O R I G I N A L A R T I C L E – U R O L O G I C O N C O L O G Y
Clinical Significance of Tumor Necrosis Factor Receptor
Superfamily Member 11b Polymorphism in Prostate Cancer
Bo-Ying Bao, PhD1, Victor C. Lin, MD4, Shu-Hung Huang, MD5, Jiunn-Bey Pao, MS7, Ta-Yuan Chang, PhD2, Te-Ling Lu, PhD1, Yu-Hsuan Lan, PhD1, Lu-Min Chen, MD8, Wen-Chien Ting, MD9, Wen-Hui Yang, PhD3, Chi-Jeng Hsieh, MS10,11, and Shu-Pin Huang, MD, PhD6,12,13
1Department of Pharmacy, China Medical University, Taichung, Taiwan;2Department of Occupational Safety and Health, China Medical University, Taichung, Taiwan;3Department of Health Services Administration, China Medical University, Taichung, Taiwan;4Department of Urology, E-Da Hospital, Kaohsiung, Taiwan;5Division of Plastic Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan;6Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan;7Department of Pharmacy Practice, Tri-Service General Hospital, Taipei, Taiwan;8Department of Obstetrics and Gynecology, China Medical University Hospital, Taichung, Taiwan;9Department of Colorectal Surgery, China Medical University Hospital, Taichung, Taiwan;10Department of Health Care
Administration, Oriental Institute of Technology, Taipei, Taiwan;11Graduate Institute of Health Care Organization Administration, College of Public Health, National Taiwan University, Taipei, Taiwan;12Department of Urology, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung, Taiwan;13Department of Urology, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
ABSTRACT
Background. Bone metastases are the most critical com-plication of prostate cancer (PCa), resulting in severe morbidity and mortality. Tumor necrosis factor receptor superfamily member 11b (TNFRSF11B) is a critical regu-lator between PCa cells and the bone environment. Recently, TNFRSF11B rs10505346 has been implicated in PCa risk in the Cancer Genetic Markers of Susceptibility genomewide association study. However, the association between this variant and biochemical failure in PCa patients receiving radical prostatectomy (RP) has not been determined. Methods. Associations of TNFRSF11B rs10505346 with age at diagnosis, preoperative prostate-specific antigen (PSA) level, Gleason score, pathologic stage, surgical margin, and PSA recurrence were evaluated in a cohort of 314 localized PCa patients receiving RP. The prognostic
significance on PSA recurrence was assessed by Kaplan– Meier analysis and Cox regression model.
Results. The mean level of preoperative PSA and the relative risks of PSA recurrence after RP were lower in individuals with T allele than in those with the G allele at TNFRSF11B rs10505346 (P = 0.019 and 0.014, respec-tively). The T allele of rs10505346 remained a protective factor against PSA recurrence (P = 0.022) in multivariate Cox regression model after considering all clinicopatho-logical risk factors except PSA level.
Conclusions. Our data suggest that TNFRSF11B rs1050 5346 is associated with PSA level and might be a prog-nostic factor for the recurrence of PSA in PCa patients receiving RP.
Prostate cancer (PCa) is most likely to metastasize to the bone, and bone metastasis occurs in approximately 90% of PCa-related deaths.1PCa bone metastases stimulate an overall increase in both bone remodeling and bone volume. Although initially thought to be osteoblastic, it is now recognized that PCa has an extensive bone resorptive component that is caused primarily by osteoclast; however, the mechanisms underlying this process are not fully understood.2
A key molecular system that influences bone remodeling consisted of tumor necrosis factor receptor superfamily Electronic supplementary material The online version of this
article (doi:10.1245/s10434-010-0994-3) contains supplementary material, which is available to authorized users.
