Purification and Characterization of Neutral Sphingomyelinase from
Helicobacter pylori
†Err-Cheng Chan,*,‡Chih-Chieh Chang,‡,§Yi-Shuane Li,‡C. Allen Chang,§Chiuan-Chian Chiou,‡ and Tzong-Zeng Wu|
School of Medical Technology, Chang Gung UniVersity, Taoyuan, Taiwan, Institute of Biotechnology, National Dong Hwa UniVersity, and the Institute of Biological Science and Technology, National Chiao Tung UniVersity, Hsinchu, Taiwan
ReceiVed NoVember 3, 1999; ReVised Manuscript ReceiVed January 31, 2000
ABSTRACT: Phospholipase activities of human gastric bacterium, Helicobacter pylori, are regarded as the pathogenic factors owing to their actions on epithelial cell membranes. In this study, we purified and characterized neutral sphingomyelinase (N-SMase) from the superficial components of H. pylori strains for the first time. N-SMase was purified 2083-fold with an overall recovery of 37%. The purification steps included acid glycine extraction, ammonium sulfate precipitation, CM-Sepharose, Mono-Q, and Sephadex G-75 column chromatography. Approximate molecular mass for the native N-SMase was around 32 kDa. When N-ω-trinitrophenylaminolauryl sphingomyelin (TNPAL-SM) was used as a substrate, the purified enzyme exhibited a Km of 6.7 µM and a Vmax of 15.6 nmol of TNPAL-sphingosine/h/mg of protein at 37°C in 50 mM phosphate-buffered saline, pH 7.4. N-SMase reaches optimal activity at pH 7.4 and has a pI of 7.15. The enzyme activity is magnesium dependent and specifically hydrolyzed sphingomyelin and phosphatidylethanolamine. The enzyme also exhibits hemolytic activity on human erythrocytes. According to Western blot analysis, a rabbit antiserum against purified N-SMase from H. pylori cross-reacted with SMase from Bacillus cereus. Sera from individuals with H. pylori infection but not uninfected ones recognizing the purified N-SMase indicated that it was produced in vivo. In enzyme-linked immunosorbent assays, the purified N-SMase used as an antigen was as effective as crude protein antigens in detecting human antibodies to H. pylori.
Helicobacter pylori, a spiral Gram-negative bacterium is strongly associated with human gastritis, pepticulcer, and gastric cancer (1-7). Infection of H. pylori begins with gastric mucus colonization, followed by attachment of the bacteria to the specific portions of epithelial cell (8). Human gastric epithelium protects itself against various noxious factor attacks by the lipid hydrophobic barrier (9, 10). Mauch et al. (11) reported that H. pylori infection damaged the phospholipid-rich epithelial layer of gastric mucosa. A related study also indicated that the gastric mucosal hydrophobicity of H. pylori-infected patients returned to normal values after eradication of the bacterium (12). Among the enzymes of H. pylori, phospholipases are implicated as virulence factors capable of incurring cellular damage by either directly hydrolyzing gastric membrane phospholipids or indirectly releasing mediators of inflammation (13). Our previous work identified one of the phospholipases, sphingomyelinase (SMase) activity, in clinical isolates of H. pylori (14). We also demonstrated the presence of H. pylori SMases in neutral and acidic forms, as defined by their optimal pH values and
cellular locations (14). Phospholipases of H. pylori are regarded as pathogenic factors owing to their disintegrating activities on the membrane of gastric epithelial cells (15, 16). SMase of H. pylori must be purified and requires further characterization because SMases from different organisms vary considerably in their kinetic properties and their actions toward lipid membrane composition (17).
Several bacterial SMases have been highly purified and used in studies of membranes structure and studies on the evolution of cytolysins produced by the diverse genera of Gram-positive pathogenic bacteria (18, 19). SMases produced by Bacillus and Staphylococcus strains are designated as β-hemolysin, and possess an activity of hemolysis (20). SMase also has other potent biological effects that may be more important than its toxic role in a membrane damage. For instance, sphingomyelin (SM) hydrolysis by SMase on cellular membrane can initiate a cellular signaling pathway (21). SM is a phospholipid preferentially concentrated in the outer layer of the plasma membrane of mammalian cells. Bacterial SMase acted on the cellular membrane may hydrolyze SM to yield ceramide and phosphocholine. The hydrolytic product, ceramide, is regarded as an active †This work was supported in part by National Science Council, ROC,
Grant NSC-87-2314-B-182-084 (E.C.C.) and Chang Gung Memorial Hospital Grant CMRP 756 (E.C.C.).
* To whom correspondence should be addressed. Phone: 886-3-3283016, ext 5220. Fax: 886-3-3288741. E-mail: chanec@mail. cgu.edu.tw.
‡Chang Gung University. §National Chiao Tung University.
|National Dong Hwa University.
1Abbreviations: SMase, sphingomyelinase; N-SMase, neutral sphingomyelinase; SM, sphingomyelin; TNPAL-SM, N- ω-trinitrophen-ylaminolauryl sphingomyelin; PS, phosphatidylserine; PC, phosphati-dylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; IEF, isoelectric focusing; SDS-PAGE, sodium dodecyl sulfate-polyacryl-amide gel electrophoresis; pI, isoelectric point; ELISA, enzyme-linked immunosorbent assay.
10.1021/bi9925423 CCC: $19.00 © 2000 American Chemical Society Published on Web 03/29/2000
participant in the cellular regulations, apoptosis, and inflam-matory responses (22, 23). Therefore, H. pylori SMase could be a proinflammatory and apoptotic mediator associated with the development of gastric ulcer diseases. Our previous work demonstrated that some patients with severe ulcer diseases have a high activity of SMase (24). However, owing to the insufficient number of clinical isolates of H. pylori tested at this stage, we cannot determine whether SMase activity correlates with pathogenesis. To further elucidate the viru-lence of H. pylori N-SMase, a purified N-SMase of H. pylori must be obtained, and this work reported the purification and characterization of the N-SMase from H. pylori.
