ELSEVIER PII: S0032-3861 (96)01040-3
Printed in Great Britain. All rights reserved 0032-3861/97/$17.00 + 0.00
Reactive compatibilization of polymer
blends of poly(butylene terephthalate) and
polyamide 6,6: 2. Morphological and
mechanical properties
Chieh-Chin Huang and Feng-Chih Chang*
Institute of Applied Chemistry, National Chiao-Tung University. Hsin-chu, Taiwan,
Republic of China
(Received 9 April 1996; revised 31 October 1996)
Bisphenol-A type solid epoxy resin has been demonstrated as a low cost and efficient compatibilizer for the immiscible and incompatible blends of polyamide 6,6 (PA66) and poly(butylene terephthalate) (PBT). The phase domains of the compatibilized blends have been reduced, while their mechanical properties substantially improved. A fraction of this difunctional epoxy resin is able to form in situ the PBT-co-Epoxy- co-PA66 mixed copolymer at the interface. This mixed copolymer with both segments structurally identical to both base polymers will anchor along the interface, and functions as an effective compatibilizer for the PA66/PBT blends. The mechanism of the mixed copolymer formation has been discussed in detail. The mechanical property improvement of the PA66-rich blends is more significant than of the PBT-rich blends after compatibilization. The nature of the reactive compatibilization tends to leave a greater fraction of the compatibilizer in the PBT phase than in the PA66 phase. Therefore, the intrinsic properties of the PBT matrix have been reduced after compatibilization. This is why the improvement in properties of the PBT-rich blends are not as great as expected. Different types of core-shell elastomers have also been employed to toughen the PA66/PBT blends. © 1997 Elsevier Science Ltd.
(Keywords: reactive compatibilizer; polyamide; PBT)
INTRODUCTION
Polymer blending from existing polymers is the most rapid and economical route to creating new materials with greater versatility and flexibility than the develop- ment of new polymers. However, most randomly chosen polymer pairs are immiscible and incompatible, and result in products with inferior properties than the average of the base polymers. In order to overcome the problems of incompatible polymer blends, methods and technologies on how to improve the compatibility have evolved. Research activity on compatibilization has grown at an exponential rate during the last two decades, and has been the subject of a few recent reviews 1-6.
The major role the compatibilizer plays is to reduce the interfacial tension in the melt, by causing an emulsifying effect and leading to finer domains of the resultant blend. Relative to the uncompatibilized blend, the compatibi- lized blend tends to widen the thickness of the interphase, and therefore improves the adhesion at phase bound- aries. Compatibilization can also stabilize the dispersed phase against coalescence during thermal annealing.
Earlier studies on compatibilization concentrated on a non-reactive type, where the compatibilizers employed were mainly the block or graft copolymers possessing segments structurally similar or miscible with the blending constituents. Since the maleic anhydride functional group
* To w h o m correspondence should be addressed
was discovered to be capable of reacting with amine endgroups of polyamides in situ, numerous compatibilized polyamide/polyolefin commercial products have been developed. It also has stimulated great interest in the area of reactive type compatibilization. The trend dearly shows that reactive compatibilization has become the mainstream in compatibilizing incompatible polymer pairs during the last few years 6.
The mechanism of a reactive compatibilization system is relatively more complex than the corresponding non- reactive counterpart. Unlike the non-reactive copolymer compatibilizer, a so-called reactive compatibilizer is itself not considered to be a phase compatibilizer of the blend. It serves only as a precursor of the finally formed copolymer compatibilizer. The eventual in situ-formed copolymer possessing segments structurally identical or chemically miscible with the respective base polymers is the major contributor in compatibilizing the incom- patible blend. The final properties of the reactively compatibilized blend depends upon the structure and distribution of the in situ-formed copolymer.
