%) showed symptoms 72 days postinoculation, but no symptoms were observed on the control plants. One hundred twenty-six days postinoculation, two ‘Powderblue’ and four ‘Southern Belle’ plants showed mild symptoms, whereas all ‘Premier’ plants were asymptomatic. Positive reisolations of the bacteria from the inoculated symptomatic plants, not from asymptomatic plants, fulfilled Koch's postulates, which confirmed X. fastidiosa was the causal bacterium of the new blueberry disorder, the bacterial leaf scorch of blueberry.
This original blueberry bush provided valuable information on the survivability of the X. fastidiosa blueberry strain. The bacterium was able to survive at 5 °C for 48 d when the bush was kept in a plastic bag before being planted in a large pot and kept in the greenhouse. On 10 July 2006, tissues from this bush were collected for isolation and ELISA and the results were positive for both methods. The blueberry industry-particularly growers-in the southeastern United States will find this information especially important, because the research suggests that the bacteria is able to survive in the aboveground tissues through the south Georgia winter because it is unlikely for the temperature to remain at 5 °C 24 h a day for a consecutive 48 d in the winter. Furthermore, the source of inoculum for transmission would likely be available year-round (11).
By 3 months after initial inoculation, all eight X. fastidiosa-injected FL 86-19 plants showed symptoms, whereas all four PW medium-only-injected plants remained asymptomatic. For the other three cultivars, only two of six ‘Powderblue’ and four of eight ‘Southern Belle’ showed mild symptoms, whereas zero of six ‘Premier’ plants were symptomatic even at 4 months postinoculation. Both ELISA and direct isolations confirmed the presence of X. fastidiosa in symptomatic plants. Yellow stems or twigs were a strong symptomatic indicator of X. fastidiosa infection. There seemed to be a different degree of susceptibility among the three cultivars and one selection with selection FL 86-19 clearly being the most susceptible consistent with what had been observed in the field (11).
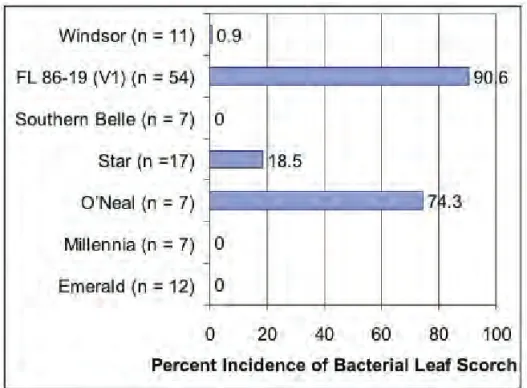
Further studies indicated that there is varietal resistance in some southern highbush blueberries. The FL 86-16 variety is particularly susceptible to infection. When compared with other southern highbush or rabbiteye varieties, the “V5” variety
showed resistance to the bacterium (Fig. 4). This is encouraging, since it indicates that breeding can be utilized to develop varieties that are highly resistant to Xylella. Likewise, surveys have shown that there are other varieties that either do not develop symptoms or that slow epidemic spread of the disease (Fig. 5). ‘FL 86-19’ is highly susceptible, as is the ‘O’Neal’ cultivar. ‘Star’ is susceptible, but it is representative of desirable cultivars that will develop the disease but still likely be economically viable; field epidemics observed in ‘Star’ and similar cultivars do not develop as rapidly, allowing adequate time to recoup investments (6).
Insect vectors for the blueberry bacterial leaf scorch disease are under investigation in Georgia and the glassy-winged sharpshooter leafhopper, Homalodisca vitripennis (formerly H. coagulata), is likely an important suspect.
Phloem-limited plant pathogenic prokaryotes
In Mollicutes, the cell wall-less and phloem-limited prokaryotes, there are two major plant pathogens: spiroplasmas and phytoplasmas. Spiroplasmas are cells with helical forms during log phase growth. Most spriroplasmas are cultivable in enriched media that contain supplemented sterols and other ingredients (9). They are facultative anaerobic or microaerophilic. They are associated with three plant diseases: citrus stubborn and horseradish brittle root disease by Spiroplasma citri, corn stunt disease by S. kunkelii, and periwinkle disease by S. phoeniceum. Phytoplasmas have been associated with more than 500 plant diseases worldwide (22) ever since the historical discovery by Doi et al. (14) of then referred as mycoplasma-like organisms (MLO) found in the pholem elements of plants infected with mulberry dwarf, potato witches’-broom, aster yellows, or paulownia witches’-broom. Phytoplasmas are still noncultivable even though they have been classified into 30 group-subgroups and four undetermined entities based on the 16S rDNA RFLP grouping (http: //plantpathology. ba. ars.usda.gov/pclass/pclass_taxonomy.html).
There were unintentional fans of phytoplasmas for as early as 1000 years ago in Song Dynasty and as recent as nowadays. When phytoplasmas infect peonies, the plants produced flowers not in the typical red or yellow colors, but in a delicate green we call virescence. The green flower was considered so attractive and valuable about 1000 years ago in China that the Song Dynasty’s imperial court received a special annual tribute composed of the blossoms. More recently, phytoplasmas have helped brighten winter holidays by transforming otherwise lanky poinsettias, with their eye-catching red