MATERIALS AND METHODS
Bacterial Isolates and CultiVation. All strains of H. pylori from ulcer patients were obtained from Chang Gung Memo-rial Hospital, Taiwan. Twelve strains were screened quan-titatively for SMase activity. The bacteria were cultured on chocolate agar plates at 37°C in a microaerophilic atmo-sphere of 10% CO2in air, and 95% humidity for 4 days as
previously described elsewhere (14). Strains of H. pylori were identified by Gram stain morphology, urease test, and histology. Strain K8 with the highest SMase activity was used throughout this work to purify the N-SMase.
N-SMase Extraction. To prepare the whole cell lysate, the washed strain K8 from chocolate agar plates was inactivated at 60°C for 15 min and then sonicated for 10 s intervals in a Branson ultrasonifier (microtip, position 5, 40% pulsed). The cell sonicate was then ultracentrifuged (100000g for 30 min), and the supernatant was stored at -20°C before use. To prepare an acid glycine extract, 2.5 mL of glycine buffer (0.2 M, pH 2.2) was added to every 100 mg of bacterial cell mass. This suspension was gently mixed for 20 min at room temperature and centrifuged (10000g for 30 min at 4°C). The supernatant was neutralized with NaOH (1.0 N) to pH 7.0 and dialyzed against 10 mM PBS buffer, pH 7.4. at 4
°C overnight. Insoluble particles were spun down, and the supernatant was stored at -20°C for subsequent purification. Purification of N-SMase. Purification process was per-formed at 4°C. To perform the ammonium sulfate precipita-tion, the neutralized acid glycine extract was slowly added with a cold, saturated ammonium sulfate solution to a final concentration of 40% ammonium sulfate and gently stirred for 1 h. After centrifuging (10000g, 10 min) the solution, the precipitation was repeated at the final concentrations of 60% and 80% ammonium sulfate. Each precipitated fraction was resuspened and determined for the activity of N-SMase, and the 80% ammonium sulfate fraction containing the highest activity of N-SMase was then dialyzed against 20 mM PBS buffer, pH 6.0, overnight and used in subsequent chromatographic purification. Cationic exchange chroma-tography was performed on a CM-Sepharose cartridge (Bio-Rad, 0.7× 2.5 cm) equilibrated in a buffer containing 20 mM sodium phosphate (pH 6.0), and proteins were eluted with the same buffer containing a linear gradient from 0.1 to 1.0 M of NaCl over 80 mL. The flow rate was controlled at 0.75 mL/min, and fractions of 4 mL were collected and monitored for UV absorbance at 280 nm. Fractions contain-ing N-SMase activities were pooled and dialyzed against 20 mM Tris-HCl buffer (pH 8.1) for the next anionic exchange process. Anionic exchange chromatography was performed
on a Mono Q cartridge (Bio-Rad, 0.7× 2.5 cm) with a buffer containing 20 mM Tris-HCl (pH 8.1), and proteins were eluted with the same buffer solution containing a linear gradient of 0 to 0.5 M NaCl. The flow rate was controlled at 1.0 mL/min, and fractions of 2 mL were collected. Size-exclusion chromatography was performed on a Sephadex 75 column (Pharmacia LKB Biotechnology Inc., 1× 80 cm) with a buffer solution containing 10 mM Tris-HCl, pH 7.4, and proteins were eluted with the same buffer containing 0.1 M NaCl. The flow rate was controlled at 0.4 mL/min, and fractions of 0.5 mL were collected. To determine the native molecular mass of N-SMase, fractions containing N-SMase activity were concentrated and applied to the gel filtration high-performance liquid chromatography (HPLC) using a Bio-Sil SEC-250 column (Bio-Rad, 7.8× 300 mm) with a buffer containing 10 mM sodium phosphate, pH 6.8. N-SMase activity was eluted with the same buffer at a flow rate of 1.0 mL/min. Molecular mass standards included blue dextran, albumin (67 kDa), ovalbumin (43 kDa), chymo-trypsinogen A (25 kDa), and ribonuclease A (13.7 kDa).
N-SMase Assay. The activity of N-SMase was determined according to the ability of the enzyme to hydrolyze the SM analogue, TNPAL-SM (Sigma), as described elsewhere (25, 26). Briefly, the enzyme preparation (0.03-0.6 mg of total protein/100µL) diluted to 200 µL with 50 mM PBS buffer (pH 7.4) was mixed with 20 nmol of TNPAL-SM (in 0.05% Triton X-100) in 50µL of magnesium chloride-PBS buffer (pH 7.4). The reaction lasted for 2 h at 37 °C and was terminated by adding 0.75 mL of 2-propanol/heptane/5 M H2SO4(40:10:1, v/v), followed by 0.4 mL of water and 0.45
mL of heptane. After phase separation, a portion of the upper phase was removed and the reaction product, TNPAL-sphingosine, was measured spectrophotometrically at 330 nm. One unit of activity was defined as the amount of N-SMase catalyzing the production of 1 nmol of TNPAL-sphingosine/h at 37°C. Specific activity was given as units of enzyme activity per milligram of protein. To examine the effect of different pH values on N-SMase activity, 10 mM sodium acetate buffer (for pH 2-5), 10 mM PBS buffer (for pH 5.5-7), and 10 mM Tris-HCl buffer (for pH 7.4-11.0) were used in the assay. To investigate the substrate specific-ity, gel diffusion assay was slightly modified according to the method of Lenchner et al. (27). The substrate solutions were prepared separately by suspending 0.1 g of SM, phosphatidylserine (PS), phosphatidylcholine (PC), phos-phatidylethanolamine (PE), and phosphatidylinositol (PI) (Sigma Co.) in 1 mL of PBS buffer (pH 7.4) containing 5 mM magnesium chloride. The solutions were sonicated for 3 min then thoroughly mixed with 9 mL of 1.1% agar and poured onto gel bond films to make the substrate gels. The purified N-SMase solution was added into punched holes of the substrate gel and incubated at 37°C for 2 h. The substrate specificity of N-SMase was demonstrated by observing the appearance of opaque zone, and the zone radius could be measured for semiquantifying the enzyme activity.