From practical and economical points of view, a com- patibilizer readily available at low cost is the obvious first choice. A few readily available polymers are miscible or nearly miscible with certain selected other polymers, and are therefore, more or less, able to function as compatibilizers for those polymer pairs. For this reason, polycaprolactone (PCL) has been used to compatibilize
7
polycarbonate (PC)/phenoxy, PC/styrene-co-acrylorfitrile
(SAN) 8 and PC/polyamide 6,6 (PA6) 9 blends. For the same reason, poly(methyl methacrylate) (PMMA) can act as a compatibilizer for blends of styrene-co-maleic anhydride (SMA)/SAN 1° and poly(vinylidene difluoride) (PVFz)/SAN 1°. Basically the compatibilization effect from the above-mentioned polymers is considered to be non- reactive, although interchange reaction may occur. Bisphenol-A type solid epoxy resin possessing two epoxide endgroups can function as a reactive compatibilizer for the PC/PA blends 11 .
Polyamide 6,6 (PA66) and poly(butylene terephtha- late) (PBT) are both high volume commercial polymeric products. Blends from PA66 and PBT should be the ideal choice to create new polymeric materials possessing certain unique properties from the base polymers. However, poor compatibility between these two classes of polymers prevents them from creating any useful product. Solid epoxy resin can, for several reasons, be considered as a potential compatibilizer for the PA66/ PBT blends. The epoxide endgroups are able to react with endgroups of PA66 and PBT to form, in situ, a
certain amount of the desired PBT-co-epoxy-co-PA66
mixed copolymers at the melt. Additionally, the chemical structure of the Epoxy resin segment is miscible or partially miscible with PBT 12, and therefore the in situ-
formed epoxy-co-PA66 or PA66-co-epoxy-co-PA66
block copolymer is also expected to be a compatibilizer for the PA66/PBT blends. This study has demonstrated that this solid epoxy resin is indeed an excellent reactive compatibilizer for the PA66/PBT blends. The chemistry, compatibilizing mechanism, rheologies and thermal properties were reported in a previous paper 13. This paper will further discuss the effect of compatibilization on the resultant morphological and mechanical properties.
EXPERIMENTAL
P66, Zytl 101L, was purchased from the Du Pont. PBT-, D-201, is a nature grade product from Shinkong Synthetic Fibers of Taiwan. Solid-state bisphenol A type epoxy resin, NPES-909, has the epoxy equivalent weight of 2060 g/equiv, that was obtained from Nan Ya Plastics of Taiwan.
Melt blending with desired component ratios were carried out using a 30-mm twin-screw co-rotating extruder. The extruded pellets were dried in an oven at 100°C for at least 24 h, and then injection moulded into standard 1/8-inch-thick ASTM specimens, using an Arburg 3-oz injection moulding machine. The detailed processing conditions for the extruder blending and 13 injection moulding were reported in a previous paper . Morphologies of the cryogenically fractured surfaces were examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) on the plane perpendicular to the injection flow direction at a region between central line and the skin. For the PBT- dominant blends, the PA66 phase was etched out by formic acid followed by coating with gold prior to the SEM examination.
Two different types of core-shell elastomers, KCA- 102 from the Kureha Chemical Co. of Japan and EXL-3386 from Rohm & Hass Co. were employed as impact modifiers of the PA66/PBT blends. KCA-102 has a polybutadiene core and PMMA shell structure, while EXL-3386 has a similar structure except that the shell contains a small amount of acrylic acid functional group.
In order to identify the elastomer phase distribution in the blends, the microtomed thin-layer specimens were stained with 2% OsO4 solution and examined by TEM. Notched Izod impact tests were carried out at ambient conditions according to ASTM-D256 standard. Standard tensile tests (ASTM-D638) were carried out at a cross- head speed of 50mmmin -~. The procedures of the previously developed method to determine impact critical strain energy release rate (Go) were carried out at ambient conditions by varying the depth of the notch 14'15.