Ó Society of Surgical Oncology 2010 First Received: 27 November 2009; Published Online: 4 March 2010 S.-P. Huang, MD, PhD e-mail: shpihu@yahoo.com.tw DOI 10.1245/s10434-010-0994-3
member 11b (TNFRSF11B, also called osteoprotegerin), receptor activator of nuclear factor jB (RANK), and RANK ligand (RANKL). RANKL, expressed by osteo-blasts and their immature precursors, is necessary and sufficient for osteoclastogenesis. RANKL activates its receptor, RANK, which is expressed on osteoclasts and their precursors, thus promoting osteoclast formation and activation, and prolonging survival by suppressing apop-tosis. The TNFRSF11B prevents association of RANKL with RANK by acting as a decoy receptor.3 Because TNFRSF11B has been found to be a potent inhibitor of osteoclastic bone resorption, it was then further investi-gated as a potential therapeutic modality for the treatment of both osteoporosis and tumor-induced bone disease.4,5
TNFRSF11B has a wide tissue distribution and is found in normal prostate, in prostate tumors, in PCa bone metastases, and in the stromal cells in prostate tumors. Although TNFRSF11B is not highly expressed in the PCa cells, its production by stromal cells in the bone may support the survival of PCa cells in the early stages of bone metastasis.6,7 The potential clinical applications of serum TNFRSF11B have also been carried out. Serum TNFRSF11B was found to be increased in patients with bone metastases and in patients whose disease progressed after androgen ablation. More importantly, this increase was detectable before increase of the classical marker, prostate-specific antigen (PSA), suggesting that serum TNFRSF11B may not only indicate changes in PCa cell survival and growth, but may also be a marker of advanced disease.8
Because of the critical functions of TNFRSF11B in bone metabolism and tumor-induced bone disease, genetic polymorphisms of TNFRSF11B might be important deter-minants in the progression and bone metastasis of PCa. However, much research has focused on the association between polymorphisms in TNFRSF11B and bone mineral density, osteoporosis, and osteoporotic fractures.9,10 Recently, millions of single nucleotide polymorphisms (SNPs) have been evaluated for associations with PCa risk in the Cancer Genetic Markers of Susceptibility (CGEMS) genomewide association study.11However, the prognostic value of those identified PCa susceptibility variants has not been determined.
In the present study, we selected 40 SNPs associated with genes that have been implicated in cancer progression and had low P values (P \ 0.01) in CGEMS (Supple-mentary Table 1). The associations of these variants with clinicopatholgical variables of PCa were evaluated in a population of 314 clinically localized PCa patients who underwent radical prostatectomy (RP). Because each patient’s tumor was accurately and systematically graded and staged using uniform criteria after RP, it offers an excellent way to assess associations of these genetic vari-ants with clinicopathological features and prognosis of
PCa. Interestingly, our primary allelic analysis revealed that only TNFRSF11B rs10505346 was associated with PSA recurrence after RP, suggesting the important roles of genetic polymorphisms in TNFRSF11B/RANK/RANKL system play in disease progression of PCa.
MATERIALS AND METHODS Patient Recruitment and Data Collection
The study population was expanded from our hospital-based PCa case–control study, which has previously been described.12–17 Briefly, patients with diagnosed and path-ologically confirmed PCa were actively recruited from three medical centers in Taiwan: Kaohsiung Medical University Hospital, Kaohsiung Veterans General Hospital, and National Taiwan University Hospital. A subset of patients with clinically localized PCa who underwent RP was followed up prospectively to investigate the potential role of genetic variants in the progression of PCa (defined by the recurrence of PSA). PSA recurrence was defined as two consecutive PSA measurements of [0.2 ng/ml at an interval of [3 months, and the PSA level of [0.2 ng/ml at the first follow-up was considered the date of recurrence.18 For more precise analysis of the effect of disease recur-rence after RP, patients who received adjuvant hormone therapy or radiotherapy were excluded, thus leaving 314 cases for final analysis.
Disease stage was determined by pathologic findings, pelvic computed tomography or magnetic resonance imaging, and radionucleotide bone scans, according to the criteria outlined by the American Joint Committee on Cancer (AJCC) tumor, node, metastasis classification sys-tem (AJCC Cancer Staging Manual, 5th edition, 1997). Pathologic grade was recorded as Gleason scores and was classified into two groups, one with Gleason scores 2–7 and the other with Gleason scores of 8–10.19 Pathology anal-yses were performed on the whole specimens with step sections (2–3 mm), and the positive surgical margin was defined as tumor cells present at the inked margin. This study was approved by the institutional review board of these hospitals, and informed consent was obtained from each participant.