Isoelectric Focusing (IEF) of N-SMase. IEF was performed on a PhastSystem electrophoresis apparatus (Pharmacia LKB Biotechnology Inc.) with PhastGel IEF 3-9 covering the pH range 3-9 according to the manufacturer’s instructions. The PhastGel IEF 3-9 medium is homogeneous polyacrylamide gel (5% T, 3% C) containing Pharmalyte carrier ampholytes (Pharmacia). Proteins migrated under an electric field to a
point in the pH gradient that corresponded to their pIs. The gel was stained by Coomassie blue, and Pharmacia broad pI calibration kit was used to determine the pI of purified N-SMase.
Hemolytic ActiVity of N-SMase. One volume of blood from healthy adults was added to 9 vol of anticoagulant buffer (0.1 mM sodium-citrate). The solution was then centrifuged (600g) at room temperature for 15 min to obtain erythrocytes. Next, the hemolytic activity of the enzyme was examined by adding purified N-SMase to 5% erythrocyte suspension (in 5 mM PBS buffer, pH 7.4) and incubated at 37°C with gentle shaking. The final activity of N-SMase in reaction solution was 0.15 units/mL. Aliquots were withdrawn from the reaction solution at various incubating times and centri-fuged at room temperature. To measure hot-cold hemolysis, aliquots from the incubation mixture were immediately diluted to 10 vol with phosphate-buffered saline precooled at 0 °C, cooled in an ice bath, and centrifuged. The supernatants were then diluted to 10 vol with 10 mM PBS buffer, pH 7.4, and were measured spectrophotometrically at 550 nm. A complete lysis of erythrocyte was obtained by adding distilled water to the erythrocyte cells.
SDS-PAGE. The protein profiles were determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electro-phoresis (PAGE) by following the method of Laemmli (28) with 12% acrylamide running gels and 5% stacking gels. Samples were denatured for 5 min at 100°C in a sample buffer containing 1% SDS and 12.5%β-mercaptoethanol, and the gels were loaded with 1-5µg of protein. Electro-phoretic migrations were carried out for 1 h at 25 V/m. Protein profiles were visualized with Coomassie blue stain. Protein Assay. Protein concentration was determined by the Bradford dye binding assay reagent from Bio-Rad using bovine serum albumin as a standard. A microassay procedure, which can obtain a linear standard curve range from 1.25 to 25 µg/mL protein, was used according to the manual instruction.
Preparation of Antisera. To obtain enough purified N-SMase for immunization, approximately 710µg of purified enzyme was prepared from 40 g of H. pylori mass according to the described purification steps. Antisera against purified N-SMase and whole cells of H. pylori were raised in female New Zealand white rabbits as described elsewhere (14). A total of 500µg of the purified N-SMase or boiled H. pylori solution (108 cells) mixed with Freund complete adjuvant
was injected subcutaneously into eight sites on the back and hind legs of each rabbit. Booster dose (100 µg) was administered in a similar manner in Freund incomplete adjuvant 14 and 28 days after initial immunization. On day 42, the rabbits were bled, and the serum was collected and stored at -20°C. Preimmune serum was taken and used as a negative control serum. For immunoblotting experiments, the rabbit antisera were diluted at 1:200.
Immunoblotting. Proteins were subjected to SDS-PAGE (10% polyacrylamide) and were transferred to nitrocellulose membrane (Millipore, Bedford, MA) by electroblotting for 1 h at 1 A. The blotted nitrocellulose was then incubated with sera, washed, incubated with alkaline phosphatase-conjugated anti-human IgG (Biogenesis Inc., Sandown, NH) or anti-rabbit IgG (Biogenesis) as described elsewhere (28, 29). To detect human anti-SMase antibodies, human sera were tested in the Western blot analysis. Human sera were
obtained from four H. pylori-uninfected individuals and 11 H. pylori-infected patients who had been confirmed by endoscopy, rapid urease test, and histologic examination at Chang Gung Memorial Hospital, Taiwan.
ELISA. On the basis of the diagnosis of a H. pylori infection by microscopy examination, culture, and rapid urease test of gastric biopsies as described elsewhere (30), positive sera from 25 infected patients and negative sera from 25 uninfected individuals were selected for ELISA. The purified N-SMase solution was diluted in 50 mM sodium carbonate buffer, pH 9.6. Prior to each experiment, round-bottomed microtiter plates were separately coated with 0.4 µg of purified N-SMase, 1 µg of acid glycine extract, and 1 µg of whole cells lysate of H. pylori/microplate well and then incubated at 4 °C overnight. Alkaline phosphatase-conjugated anti-human IgG (Biogenesis) was used as the secondary antibody. Another procedure of ELISA has been described elsewhere (31). The antibody titer of human serum was defined as the reciprocal of the highest dilution that produced an absorbance of g0.15. Sera from H. pylori-uninfected and H. pylori-infected patients with titers exceed-ing and lyexceed-ing below the mean titer of all sera from uninfected patients by more than three standard deviations were defined as “false positive” and “false negative” sera, respectively. Finally, the sensitivity, specificity, positive, and negative predictive values were calculated.
RESULTS
N-SMase ActiVities of Clinical Isolates. Twelve clinical isolates of H. pylori from humans were screened quantita-tively for N-SMase activity, and the specific activities varied from 2.0 to 16.6 nmol of TNPAL-sphingosine/h/mg of protein with a mean of 6.2 ( 0.8 nmol of TNPAL-sphingosine/h/mg of protein. Among the clinical isolates, strain K8 was used to purify and characterize N-SMase because it possessed the highest N-SMase activity on repeated subculture.