RESULTS AND DISCUSSION
Morphologies
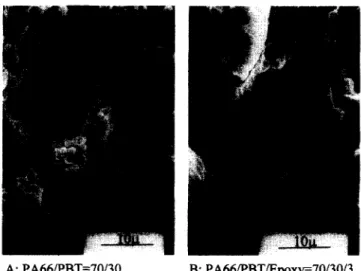
Figure 1 gives the non-etched SEM micrographs of the
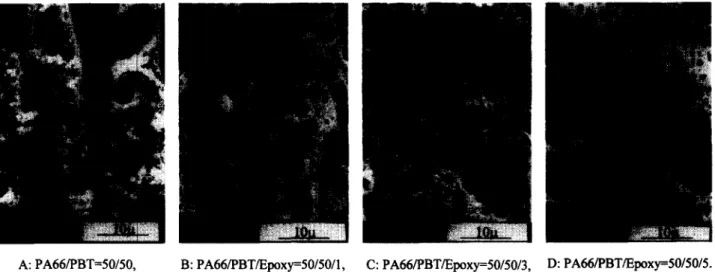
uncompatibilized and compatibilized PA66/PBT = 70/30 blends. The average size of the dispersed PBT spherical particles of the uncompatibilized blend is approximately 1 2 #m, while the phase contrast disappears from the compatibilized blend. Figure 2 illustrates the effect of the compatibilizer quantity on the resultant phase morphol- ogies of the PA66/PBT = 50/50 blends. The uncompati- bilized blend (Figure 2A) is co-continuous, showing evidence of mutual inclusions. The PA66 component becomes the dispersed phase in all the compatibilized blends, while the size of the dispersed particles decreases with increasing compatibilizer quantity (Figures 2B-2D).
Pure PA66 is slightly more viscous than that of the pure PBT as mentioned earlier 13. The shift from a co- continuous morphology into the dispersed PA66 mor- phology after compatibilization is probably due to further increase of the viscosity ratio of
T/pA/~PBT
after compa- tibilization. The continuous decline on the domain size of the dispersed particles up to 5 phr epozy resin indicates that the in situ-formed PBT-co-epoxy-co-PA66 copoly- mers at the interface have not yet reached the interfacial saturation limit. As mentioned before 13, only a small fraction of the original epoxy is able to produce the desirable PBT-eo-epoxy-co-PA66 mixed copolymer at the interface. Therefore, this PBT-co-epoxy-co-PA66 should be highly efficient in compatibilizing the PA66/PBT blends. A substantial fraction of the original epoxy tends to form the epoxy-co-PBT or PBT-co-epoxy-co-A: PA66/PBT=70/30, B: PA66/PBT/Epoxy=70/30/3. Figure 1 SEM micrographs of uncompatibilized and compatibilized blends. (A) PA66/PBT = 70/30. (B) PA66/PBT/epoxy = 70/30/3
PBT copolymer within the PBT phase, because the epoxy is first dissolved in the PBT phase and has the first opportunity to contact and react with PBT during melt blending. Formation of epoxy-co-PA66 or PA66-co- epoxy-co-PA66 copolymer is also possible, but its quantity should be negligible.
Figure 3
shows the SEM micrographs of the uncompati- bilized and compatibilized PA66/PBT = 30/70 blends. A similar trend has also been observed that shows the size decrease of the dispersed domains by increasing the compatibilizer quantity.The above morphological information clearly demon- strates that this epoxy resin does indeed act as an effective emulsifying agent of the PA66/PBT blends, by reducing the interfacial tension at the melt and the resultant domain size. Whether this epoxy resin can also serve as a mechanical compatibilizer will be answered later, based on the resulting mechanical properties.