Genotyping
Genotyping was performed by Sequenom iPLEX matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry technology at the National Genotyping Center, Academia Sinica, Taiwan. Briefly, primers for locus-specific polymerase chain reaction and allele-specific extension were designed by MassARRAY
AssayDesign 3.0 software (Sequenom, San Diego, CA). The sample DNAs were amplified by primers flanking the targeted sequence, followed by dephosphorylation and allele-specific primer extension. The extension products were purified, loaded into a 384-format SpectroChip, and subjected to MALDI-TOF mass spectrometry. The resulting data were analyzed by the Sequenom MassARRAY TYPER software. Quality control included genotyping of 39 blind duplicate samples, revealing a 100% agreement on geno-typing calls. The SNP was in Hardy–Weinberg equilibrium (P [ 0.05), and the genotyping call rate was 100%.
Statistical Analysis
The linear regression model was used to estimate the effects of alleles and genotypes on age at time of first diagnosis and age-adjusted preoperative PSA levels. Logistic regression analyses were performed to compute odds ratios and the 95% confidence intervals (95% CI) for estimating the associations of individual SNP alleles as well as genotypes to the risk of clinicopathological features and PSA recurrence, while adjusting for age (age-adjusted odds ratio [aOR]). The Kaplan–Meier method was used to compare the influence of alleles and genotypes in the PSA-free survival interval, and significance was determined by the log rank test. Univariate and multivariate analyses to determine the interdependency of alleles/genotypes and the risk parameters, such as age, preoperative PSA, Gleason score, pathologic stage, and surgical margin, were carried out by Cox proportional hazard regression. SPSS software version 16.0.1 (SPSS, Chicago, IL) was used for statistical analyses. A two-sided P value of \ 0.05 was considered statistically significant.
RESULTS
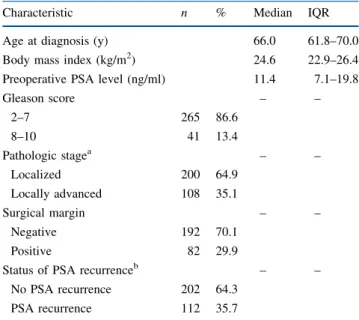
The demographic characteristics of 314 PCa patients who received RP are summarized in Table1. The median of age, body mass index, and PSA level were 66.0 years, 24.6 kg/m2, and 11.4 ng/ml, respectively. Most PCas were at Gleason score of 2–7 (86.6%), localized pathologic stage (64.9%), and negative surgical margin (70.1%); however, 112 (35.7%) experienced PSA recurrence during the 38.5-month (mean) and 30.8-month (median) follow-up periods.