Extraction of Superficial Proteins. Acid glycine extract and saline extract were prepared from H. pylori strain K8 and used to isolate N-SMase. Acid glycine extract contained the 68% recoveries of N-SMase activity, which was 15% higher than saline extract did. Acid glycine extraction resulted in loss of flagella, but otherwise did not lead to significantly modify the shape of the bacterial cells as checked on Gram-stained smears (figure not shown). In addition, according to plate colony counts, 95% of the treated bacterial cells remained viable. Acid glycine extract was used as starting material for the subsequent purification of N-SMase.
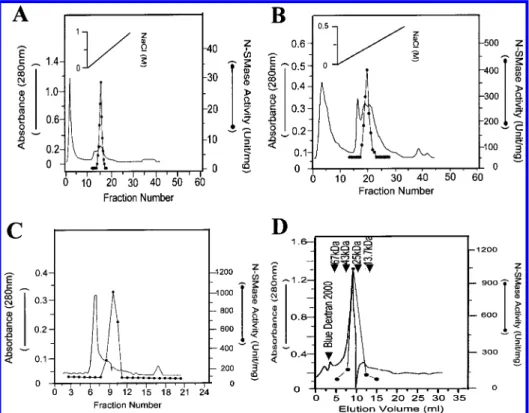
Purification of N-SMase. After acid glycine extract was neutralized with NaOH and dialyzed overnight, N-SMase activity was then precipitated with ammonium sulfate. A major portion of N-SMase activity was precipitated in a fraction between 60 and 80% saturated solution of am-monium sulfate. When the resuspended precipitated proteins were applied to a CM-Sepharose cation-exchange column, all of the N-SMase bound to the column, and N-SMase was then eluted as a single peak at approximately 0.25 M NaCl (Figure 1A). Fractions 14, 15, 16, and 17 were pooled and chromatographed on a Mono-Q anion-exchange column, and N-SMase activity was eluted at 0.2 M NaCl and was detected mainly in fractions between 18 and 22 as shown in Figure
1B. These fractions were then applied to a Sephadex G-75 gel filtration column, and N-SMase activity eluted as a single peak (Figure 1C). For confirming the purity of N-SMase, the fraction 9 from Sephadex G-75 chromatography was applied to a gel filtration HPLC column and resulted in an elution of a single N-SMase activity peak with an estimated molecular mass of 32 kDa (Figure 1D). Analysis of the specific activities at each step in the purification process revealed that the N-SMase was purified more than 2000-fold from the whole cell lysate (Table 1). The denatured proteins profile of each purification step was analyzed by SDS-PAGE shown in Figure 2A, which indicated the purification to homogeneity of a 32 kDa protein.
Isoelectric Point (pI) of N-SMase. The pI of N-SMase was determined by electrophoretic isoelectric focusing with seven standard proteins of known pIs ranging between 4.55 and
8.65. Correlating the migration distance of protein with that of standard proteins allowed us to determine a value of pI 7.15 for purified H. pylori N-SMase (Figure 2B).
Characteristics of Enzyme Kinetics. The rate of TNPAL-SM hydrolysis at 37°C in 50 mM phosphate-buffered saline (pH 7.4) was monitored as a function of TNPAL-SM concentration from 0.0015 to 6 mM. Lineweaver-Burk analysis (32) yielded a Kmof 6.7 µM and a Vmax of 15.6
nmol of TNPAL-sphingosine/h/mg of protein for purified N-SMase.
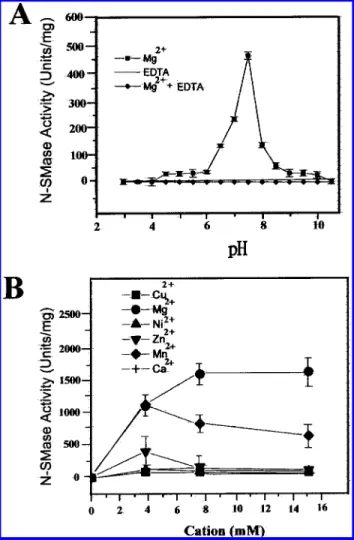
Effect of pH on SMase ActiVity. In the presence of Mg2+,
purified SMase activity of H. pylori was measured at 37°C with buffers ranging from pH 2 to 11. The optimal pH value for activity was determined at around pH 7.4, as shown in Figure 3A. Therefore, the SMase was defined as a neutral form of SMase (N-SMase). According to this figure, the FIGURE1: Chromatography of N-SMase from H. pylori. (A) Ammonium sulfate-precipitated supernatant was desalted and applied to the CM-Sepharose column, which was then eluted with a linear gradient of NaCl (solid diagonal line). (B) Fractions containing N-SMase activity were dialyzed overnight and applied to the Mono Q column, which was then eluted with a linear gradient of NaCl (solid diagonal line). (C) Fractions containing N-SMase activity were concentrated, dialyzed overnight, and applied to the Sephadex G-75 column, which was then eluted isocratically. (D) Fraction 9 from Figure 1C was concentrated and applied to the Bio-Sil SEC-250 column, which was then eluted as described in the Materials and Methods. Column elutes were monitored for absorbance at 280 nm (solid line), and assayed for N-SMase activity (solid circles). The arrows indicate the elution volume of molecular standards which were ribonuclease A (13.7 kDa), chymotrypsinogen A (25 kDa), ovalbumin (43 kDa), albumin (67 kDa), and blue dextran 2000.