Figure 4
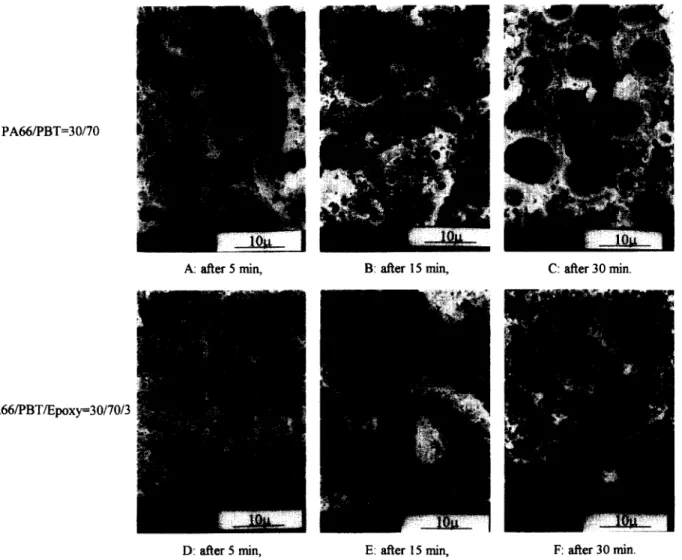
illustrates the effect of annealing time at 200°C on the phase stability against coalescence of the dispersed PA66 phase of the uncompatibilized and compatibilized PA66/PBT = 30/70 blends. Coalescence of the dispersed phase with the annealing time of the uncompatibilized blend is evident by comparingFigures
3A, 4A, 4B
and4C.
On the contrary, there is no clear sign of phase coarsing from the compatibilized blend (compareFigures 3C, 4D, 4E
and4F).
Figure 5
shows the TEM micrographs of the elastomer- modified PA66/PBT = 30/70 blends. Two different types of elastomers were employed in this study. The KCA-102 is a core-shell type elastomer with a PMMA shell, while the EXL-3386 has a small fraction of the shell in acid form. It is not clear why the domain size of the dispersed PA66 phase of the KCA-102 elastomer-modified blend is smaller than that of the EXL-3386 elastomer-modified blend under identical processing conditions.Figure 5A
shows that the KCA-102 elastomer particles are distrib- uted mainly in the PBT phase, as would be expected. The EXL-3386 elastomer particles reside mostly along the interface with small fraction in either PBT or PA66 phase. These acid-containing elastomer particles (EXL-3386) are expected to be distributed firstly in the PBT phase during the earlier stages of melt blending, because the shell structure (mainly PMMA) of the elastomer is more compatible with PBT than with PA66. Additionally, PBT has a lower melting temperature than that of PA66. The acid functional group of the elastomer has the chance to make contact and react with the amine endgroup of
A: PA66/PBT=50/50, B: PA66/PBT/Epoxy=50/50/1, C: PA66/PBT/Epoxy=50/50/3, D: PA66/PBT/Epoxy=50/50/5. Figure 2 SEM micrographs of uncompatibilized and compatibilized blends. (A) PA66/PBT = 50/50. (B) PA66/PBT/epoxy = 50/50/1. (C) PA66/ PBT/epoxy = 50/50/3. (D) PA66/PBT/epoxy = 50/50/5
A: PA66/PBT=30/70, B: PA66/PBT/Epoxy=30/70/1 C: PA66/PBT/Epoxy=30/70/3, D: PA66/PBT/Epoxy=30/70/5. Figure 3 SEM micrographs of uncompatibilized and compatibilized blends. (A) PA66/PBT = 30/70. (B) PA66/PBT/epoxy = 30/70/1. (C) PA66/ PBT/epoxy = 30/70/3. (D) PA66/PBT/epoxy = 30/70/5
PA66/PBT=30/70
A: after 5 rain, B: atter 15 min, C: after 30 min.
PA66/PBT/Epoxy=30/70/3
D: after 5 min, E: after 15 min, F: atter 30 min.