A total of 40 SNPs (associated with genes that have been implicated in cancer progression and had low P values [P \ 0.01] in CGEMS, Supplementary Table 1) were selected, and their prognostic significance on disease progression was evaluated in a cohort of 314 patients with clinically localized PCa who underwent RP. Our primary allelic analysis revealed that only TNFRSF11B
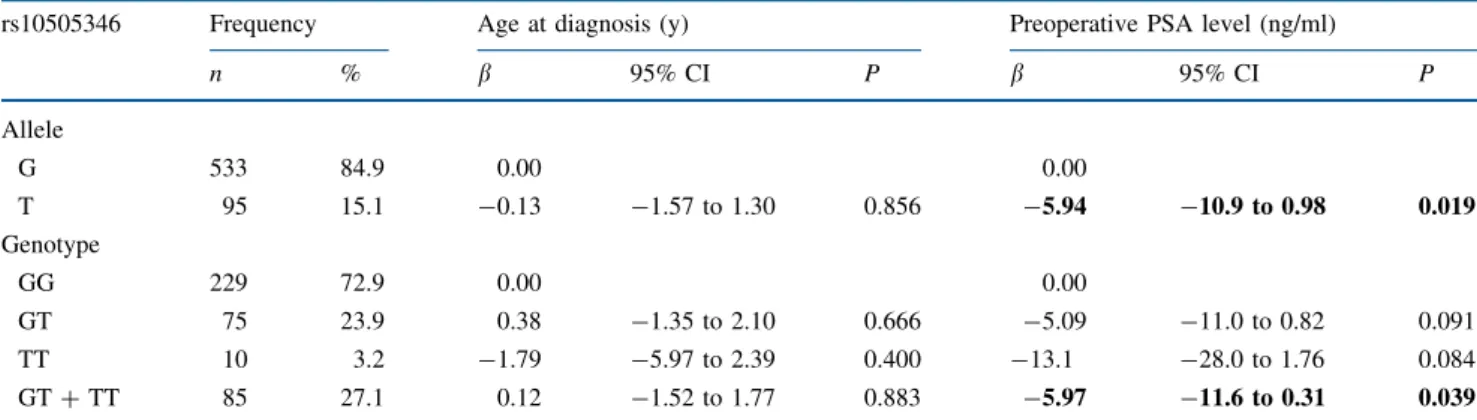
rs10505346 was associated with PSA recurrence after RP (P = 0.042). To explore the potential prognostic role of TNFRSF11B rs10505346 on the recurrence of PSA after RP, we compared the allele and genotype distributions with the various clinicopathological features (Tables2and
3). We found no allele and genotype association with patients’ age at diagnosis, high Gleason score, advanced pathologic stage, and positive surgical margin. The mean level of preoperative PSA was 5.94 and 5.97 ng/ml lower in individuals with T allele and GT ? TT genotype than in those with the G allele and GG genotype at TNFRSF11B rs10505346, respectively. The T allele of TNFRSF11B rs10505346 was also statistically signifi-cantly associated with lower relative risks of PSA recurrence after RP (aOR 0.53, 95% CI 0.32–0.88), but only a weak association with PSA recurrence in GT ? TT carriers (aOR 0.59, 95% CI 0.34–1.02).
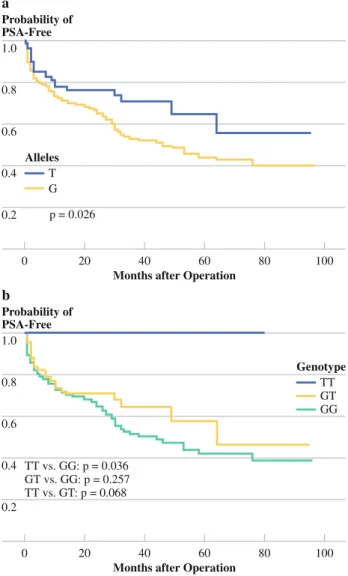
The associations between alleles and genotypes of TNFRSF11B rs10505346 and time to disease recurrence were presented in Fig.1. Kaplan–Meier survival analysis and log rank test revealed a statistically significant asso-ciation of T allele and TT genotype with PSA-free survival. The median estimated cumulative survivals were signifi-cantly higher in T allele and TT genotype carriers than in those with the G allele and GG genotype ([96 vs. 46 months, P = 0.026 for T vs. G allele; and [96 vs. 44 months, P = 0.036 for TT vs. GG genotype).