Table 1: Purification of N-SMase from H. pylori K8
purification step total protein (mg) total activity (nmol of TNPAL-sphingosine/h) specific activity (nmol of TNPAL-sphingosine/h/mg) recovery (%) purification (n-fold) whole cells lysate (10 mL) 224 87.2 0.39 100 1.0
acid glycine extract extract (50 mL) 74 59.1 0.8 67.7 2.1 ammonium sulfate precipitate (30 mL) 20 49.5 2.49 56.8 6.4 CM Sepharose (10 mL) 1.4 41.3 29.5 47.4 75.6 Mono Q (5 mL) 0.1 37.3 373 42.8 954.4 Sephadex G-75 (1 mL) 0.04 32.5 813 37.3 2083.3
activity of N-SMase was completely inhibited in the presence of EDTA. Moreover, adding an excess amount of Mg2+could
restore the EDTA-inhibited N-SMase activity (data not shown).
Cations Effect on SMase ActiVity. Adding Mg2+, Mn2+,
or Zn2+ to the assay solution caused a dose-dependent
activation of N-SMase with maximal activities at 1600, 1100, and 400 units/mg of protein, respectively (Figure 3B). The Kmvalues for Mg2+, Mn2+, and Zn2+were 3, 2.5, and 2 mM,
respectively. This figure revealed that higher concentrations of Mn2+ and Zn2+ lost their ability to activate N-SMase.
Cu2+, Ca2+, and Ni2+did not stimulate the enzyme activity.
Moreover, combining Mn2+ or Zn2+ with Mg2+ did not
synergistically enhance the enzyme activity.
Substrate Specificity and Hemolytic ActiVity. Substrate gels each containing 0.1 g of SM, PS, PE, PC, and PI were
prepared and incubated with the purified bacterial N-SMase for 2 h as described in Materials and Methods. N-SMase of H. pylori exhibited a highly substrate specificity for SM and a low specificity for PE. Under the standard assay examined, PS, PI, and PC were not hydrolyzed by the purified enzyme to any appreciable extent. On the other hand, N-SMase from B. cereus showed a significant ability to hydrolyze substrates SM, PE, and PS. Purified N-SMase of H. pylori hemolyzed human red blood cells with 59% of hot lysis and 93.5% of hot-cold lysis after 120 min incubation (Table 2).
Immunological Characterization. According to Western blot analysis, antisera raised against purified N-SMase from H. pylori recognized the N-SMase and no other constituents FIGURE 2: (A) SDS-PAGE (10% polyacrylamide) of protein
profile from each step of H. pylori N-SMase purification and (B) pI determination of N-SMase by isoelectric focusing (IEF) elec-trophoresis. (A) Lane a, proteins from acid glycine extract; lane b, proteins from 80% saturated ammonium sulfate precipitated frac-tion; lane c, proteins from fraction 16 from CM-Sepharose chromatography (as shown in Figure 1A); lane d, proteins from fraction 20 from Mono Q chromatography (as shown in Figure 1B); lane e, proteins from fraction 9 from Sephadex G-75 chromatog-raphy (as shown in Figure 1C). Lane M denotes the positions and molecular masses (kDa) of standards. Analyses were performed as described in the Materials and Methods, and bands were visualized by Coomassie blue. (B) pI was measured with PhastGel IEF 3-9 media in a PhastSystem (Pharmacia LKB Biotechnology, Inc.) followed by Coomassie blue staining, and other conditions were described in the Materials and Methods. Lane L indicates purified N-SMase from H. pylori, lane S indicates purified N-SMase from B. cereus, lane M indicates pI calibration standards, and arrows indicate pI values of proteins.
FIGURE3: Effect of pH and cations on the N-SMase activity of H. pylori. (A) Activity measurements of 25µg of purified N-SMase were taken after 20 min incubation at 37°C with buffers ranging from pH 2 to 11 in the absence of EDTA (filled squares) or in the presence of 20 mM EDTA (filled diamonds). (B) N-SMase activity was measured in a standard enzyme assay by adding the indicated cations in an assay solution. The results are the means ( SEM of four separate experiments, each performed in at least triplicate.
Table 2: Hemolysis of Human Erythrocytes Induced by N-SMase of H. pylori time (min) 0 30 60 90 120 hot lysis (%) 2.3 4.9 10.6 35.6 59 hot-cold lysis (%) 2.3 26.7 31.9 63.3 93.5 negative controla(%) 0.5 0.5 3.0 5.0 5.3
aNegative control experiment was performed by adding
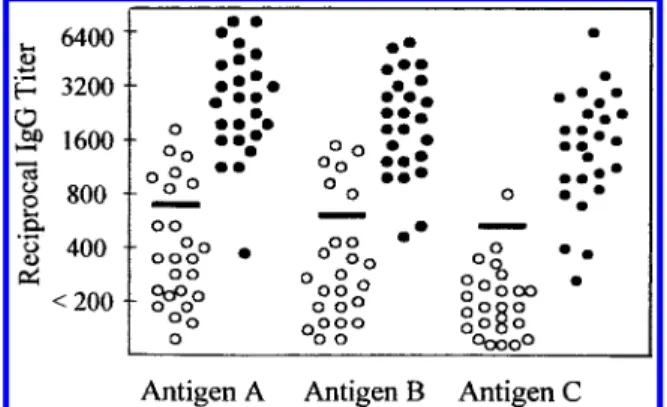
of acid glycine extract (Figure 4A). The antiserum also recognized the purified N-SMase from B. cereus, suggesting that N-SMases of H. pylori and B. cereus might be antigenically related. To investigate the serum antibody response to N-SMase in humans, the purified N-SMase was used as an antigen in the Western blot test. Ten of eleven sera from H. pylori-infected patients showed positive results, and all four sera from uninfected persons did not recognize the purified enzyme (Figure 4B). By ELISA using either the sonicated whole cells (antigen A), acid glycine extract (antigen B), or purified N-SMase (antigen C) as an antigen, the individual reciprocal IgG titers in the H. pylori-infected and uninfected humans are illustrated in Figure 5. Table 3 summarizes the outcome of the tests with 50 sera giving discrepant results with various antigens. Low numbers of false positive and false negative results of ELISA with a specificity of 96% and a sensitivity of 89% was observed when the purified H. pylori N-SMase was used. Although the crude antigens offered a better sensitivity than the purified N-SMase, there were unacceptably high numbers of false positive results of ELISA with a specificity of only 72% and a low predictive value of positive test.