Figure 4 SEM micrographs of uncompatibilized and compatibilized specimens, after annealing at 200°C. (A) PA66/PBT = 30/70 after 5 min. (B) P A 6 6 / P B T = 3 0 / 7 0 after 15min. (C) P A 6 6 / P B T = 3 0 / 7 0 after 30min. (D) P A 6 6 / P B T / e p o x y - 3 0 / 7 0 / 3 after 5min. (E) PA66/ PBT/epoxy = 30/70/3 after 15 min. (F) PA66/PBT/epoxy = 30/70/3 after 30 min
A: PA66/PBT/KCA-102=30/70/10, B: PA66/PBT/EXL-3386=30/70/10. Figure 5 TEM micrographs of elastomer-modified blends. (A) PA66/ PBT/KCA-102 = 30/70/10. (B) PA66/PBT/EXL-3386 = 30/70/10
PA66 to form various PA66-bonded elastomers at inter- face during the later stages o f melt mixing. The portions of these elastomer particles may not have the chance to react with PA66 that tend to remain in the PBT phase. Those excessively reacted PA66-bonded elastomer particles tend to reside in the PA66 phase. However, most o f the elastomer particles are only lightly reacted that tend to reside along the interface. It appears that the phase
distribution of these acid functionalized elastomer parti- cles depends on the extents of the interfacial reactions between the acid groups of the elastomer with the amine endgroups of PA66. An extended time of melt mixing should result in more elastomer particles in the PA66 phase. Under processing conditions employed in this study, most of the elastomer particles are only slightly reacted, and therefore the elastomer particles are mainly distributed at the interface.
Elastomer phase distribution in a binary blend is a very important factor in dictating the toughness of the
16
resulted blend . Toughness o f these elastomer-modified blends will be discussed later.
Mechanical properties
We can roughly divide the material mechanical properties into two classes, strength and toughness. Tensile strength and modulus can be considered as the material strength, while tensile elongation and impact fracture energy are the material toughness. Modification o f a polymeric material usually results in improving one property, but adversely affects the other. It is relatively rare to have both properties enhanced simultaneously in any type o f polymer modification. Elastomer-toughened polymers usually improve their toughness at the cost of lower strength. On the contrary, mineral-filled polymers usually increase their strength, but decrease their toughness. This general trend has also been frequently
observed in many compatibilized blends, relative to their uncompatibilized counterparts. Both strength and toughness have been found to be improved in this compatibilized PA66/PBT system. Figure 6 shows the
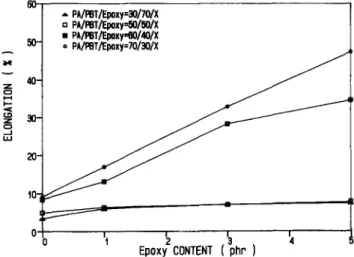
effect of the compatibilizer quantity on the tensile strength of the PA66/PBT blends. The presence of 1 phr epoxy resin is enough to approach the maximum achievable tensile strength improvement. The trend in tensile modulus is similar to the tensile strength, as shown in Figure 7. The results of the tensile elongation
shown in Figure 8 are somewhat more complicated.
Compatibilization shows little or no improvement at all on tensile elongation from those blends in which the PA66 is the minor dispersed phase. Those blends having the PA66 as the major continuous phase show tensile elongation improvement with increase of compatibilizer quantity. Figure 9 shows the effect of compatibilizer on
the impact strength of the blends. Again, the improve- ment of the impact strength achieved is more substantial for PA66-rich blends than for PBT-rich blends. It also needs only 1 phr of the compatibilizer in order to approach the maximum achievable improvement.