TABLE 1 Demographic and clinicopathological characteristics of 314 prostate cancer patients who received radical prostatectomy
Characteristic n % Median IQR
Age at diagnosis (y) 66.0 61.8–70.0
Body mass index (kg/m2) 24.6 22.9–26.4
Preoperative PSA level (ng/ml) 11.4 7.1–19.8
Gleason score – – 2–7 265 86.6 8–10 41 13.4 Pathologic stagea – – Localized 200 64.9 Locally advanced 108 35.1 Surgical margin – – Negative 192 70.1 Positive 82 29.9
Status of PSA recurrenceb – –
No PSA recurrence 202 64.3
PSA recurrence 112 35.7
IQR interquartile range, PSA prostate-specific antigen
a Tumor, node, metastasis system staging by American Joint Com-mittee on Cancer (1997): localized, T1/T2 N0 M0; locally advanced, T3/T4 N1 M0
b Mean follow-up of 38.5 months and median follow-up of 30.8 months
To assess the predictive effects of TNFRSF11B rs10505346 on PSA recurrence after RP, currently used clinicopathological parameters, including age at diagnosis, preoperative PSA, Gleason score, pathologic stage, and surgical margin, were evaluated together by Cox propor-tional hazard analysis (Table4). Our univariate analyses found that high preoperative PSA level, Gleason score of 8 to 10, advanced pathologic stage, positive surgical margin, and the T allele of TNFRSF11B rs10505346 significantly influenced post-RP PSA-free survival time (P \ 0.001 to P = 0.030). However, the T allele of TNFRSF11B rs10505346 did not reach significance after adjusting for all clinicopathological risk factors in the multivariate analyses (P = 0.130). Because there was a strong correlation between TNFRSF11B rs10505346 and PSA level (Tables2
and3), the T allele of TNFRSF11B rs10505346 showed a significant association with better PSA-free survival (P = 0.022) without adjusting for PSA level in the multi-variate analysis. Similarly, a weak association was also
observed in the GT ? TT genotypes of TNFRSF11B rs10505346 (P = 0.052).
DISCUSSION
Several theories have been proposed to explain the marked predisposition of bone metastasis in PCa. Most investigators favor the ‘‘seed and soil’’ hypothesis, in which local microenvironmental factors provide proliferative and survival advantages for particular types of cancer cells. Because of the critical role of TNFRSF11B in bone metabolism and PCa bone metastasis, genetic variants in TNFRSF11B might influence disease development and progression. In the present study, we found that the minor allele, T, in carriers of TNFRSF11B rs10505346 had sta-tistically significantly lower preoperative PSA levels and risks for disease recurrence after RP than in those with the major allele, G (Tables 2and3). In addition, the T allele of TNFRSF11B rs10505346 was statistically significantly TABLE 2 Association of TNFRSF11B rs10505346 with age at diagnosis and preoperative PSA level among prostate cancer patients who received radical prostatectomy
rs10505346 Frequency Age at diagnosis (y) Preoperative PSA level (ng/ml)
n % b 95% CI P b 95% CI P Allele G 533 84.9 0.00 0.00 T 95 15.1 -0.13 -1.57 to 1.30 0.856 -5.94 -10.9 to 0.98 0.019 Genotype GG 229 72.9 0.00 0.00 GT 75 23.9 0.38 -1.35 to 2.10 0.666 -5.09 -11.0 to 0.82 0.091 TT 10 3.2 -1.79 -5.97 to 2.39 0.400 -13.1 -28.0 to 1.76 0.084 GT ? TT 85 27.1 0.12 -1.52 to 1.77 0.883 -5.97 -11.6 to 0.31 0.039
TNFRSF11B tumor necrosis factor receptor superfamily member 11b, PSA prostate-specific antigen, b b estimate, 95% CI 95% confidence interval. Bold indicates P B 0.05
TABLE 3 Association of TNFRSF11B rs10505346 with Gleason score, pathologic stage, surgical margins, and PSA recurrence among prostate cancer patients who received radical prostatectomy
rs10505346 Gleason score 8–10 Locally advanced pathologic stage Positive surgical margin PSA recurrence
aOR (95% CI) P aOR (95% CI) P aOR (95% CI) P aOR (95% CI) P
Alleles G 1.00 1.00 1.00 1.00 T 0.81 (0.41–1.60) 0.540 0.81 (0.50–1.29) 0.372 1.17 (0.72–1.89) 0.531 0.53 (0.32–0.88) 0.014 Genotypes GG 1.00 1.00 1.00 1.00 GT 0.84 (0.38–1.86) 0.669 0.91 (0.52–1.59) 0.744 1.25 (0.70–2.25) 0.447 0.70 (0.40–1.23) 0.215 TT 0.62 (0.08–5.12) 0.655 0.44 (0.09–2.14) 0.310 1.06 (0.26–4.28) 0.931 –a GT ? TT 0.81 (0.38–1.74) 0.593 0.85 (0.50–1.44) 0.538 1.23 (0.70–2.15) 0.469 0.59 (0.34–1.02) 0.058 PSA prostate-specific antigen, aOR age-adjusted odds ratio, 95% CI 95% confidence interval; boldface indicates P B 0.05
associated with better PSA-free survival in Kaplan–Meier survival analysis and in multivariate Cox proportional hazard analysis after considering other clinicopathological risk covariates, except PSA levels (Fig.1and Table 4).