DISCUSSION
Although phospholipases of H. pylori are regarded as pathogenic factors causing the ulceration of human gastric cells, the properties of those enzymes have not been thoroughly examined at a biochemical level owing to the lack of a purified enzyme. In this study, we first purified N-SMase from H. pylori and characterized it. The activity of N-SMase was present on the surface of H. pylori. Therefore, superficial proteins from acid glycine extract of whole H. pylori cells were purified sequentially with ammonium sulfate precipitation, CM-Sepharose, Mono-Q, and Sephadex G-75 column chromatography. The purifica-tion of N-SMase resulted in a greater than 2000-fold increase in specific activity. Although cell surface proteins of H. pylori could be extracted by various methods, acid glycine extract contained more H. pylori-specific proteins and was widely used as antigens in ELISA, (31, 33, 34). The mechanism of N-SMase released by acid glycine extraction is unknown. According to our results, the acid glycine extraction led to a decrease of less than 5% in the number of viable bacteria and produced a high yield (68%) of N-SMase. Thus, N-SMase appeared to be surface exposed, but not located at the cytoplasm of H. pylori. Hjelmeland postulated that integral membrane proteins elute in the void volume by a size-exclusion chromatography (35). Since our results did not detect any N-SMase activity in the void volume from FIGURE4: Immunoblot of H. pylori N-SMase and B. cereus SMase.
(A) After SDS-PAGE (in 10% acrylamide), proteins were elec-trotransferred to a nitrocellulose membrane, and then incubated with immune or preimmune rabbit serum. All primary antisera were diluted at 1:200, and the secondary antibody was alkaline phos-phatase-conjugated goat anti-rabbit serum. Lane a, acid glycine extract reacted with antiserum against whole cells of H. pylori; lane b, acid glycine extract reacted with antiserum against Sephadex 75 purified H. pylori N-SMase; lane c, purified N-SMase of H. pylori reacted with antiserum against Sephadex 75 purified H. pylori N-SMase; lane d, purified SMase of B. cereus reacted with antiserum against Sephadex 75 purified H. pylori N-SMase; lane e, purified SMase of H. pylori reacted with preimmune serum. (B) Western blot analysis for purified N-SMase antigen using sera from H. pylori-infected and uninfected persons. Each membrane strip contained 0.25µg of antigen, and a secondary antibody was goat anti-human IgG polyclonal antibody.
FIGURE5: Comparison of H. pylori antibody titers in human sera using three antigen preparations. Sera from 25 H. pylori-infected patients (filled circles) and from 25 uninfected persons (open circles) were incubated with one of three antigen preparations in an enzyme-linked immunosorbent assay as described in the Materials and Methods. Antigen preparations were A, sonicates of four H. pylori strains; B, acid glycine extract from H. pylori K8; and C, purified N-SMase from Sephadex G-75 column. (s) 3 SD exceeding mean titer of sera from H. pylori uninfected persons.
Table 3: Specificity, Sensitivity, and Predictive Values of H. pyloir-IgG ELISAs with Different Antigen Preparationsa
antigen whole cells acid glycine extract purified N-SMase sensitivity (%) 96 92 89 specificity (%) 72 76 96
positive predictive value (%) 77 79 96
negative predictive value (%) 95 90 89
efficiency of test (%) 84 84 92
aSensitivity ) positivity in disease; specificity ) negativity in health;
predictive value of positive test ) % of patients with positive test results who are diseased; predictive value of negative test ) % of patients with negative test results who are nondiseased; efficiency of test ) % of patients correctly classified as diseased and nondiseased.
the size-exclusion chromatography, the enzyme is unlikely an integral component of the outer membrane of H. pylori. The acid glycine extract of bacteria was not expected to either include integral membrane proteins or be contaminated with lipids, lipopolysaccharide, and nucleic acids. Because thor-ough disruption of bacterial cells prior to isolation of H. pylori N-SMase was unnecessary, we avoid a complicated purification process as reported elsewhere for other mam-malian SMases (36, 37).
The water-soluble property and without a hydrophobic chromatography step leading to a successful purification indicate that N-SMase of H. pylori is hydrophilic. This feature differs from that of the mammalian SMase as a highly hydrophobic, lipid-conjugated, integral membrane enzyme as described in a previous study (36, 37). A single band migrating at 32 kDa in SDS-PAGE and N-SMase activity eluted from the size-exclusion HPLC column being associ-ated with a protein of molecular mass of 32 kDa suggest that the native N-SMase of H. pylori consists of only one polypeptide. The purified enzyme exhibited an optimal activity at pH 7.4 and was completely inactivated at a pH range less than 4. This feature suggests H. pylori N-SMase cannot function in the stomach to damage the epithelial layer of gastric mucosa. However, the local pH neutral environ-ment created by the superficial urease of H. pylori may restore N-SMase activity, and subsequently, be a potential virulence factor. Thus, the surface-exposed N-SMase of H. pylori may be considered as a cytolytic factor of the gastroduodenal mucosa.