The question arises why the compatibilization is effective on the blend toughness (elongation and impact strength) only for PA66-rich blends, even though both PA66 and PBT matrices are very tough individually. An effective compatibilizer can increase the
interfacial adhesion of a blend, and consequently also improves its mechanical properties. Most literature concentrates on the interfacial property improvement for a compatibilized blend relative to that of the uncompatibilized counterpart, but fails to address the change of matrix intrinsic properties by the compati- bilizer. A fraction of the compatibilizer (reacted or unreacted), more or less, is expected to be dissolved in both matrices at a molecular level, which will certainly change the individual intrinsic properties of the blend components. This may increase or decrease the matrix intrinsic properties depending on the system. Styrene- maleic anhydride copolymer (SMA) in PA66 is known to be detrimental and causes toughness reduction of SMA compatibilized PA66/PS blends 17. In the case of a detrimental effect, the compatibilized blend may improve or deteriorate in mechanical properties, depending on the competition between the advantages due to better adhesion" (and dispersity), and the disadvantages dUel7 to the loss of inherent toughness of blend components . In this epoxy reactively compatibilized PA66/PBT system, a substantially greater fraction of the added epoxy resin is dissolved in the PBT than in the PA66. The epoxy resin is more compatible with PBT than with PA66 and PBT also has a lower melting temperature than PA66. Not all of the epoxy initially dissolved or distributed in the PBT phase has the chance to make contact and react with
60 55-
i°-
i ,o-
=;
zo
o
,,= PA/~BT/~oxy=20~O/X - PA/PBT/F.poxy=5OJSO/X ,'I p/~PBT~:I~Xy=EO/40/X • PMRBT/Epoxy=70/20/X'1
EPOXY
2 CONTENT (~hr }
14
Figure 6 Effect of compatibilizer content on tensile strength
60 5O- 40-
so-
o
uJ 20- 10- 0 ,,. PA/RBT/Epoxy=30/70/X o pA/PBT/F.poxy =50150/X • PM'RBT/~.poxy=BO/40/X * PA/PBT/Epoxy=70/30/X i'1
'2
( 3hrp )
'4
Epoxy CONTENTFigure 8 Effect of compatibilizer content on tensile elongation
2800 2800- i 27~)- LU ..J z ~ o - p-. 2 4 0 ) Figure 7 -,, PA/PBT/Epoxy:20/70/X n PAIPBT/Epoxy=50/5OIX • PA/PBTIEpoxy=8OI40/X , PA/PBT/Epoxy=70/30/X
',
'2
3'
'4
EPOXY CONTENT [phr }Effect of compatibilizer content on tensile modulus
_I i
- PA/PBT=30/70 -, pA/Per-5o/5o o PklPST=60/40 • Pk/PBT=70120 n 4O-iJ
' ' 'd 0 1 EPOX2Y CONTENT ~phr IFigure 9 Effect of compatibilizer on notched impact strength
PA66 at the interface. Epoxy resin residing in the PA66 phase is therefore substantially less likely. The presence of epoxy resin in the PBT phase, either in its unreacted form or as in situ-formed copolymers, may cause the
inherent properties of the matrix PBT to deteriorate. This is probably why the mechanical properties of the PBT-dominate blends show little or no improvement at all. The expected property improvement due to the enhancement of the interfacial adhesion may be partially offset by the deterioration of the matrix intrinsic prop- erties. The major component matrix of a blend normally dictates the final properties of the blend, while the dispersed phase plays a less important role. In PA66-rich blends, the originally tough PA66 phase is more or less totally uncontaminated with epoxy resin, and its intrinsic properties are less affected. This is probably why the observed mechanical property improvements of those PA66-rich blends are more substantial.
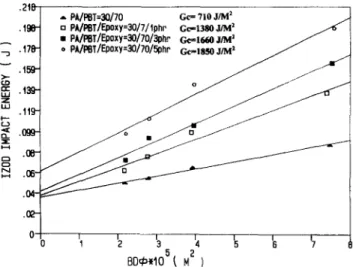
Figure 10 gives the plots of BDO vs impact energy of the
uncompatibilized and compatibilized PA66/PBT=30/70 blends. B is the specimen thickness, D is the unnotched width and ~b is the geometrical factor ~4. The slope of the regression line is the impact critical strain energy release rate (Go). Figure 10 clearly shows the progressive improve-
ment of Gc caused by increasing the amount of this epoxy compatibilizer.