The human TNFRSF11B gene is located at chromosome 8q24, but it is [8 Mb upstream of the loci that has been identified to associate with risk of prostate, colorectal, breast, ovarian, and bladder cancers.20–24By studying two genetic polymorphisms in the putative promoter region of TNFRSF11B, rs3134071 and rs2073617, Narita et al. found that the T allele of the rs2073617 is a predicting factor for cancer-specific survival in metastatic PCa patients.25Notably, the SNP, rs10505346, that we analyzed in this study is located in intron 1. Although it is just 440 bp downstream of rs2073617, we found that the r2was 0.078 according to HapMap CHB (Han Chinese in Beijing,
China) population data, suggesting no linkage disequilib-rium between rs10505346 with those two SNPs reported previously. Therefore, we believe that the TNFRSF11B rs10505346 we analyzed in this study could serve as a novel biomarker for PCa progression.
Because the TNFRSF11B rs10505346 polymorphism is situated in the first intron of the TNFRSF11B gene, it is reasonable to hypothesize that this SNP might alter the consensus splicing site sequence or the binding site for transcription factors, thereby influencing TNFRSF11B splicing and expression. In fact, there are several untyped HapMap SNPs that can be captured by rs10505346, such as rs10505349, rs10955911, rs11573838, rs11573896, rs11984630, rs1485287, rs1994276, and rs2875845, located from promoter region to the first intron of TNFRSF11B; many of them were predicted to create or break binding sites for transcription factors. Most interestingly, rs11573896 (A to T) was predicted to create two binding sites for heat-shock transcription factor 1 (HSF1) by FASTSNP.26Upregulation of HSF1 has been found in metastatic variant of PC-3 human PCa cell lines and in mouse PCa models, especially in metastases.27–29 In addition, positive TNFRSF11B immu-nostaining in the tumor epithelium has been suggested as a prognostic marker for biochemical recurrence in RP patients.30Therefore, further functional study is required to investigate whether these polymorphisms captured by TNFRSF11B rs10505346 as well as itself would alter TNFRSF11B function and influence the recurrence of PSA. PSA, also known as kallikrein 3, is an androgen-regulated serine protease synthesized in the epithelium of the prostate and is a widely used tumor marker that facilitates the detection of PCa. However, results from the Prostate Cancer Prevention Trial have shown that the sensitivity and speci-ficity of PSA test for PCa is not ideal.31We and others have found that several PCa risk variants were associated with PSA levels, such as TNFRSF11B rs10505346 in this study, rs10486567 at 7p15, rs10993994 at 10q11, rs4962416 at 10q26, rs2735839 at 19q13, and rs5945619 at Xp11.32These findings might have an important clinical implication to improve the discriminatory performance of PSA by accounting for the variation in PSA levels due to genetic variants. If men inherit fewer alleles that are associated with higher PSA levels, currently used uniform PSA cutoff values might lead to a lower sensitivity in PCa detection. Although it has been found that PSA can stimulate TNFRSF11B production, how polymorphisms in TNFRSF11B affect PSA level deserves further investigation.33
In conclusion, we provide the first evidence for the association of TNFRSF11B rs10505346 with preoperative PSA levels and relative risks for PSA recurrence after RP. More importantly, our data demonstrated that the presence of the variant T allele might have a considerable protective effect against the recurrence of PSA in PCa patients who 1.0 0.8 0.6 0.4 0.2 0 80 100
Months after Operation
40 60 20 Probability of PSA-Free a T G Alleles p = 0.026 1.0 0.8 0.6 0.4 0.2 0 80 100