To avoid the radiometric procedure, we adopted a spec-trophotometric method described by Gatt et al. (25) to determine N-SMase activity. A colored derivative SM compound, TNPAL-SM, was used as a substrate, in which its rate of hydrolysis is the same order of magnitude as that of radioactive-labeled SM (data not shown). The Vmaxand
Kmdetermined for purified N-SMase (15.6 nmol of
TNPAL-sphingosine/h/mg of protein, and 6.7 µM, respectively) resembled that determined for whole cell lysate. The low Km of H. pylori N-SMase suggests that the enzyme can
efficiently function at the micromolar concentrations of SM present in the human gastric epithelial membrane. The catalytic action of N-SMase on sphingomyelin was stimulated in the presence of Mg2+, Mn2+, or Zn2+. The activating effect
of those cations may be explained by their ability to alter the physical state of the sphingomyelin micelles to be favorable for the attack of N-SMase of H. pylori (38).
Results in this study demonstrate that N-SMase of H. pylori is a hot-cold hemolysin, which has similar character-istics to those of the β-hemolysin from S. aureus and B.
cereus. β-Hemolysin is cytotoxic for a variety of tissue culture cells, and large dosages are toxic for experimental animals (20, 39). Our laboratory is currently examining the mode of lytic action on human gastric cells of this enzyme. Several studies have confirmed that exogenously adding SMase of B. cereus to different cultured cells could activate the sphingomyelin pathway and its subsequent signaling events causing cellular apoptosis (40-44). On the other hand, a conflicting study indicated that bacterial SMase did not incur apoptosis and death of the monocytic leukemia cell line (45). The disagreement of the SMase action on cellular signaling events may be attributed to either various responses of different cell types or differential properties of SMase from
different bacteria. Further investigation is required to deter-mine whether H. pylori SMase plays an important role in inducing a sphingomyelin signaling pathway and an apoptosis of gastric cells.
According to Western blot analysis, antiserum raised by the purified N-SMase from H. pylori also recognized SMase from B. cereus. This finding strongly suggests that N-SMases produced by both B. cereus and H. pylori share common antigenic epitopes, although we have not yet identified the epitope regions. We also found that antiserum against the purified N-SMase from H. pylori did not recognize any antigen preparation from Escherichia coli, Pseudomonas aeruginosa, or Staphylococcus aureus species (data not shown). This result indicates that either N-SMase is not present in those species or there is different antigenicity of N-SMase from those species. Currently, we are identifying the N-SMase gene by employing antibody screening onto the H. pylori genomic library. Once the complete gene encoding N-SMase is sequenced, more characteristics of this enzyme can be learned by comparing its amino acid sequence with that of other sources.
Sera from H. pylori-infected patients, but not uninfected ones, recognized the purified N-SMase. The presence of antibodies to N-SMase in sera from H. pylori-infected persons confirms that the N-SMase of H. pylori is produced in vivo. This observation also implies that the N-SMase purified in this study is not a contaminant from the bacterial growth medium. Interestingly, according to our results, sera from patients with severe gastric ulcer diseases appeared to have higher antibody titers to N-SMase than that from patients with mild symptoms. However, the sera set was insufficiently large for us to draw this conclusion. Whole cell, partially purified, and purified proteins are three categories of antigens commonly used for serodiagnosis of H. pylori infection. Whole cell lysate prepared from pooled strains of H. pylori possess the theoretical advantage of exposing a maximum number of surface antigens. This situation seems to be desirable in view of the extremely variable immune response of H. pylori; however, it also increases the risks of nonspecific binding of immunoglobu-lins and cross-reaction with other Campylobacter-like species. Indeed, a sensitivity of 96% could be obtained when using a whole cell antigen (preparation A). However, there were high numbers of false positive results of ELISA with a specificity of 72%. In screening for diseases, the positive predictive value is of utmost importance. The predictive value of a positive test result indicates the frequency of diseased patients in all patients with positive test results, and the formula for calculating predictive value is presented as (true positives)/(true positives + false positives). On the basis of this formula, it can readily be seen that the higher false positive results (low specificity) will cause the lower predictive value. A diagnostic test with a low predictive value (low specificity) frequently fails as a screening test when the decrease occurs with a high prevalence. In comparison to the whole cell antigen, using the purified N-SMase as an antigen slightly decreased the sensitivity, but significantly increased the specificity. The purified N-SMase revealed an acceptable discriminative power between positive and nega-tive sera. The purified N-SMase indicated a higher predicnega-tive value of the positive result and a higher efficiency of test than the whole cell lysate. A distinct advantage of purified
antigen is its better definition and possibility of standardiza-tion. Further studies in much larger sets of different sera are needed to evaluate the purified N-SMase when diagnosing H. pylori infection.
In conclusion, N-SMase of H. pylori consists of one polypeptide with an approximate molecular mass of 32 kDa and requires divalent cations to optimally hydrolyze SM at pH 7.4. N-SMase of H. pylori is water-soluble and present in bacterial superficial components and is produced under both in vitro and in vivo conditions. N-SMase of H. pylori is a hot-cold hemolysin to human red blood cells, and it specifically hydrolyzes SM but not other phospholipid. Using the purified N-SMase as an antigen in serologic test allows us to detect the specific antibodies against H. pylori with fairly high sensitivity and specificity. The identification of the gene encoding N-SMase from H. pylori is undertaking. Future study of the surface exposed N-SMase of H. pylori on the gastric cell-lines may provide further insight into the pathogenic factors of H. pylori-associated ulcer diseases.
ACKNOWLEDGMENT
We thank Dr. Ning Lee of the Chang Gung Memorial Hospital for offering H. pylori isolates and performing bacterial culturing, urease tests, and histology.
REFERENCES
1. Goodwin, C. S., Armstrong, J. A., Chilvers, T., Peters, M., Collins, M. D., Sly, L., McConnel, W., and Harper, W. E. S. (1989) Int. J. Syst. Bacteriol. 39, 397-405.
2. Price, A. B., Levi, J., Dolby, J. M., Duncombe, P. L., Smith, A., Clark, J., and Stephenson, M. L. (1985) Gut 26, 1183-1888.