This epoxy resin has been demonstrated to be an effective emulsifier (based on morphologies) and also an efficient mechanical compatibilizer (based on mechanical properties) for PA66/PBT blends. Additionally, it has also been demonstrated to stabilize the dispersed phase from being coalesced during thermal annealing.
Elastomer toughening
Figure 11 illustrates the effect of elastomer-modification
on the resultant tensile strength. It is quite interesting to notice that the tensile strength improvement caused by the presence of the elastomer and compatibilizer occurs only in those PBT-rich blends. This result is due to the increase in tensile elongation, because the corresponding uncom- patibilized and unmodified blend is very brittle with extremely low tensile elongation. For those PA66-rich and uncompatibilized blends, they possess high elongation with their tensile elongation already exceeding their yield strains. Addition of a soft elastomer in any polymeric
A ,,=, .210 .196- • 178- . I ~ .13&- .11~ .099- . ~ - .04- 0 .
PA/PST=30/70
Gc = 710 J/M ~ a PklPSTIEpoxy=3OI7/tphr Gc.=1380J~ ~• PA/PST/F-poxy=30/70/3phn Ge==It/aO JIM 2
*PA/l~T/Ep0xy=30/70/5phn
G c , , . , 8 . ~ j ~ ~ u j /o
BD¢),105~
M z )Figure l0 Plots of impact energy vs BDdJ, to determine Gc for the
uncompatibilized and compatibilized PA66/PBT = 30/70 blends
material usually decreases its yield stress. Therefore, the addition of an elastomer into the blends results in a slight decrease of their tensile strength (actually the yield stress), that is not unexpected. Figure 12 shows the overall
decrease in tensile modulus of the elastomer-modified blends, as would be expected. Figure 13 illustrates the
effect on tensile elongation, where the PBT-rich blends again show only minor improvement (from 4 to 8%) after
60 3O- 60 Figure l l .= PW/PST/Em~ ~=~'/toz 1 o p t ¢ ~ , ,o
Effect of elastomer on tensile strength
m P ~ ~ N I S • P ~ = e T / a ~ am'
- - = P A ~ T / I ~ am'/to2 1o pw . j
g
Figure 12 Effect o f elastomer on tensile m o d u l u s
• p ~ r ataes • PI/PST/~(]O(Y 8plir = P ~ T / 8 ~ Y apt¢/l~ lo W / * 2O" t0- 0
Figure 13 Effect o f elastomer on tensile elongation
WO ~ t60-
i"
t20- 0o ~'OT eta=
/'
• ~/spm 3p~r/mz t~
/
,, PA/m/r=po~
~
Figure 14 Effect of elastomer on notched impact strength
- 3 > - LO r ' r " la.J Q . .479- .419- .35g- . 2 ~ - .239- .t7g- . t 2 - .06- 0 Figure 15 rate (Go) • ,,. PA/PBT--.30/70 Ge= 710 J/M z o PAIPBT/Epoxy/IO2--30/70/~IhP G¢=1660 J/M = • PA/POT/Epoxy/I02=- 30/70/3pttr/Sphr Ge=4t'/0 J/M = . ~ • PA/PBT/Epoxy/I02= 30/70/3phr/lOphr G¢~1"/90 J / M = • PA/PBT/EI~xy/10L~=- 301701~hrllSphr ~ anvl = /
',
'2
'3
'4
~
'6
B0c#,~05t i~ 2 )
Effect of elastomer on impact critical strain energy release
elastomer modification and compatibilization. Such an elongation increment results in a higher tensile strength for brittle polymers, as shown in
Figure 11.