Months after Operation
40 60 20 Probability of PSA-Free b TT GT GG TT vs. GG: p = 0.036 GT vs. GG: p = 0.257 TT vs. GT: p = 0.068 Genotype
FIG. 1 Kaplan-Meier analysis of time to prostate-specific antigen (PSA) recurrence after radical prostatectomy, stratified by (a) alleles and (b) genotypes at tumor necrosis factor receptor superfamily member 11b (TNFRSF11B) rs10505346
received RP. This effect is not associated with other cur-rently used outcome predictors, such as age at diagnosis, pathologic stage, Gleason score, and surgical margin, but might be due to the modification of disease progression through the potential functions of TNFRSF11B. However, this study is limited by sample size in analyses of outcomes and in subset analyses. In addition, our homogeneous Chinese Han population may make our findings less gen-eralizable to other ethnic groups. Thus, further functional analyses and large independent studies in other ethnic populations are necessary to dissect these relationships. ACKNOWLEDGMENT Supported by the National Science Council, Taiwan (grant NSC-98-2320-B-039-019-MY3), and China Medical University (grants CMU97-183, N1-21, and CMU98-C-12). We thank the National Genotyping Center of National Research Program for Genomic Medicine, National Science Council, Taiwan, for their technical support.
REFERENCES
1. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64.
2. Clarke NW, McClure J, George NJ. Disodium pamidronate identifies differential osteoclastic bone resorption in metastatic prostate cancer. Br J Urol. 1992;69:64–70.
3. Hofbauer LC, Schoppet M. Clinical implications of the osteo-protegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–5.
4. Corey E, Brown LG, Kiefer JA, et al. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005;65:1710–8.
5. Zhang J, Dai J, Qi Y, et al. Osteoprotegerin inhibits prostate cancer–induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–44.
6. Brown JM, Vessella RL, Kostenuik PJ, et al. Serum osteopro-tegerin levels are increased in patients with advanced prostate cancer. Clin Cancer Res. 2001;7:2977–83.
7. Nyambo R, Cross N, Lippitt J, et al. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J Bone Miner Res. 2004;19:1712–21.
8. Eaton CL, Wells JM, Holen I, et al. Serum osteoprotegerin (OPG) levels are associated with disease progression and response to androgen ablation in patients with prostate cancer. Prostate. 2004;59:304–10.
9. Richards JB, Rivadeneira F, Inouye M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–12.
10. Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, et al. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358:2355–65.
11. Yeager M, Orr N, Hayes RB, et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–9.
12. Huang SP, Chou YH, Wayne Chang WS, et al. Association between vitamin D receptor polymorphisms and prostate cancer risk in a Taiwanese population. Cancer Lett. 2004;207:69–77. 13. Huang SP, Huang CY, Wang JS, et al. Prognostic significance of
p53 and X-ray repair cross-complementing group 1 polymor-phisms on prostate-specific antigen recurrence in prostate cancer post radical prostatectomy. Clin Cancer Res. 2007;13:6632–8. 14. Huang SP, Huang CY, Wu WJ, et al. Association of vitamin D
receptor FokI polymorphism with prostate cancer risk, clinico-pathological features and recurrence of prostate specific antigen after radical prostatectomy. Int J Cancer. 2006;119:1902–7. 15. Huang SP, Huang LC, Ting WC, et al. Prognostic significance of
prostate cancer susceptibility variants on prostate-specific antigen TABLE 4 Cox proportional hazard analysis of factors associated with PSA recurrence after radical prostatectomy