3. Buck, G. E., Gourely, E. G., Lee, W. K., Subramanyam, K., Latimer, L. M., and Dinuzzo, R. (1986) J. Infect. Dis. 153, 664-669.
4. Anderson, L. P., Holck, S., Prulsen, C. C., Elsborg, L., and Justensen, T. (1987) Scand. J. Gastroenterol. 22, 219-224. 5. Leunk, R. D., Johnson, P. T., David, B. C., Kraft, W. G., and
Morgan, R. D. (1987) J. Med. Microbiol. 26, 93-99. 6. Marshall, B. J. (1988) Scand. J. Gastroenterol. 23 (Suppl.),
58-66.
7. Forman, D., Newell, D. G., Fullerton, F., Yarnell, J. W. G., Stacey, A. R., Wald, N., and Sitas, F. (1991) Br. Med. J. 302, 1302-1305.
8. Blaser, M. L. (1993) Trends Microbiol. 1, 255-260. 9. Hill, B. A. (1985) Am. J. Physiol. 249, G342-G349. 10. Slomiany, B. L., Kasinathan, C., and Slomiany, A. (1989) Am.
J. Gastroenterol. 84, 1273-1277.
11. Mauch, F. G., Ditschuneit, H., and Malfertheiner, P. (1993)
Gastroenterology 105, 1698-1704.
12. Goggin, P. M., Marrero, J. M., Spychal, R. T., Jackson, P. A., and Corbishley, C. M. (1992) Gastroenterology 103, 1486-1490.
13. Wallage, J. L. (1990) Am. J. Physiol. 258, G1-G11.
14. Lin, Y. L., Liu, J. S., Chen, K. T., Chen, C. T., and Chan, E. C. (1998) FEBS Lett. 423, 249-253.
15. Langton, S. R., and Cesareo, S. D. (1992) J. Clin. Pathol. 45, 221-224.
16. Czinn, S., Carr, H., and Yang, P. (1990) Gastroenterology 98, A35.
17. Brockerhoff, H., and Jensen, R. G. (1974) Lipolytic Enzymes, pp 35-59, Academic Press, New York.
18. Geoffroy, C., and Alouf, J. E. (1984) Bacterial toxins and cell
membranes, pp 241-243, Academic Press, London.
19. Smythe, C. J., and Duncan, J. L. (1978) Bacterial toxins and
cell membranes, pp 129-183, Academic Press, London.
20. Arbuthmott, J. P. (1982) Molecular Action of Toxins and
Viruses: Bacterial cytolysis, pp 107-129, Elsevier Biomedical
press, New York.
21. Kolesnick, R., and Golde, D. W. (1994) Cell 77, 325-328. 22. Okazaki, T., Bell, R. M., and Hannun, Y. A. (1989) J. Biol.
Chem. 264, 19076-19080.
23. Hannun, Y A (1994) J. Biol. Chem. 269, 3125-3128. 24. Lin, Y. L., and Chan, E. C. (1996) J. Clin. Pathol. 49, 1-3. 25. Gatt, S., Dinur, T., and Barenholz, Y. (1978) Biochim. Biophys.
Acta 530, 503-507.
26. Dole, V. P. (1956) J. Clin. InVest. 35, 350-357.
27. Lenchner, M., Kupe, T., Stefanovic, S., and Gotz, F. (1989)
Mol. Microbiol. 3, 621-626.
28. Laemmli, U. K. (1970) Nature 227, 680-685.
29. Cover, T. L., and Blaser, M. J. (1992) J. Biol. Chem. 267, 10570-10575.
30. Von Wulffen, H., Heesemann, J., Butzow, G. H., Loning, T., and Laufs, R. (1986) J. Clin. Microbiol. 24, 716-720. 31. Hirschl, A. M., Rathbone, B. J., Wyatt, J. I., Berger, J., and
Rotter, M. L. (1990) J. Clin. Pathol. 43, 511-513. 32. Wilkinson, G. N. (1961) Biochem. J. 80, 324-332. 33. Logan, S. M., and Trust, T. J. (1983) Infect. Immun. 42,
675-682.
34. Guruge, J. L., Schalen, C., Nilsson, I., Ljungh, A., Tyszkiewicz, T., Wikander, M., and Wadstrom, T. (1990) Scand. J. Infect.
Dis. 22, 457-465.
35. Hjelmeland, L. M., and Chrambach, A. (1984) Methods
Enzymol. 104, 305-318.
36. Liu, B., Hassler, D. F., Smith, G. K., Weaver, K., and Hannun, Y. A. (1998) J. Biol. Chem. 273, 34472-34479.
37. Kurth, J., and Stoffel, W. (1991) Biol. Chem. Hoppe-Seyler
372, 215-223.
38. Beil, W., Bierbaum, S., and Sewing, S. F. (1993)
Pharmacol-ogy 47, 141-144.
39. Wadstrom, T., and Mollby, R. (1971) Biochim. Biophys. Acta
242, 288-307.
40. Ji, L., Zhang, G., and Hirabayashi, Y. (1995) Biochem.
Biophys. Res. Commun. 215, 489-496.
41. Raines, M. A., Kolesnick, R. N., and Golde, D. W. (1993) J.
Biol. Chem. 268, 14572-14575.
42. Sasaki, T., Hazeki, K., Hazeki, O., Ul, M., and Katada, T. (1995) Biochem. J. 311, 829-834.
43. Tamura, H., Noto, M., Kinoshita, K., Ohkuma, S., and Ikezawa, H. (1994) Toxicon 32, 629-633.
44. Santana, P., Lanes, L., Hernandez, I., Gallardo, G., Quintana, J., Gonzalez, J., Estevez, F., De Galarreta, C. R., and Fanjul, L. F. (1995) Endocrinology 136, 2345-2348.
45. Zhang, P., Liu, B., Fenkins, G. M., Hannun, Y. A., and Oveid, L. M. J. Biol. Chem. 272, 9609-9612.