The greatest improvement occurs on the PA66-rich blend (PA66/ P B T = 70/30) using the KCA-102 elastomer.Figure 14
shows the effect of notched Izod properties. The KCA-102 elastomer-modified blends show substantially greater improvements on impact strength than those of the EXL-3386 elastomer-modified blends. We originally hoped that the acid-containing EXL-3386 elastomer would reside in the PA66 phase, to toughen the PA66 phase. A TEM micrograph
(Figure 5)
shows the elastomer particles mainly at the interface.Figure 15
shows the increase of Gc caused by increasing the KCA-102 elastomer content.CONCLUSIONS
This solid epoxy resin has been demonstrated to be an efficient compatibilizer for the PA66/PBT blends. To
function as an emulsifying agent, the
in situ-formed
mixed
PBT-co-epoxy-co-PA66
copolymers distributed atthe interface can reduce the interfacial tension and cause substantial reduction of the phase domains. To act as a mechanical reinforcing agent, this epoxy resin also gives a significant improvement of mechanical properties. Mechanical property improvement by compatibilization is more substantial for PA66-rich blends than for PBT- rich blends. The nature of the compatibilizer copolymer formation mechanism during melt mixing suggests that a substantial fraction of the epoxy resin, reacted or unreacted, resides in the PBT phase, and thus decreases the intrinsic properties of the PBT matrix. On the contrary, the epoxy resin distributed in the PA66 phase is less likely during melt mixing, and therefore the intrinsic properties of the PA66 phase are not being affected after compatibilization. Two types of core-shell elastomers have been employed to toughen the compatibilized PA66/PBT blends. The first one has a PMMA shell. The second one also has the PMMA shell, but also contains a small amount of acid functional groups. The elastomer particles with P M M A shells tend to reside mainly in the PBT phase, while the elastomer containing the acid groups tends to reside mainly along the interface. The elastomer with the PMMA shell is significantly better than the one containing the acid, in toughening the compatibilized PA66/PBT blends.
A C K N O W L E D G E M E N T
The authors are grateful to the National Science Council, Republic of China for financial support for this work through grant NSC-83-0405-E009-012.
REFERENCES
1. Xanthos, M. and Degli, S. S., Polym. Eng. Sci. 1991, 31, 929.
2. Liu, N. C. and Baker, W. W., Adv. Polym. Technol. 1992, l l ,
929.
3. Gaylord, N. G., Chemtech, July 1989, 435.
4. Folkes, M. J. and Hope, P. S., Polymer Blends and Alloys (ed.),
Blackie Academic & Professional, London, 1993, Chapter 13. 5. Adedeji, A. and Jamieson, A. M., Composite Interfaces, 1995, 3,
51.
6. Chang, F. C., in Handbook of Thermoplastics, ed. O. Olabisi.
Marcel Dekker, New York, 1996, p. 491.
7. Christiansen, W. H., Paul, D. R. and Barlow, J. W., J. Appl. Polym. Sci., 1987, 34, 537.
8. Kim, W. Y. and Lee, D. S., Polym. Bull., 1991, 26, 701.
9. Kim, W. N., Park, C. E. and Burns, C. M., J. Appl. Polym. Sci.,
1993, 49, 1003.
10. Machado, J. M. and Lee, C. S., Polym. Eng. Sci., 1994, 34, 59.
11. Chang, F. C., in Toughened Plastics 11." Science and Engineering,
ed. C. K. Riew and A. J. Kinlock. ACS Ser., Washington DC, 1996, Chap. 18, p. 279..
12. UCA R Phenoxy Resin for Solution and Coatings, Tech. literature
from Union Carbide Corp., 1986, p. 11.
13. Huang, C. C. and Chang, F. C., Polymer, in press.
14. Plati, E. and Williams, J. G., Polym. Eng. Sci., 1975, 15, 470.
15. Chang, F. C. and Yang, M. Y., Polym. Eng. Sci., 1990, 30, 543.
16. Lin, K. P. and Chang, F. C., Polym. Networks and Blends, 1994,
4(2), 51.
17. Chang, F. C. and Hwu, Y. C., Polym. Eng. Sci., 1991, 31, 1509.