Variable Univariate analysis Multivariate analysisa
HR (95% CI) P HR (95% CI) P HR (95% CI) P HR (95% CI) P
Age (y) 0.99 (0.97–1.01) 0.384 0.99 (0.96–1.01) 0.227 0.98 (0.96–1.01) 0.137 0.98 (0.95–1.01) 0.238 Preoperative PSA (ng/ml) 1.02 (1.02–1.03) £0.001 1.02 (1.02–1.03) £0.001 Gleason score 2–7 1.00 1.00 1.00 1.00 8–10 3.71 (2.69–5.11) £0.001 2.55 (1.76–3.71) £0.001 2.53 (1.74–3.67) £0.001 2.48 (1.46–4.18) 0.001 Pathologic stage Localized 1.00 1.00 1.00 1.00 Locally advanced 3.55 (2.70–4.67) £0.001 2.22 (1.58–3.14) £0.001 2.40 (1.73–3.34) £0.001 2.40 (1.51–3.83) £0.001 Surgical margin Negative 1.00 1.00 1.00 1.00 Positive 2.80 (2.10–3.74) £0.001 1.46 (1.03–2.07) 0.034 1.72 (1.23–2.40) 0.002 1.76 (1.09–2.82) 0.020 rs10505346 G 1.00 1.00 1.00 T 0.62 (0.40–0.96) 0.030 0.69 (0.42–1.12) 0.130 0.58 (0.36–0.93) 0.022 GG 1.00 1.00 GT ? TT 0.67 (0.43–1.07) 0.092 0.60 (0.36–1.01) 0.052
PSA prostate-specific antigen, HR hazard ratio, 95% CI 95% confidence interval; boldface indicates P B 0.05
a Age, preoperative PSA, Gleason score, pathologic stage, surgical margin, and rs10505346 alleles or genotypes were included in the multi-variate analysis
recurrence after radical prostatectomy. Cancer Epidemiol Bio-markers Prev. 2009;18:3068–74.
16. Huang SP, Ting WC, Chen LM, et al. Association analysis of Wnt pathway genes on prostate-specific antigen recurrence after radical prostatectomy. Ann Surg Oncol. 2010;17:312–22. 17. Huang SP, Wu WJ, Chang WS, et al. p53 Codon 72 and p21
codon 31 polymorphisms in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2217–24.
18. Freedland SJ, Sutter ME, Dorey F, et al. Defining the ideal cut-point for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:365–9.
19. Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64.
20. Amundadottir LT, Sulem P, Gudmundsson J, et al. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38:652–8.
21. Tomlinson I, Webb E, Carvajal-Carmona L, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39:984–8. 22. Easton DF, Pooley KA, Dunning AM, et al. Genome-wide
association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–93.
23. Ghoussaini M, Song H, Koessler T, et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst. 2008;100:962–6.
24. Kiemeney LA, Thorlacius S, Sulem P, et al. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet. 2008;40:1307–12.
25. Narita N, Yuasa T, Tsuchiya N, et al. A genetic polymorphism of the osteoprotegerin gene is associated with an increased risk of advanced prostate cancer. BMC Cancer. 2008;8:224.
26. Yuan HY, Chiou JJ, Tseng WH, et al. FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006;34:W635–41.
27. Hoang AT, Huang J, Rudra-Ganguly N, et al. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857–64. 28. Tang D, Khaleque MA, Jones EL, et al. Expression of heat shock
proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10:46–58.
29. O’Callaghan-Sunol C, Sherman MY. Heat shock transcription factor (HSF1) plays a critical role in cell migration via main-taining MAP kinase signaling. Cell Cycle. 2006;5:1431–7. 30. Perez-Martinez FC, Alonso V, Sarasa JL, et al. Receptor activator
of nuclear factor-kappaB ligand (RANKL) as a novel prognostic marker in prostate carcinoma. Histol Histopathol. 2008;23:709–15. 31. Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294:66–70.
32. Wiklund F, Zheng SL, Sun J, et al. Association of reported prostate cancer risk alleles with PSA levels among men without a diagnosis of prostate cancer. Prostate. 2009;69:419–27. 33. Yonou H, Horiguchi Y, Ohno Y, et al. Prostate-specific antigen
stimulates osteoprotegerin production and inhibits receptor acti-vator of nuclear factor-kappaB ligand expression by human osteoblasts. Prostate. 2007;67:840–8.