中文摘要 加熱程序在奶粉製造的過程中已是既定的行程,然而在加熱的過程中卻會造 成某些蛋白質結構上的改變,然而這些改變是非常細小且微妙的,能否利用免疫化 學的方式來偵測這些微妙的改變,就變成了一個挑戰。在去年研究計劃中證明多株 抗體無法有效區分奶粉及生乳,今年本計劃研究的目的便是藉由製造能夠辨別結構 改變的單株抗體,來判別鮮奶中是否有添加奶粉。為了測試可行性,我們以奶粉為 抗原,製造大量的單株抗體,並利用 ELISA 來篩選能辨識奶粉及生乳的單株抗 體。960 株融合瘤細胞中,有 68 株對於奶粉及生乳皆會辨認,有 4 株(1B5F2, 1C10F10, 1D8F8, 2F2D9)只會辨認奶粉對於生乳並無反應,。實驗結果發現生乳中 只要添加 5%的奶粉,便能利用此 4 株單株抗體偵測。更進一步利用西方墨點法的 結果發現,這 4 株特異性單株抗體皆會直接辨識奶粉中 â-lactoglobulin (LG)以及與 LG 結合的其他蛋白質。另外在 ELISA 的結果中,當生乳經過 95℃加熱 15 分鐘 後,此 4 株單株抗體便能偵測的到,然而不論是加熱過的生乳或奶粉,再經過還原 劑處理後,便又無法偵測。由這樣的結果推測,在奶粉製造的加熱程序中,LG 表 面產生了一個新的 epitope,而且 LG 與其他蛋白質結合所憑藉的雙硫鍵,對於這 4 株單株抗體的辨識,扮演著舉足輕重的角色。由上述實驗今年度本實驗室在生乳與 奶粉中有重大的研究成果,並更進一步對單株抗體與奶粉間作用並加以討論之,此 成果在乳業界為一重大的突破。 關鍵字:單株抗體、â-lactoglobulin ABSTRACT
It is well established that the heating process during the preparation dry milk causes some structural changes of the native proteins. Since such changes are subtle, whether or not it can be detected by an immunochemical approach remains to be of challenge. The purpose of this study was to develop conformationally dependent monoclonal antibodies (mAb) that were able to distinguish the dry milk from freshly prepared raw milk. To test this possibility, we immunized the mice with commercially prepared dry milk and produced a panel of mAb. These mAb were then screened against both dry and raw milk using an ELISA. The monoclonals that specifically recognized the dry milk antigens were selected and characterized without bias. From 960 hybridomas screened, there were 68 clones reacted equally with dry and raw milk, but 4 clones were found to be only reacted with dry milk. The later mAb were able to detect the dry milk spiked into the raw milk as low as 5% in concentration (V/V). Westernblot analysis shows these specific mAb (1B5F2, 1C10F10, 1D8F8, 2F2D9) were all direct against â-lactoglobulin (LG) and LG conjugates in dry milk. Interestingly, polyclonal antibodies prepared against LG could not discriminate the immunoreactivity between the dry and raw milk. On ELISA, these specific mAb bound the raw milk that was heated at 95℃for 15 min. The binding, however, was abolished when heated or dry milk was treated with reducing reagent. Thus, the data suggest that a new antigenic epitope was exposed while processing the dry milk (a heating procedure) and the disulfide of LG crossly linked with other milk protein moiety played a provocative role in our specific mAb recognition. A hypothetical model with respect to the interaction between the mAb and dry milk is proposed and discussed in this report.
Zeon PDF Driver Trial
Keywords: Monoclonal antibody, â-lactoglobulin
INTRODUCTION
Dairy industries are interested to know an appropriate heat treatment in milk for controlling the quality of drinking milks or to control their heating system. On the contrary, the consumers are concerned whether or not the dry milk (powdered milk) has been supplemented to pasteurized raw milk. It happens when particularly the supply of raw milk is not sufficient in the summer where the demanding of consumption increases and the production of cow milk decreases. Since ultra heat treatment (UHT) procedure has been widely used in preparing milk powder, effort using heat-denatured milk proteins as a bioindicator has been probed to estimate such false practice (1-3). For examples, Olieman and Recio (4) show that the amount of heat-denatured proteins can be estimated by analyzing the casein fraction using a capillary zone electrophoresis. A fluorescent probe using intrinsic basis states analysis has been employed for quantitative estimation of the stability of proteins in aqueous solution as a function of temperature (5). Monoclonal antibodies (mAb) prepared against LG has been utilized for studying conformational changes of LG occurring upon physical treatment and its biological properties such as its interaction with ligands and hypersensitivity reactions (6-11). Negroni et al have used an appropriate LG mAb to detect the presence of bovine milk in goat milk by an enzyme linked immunosorbent assay (ELISA) (12-17).
The purpose of the present report was to use dry milk as an antigen(s) to randomly produce a penal of mAb and then to distinctively select those monoclonals (if any) that were able to discriminate the dry and raw milk. Subsequently using an ELISA, we established 4 monoclonals possessed such a unique property. Further characterization revealed that these mAb were direct toward to LG epitope on Westernblot analysis. Meanwhile, we demonstrated that the specificity of these dry milk antibodies was achieved, in part, as a result of the cross-linking of LG with other milk proteins. A hypothetical model explaining their specificity is described in details.
Zeon PDF Driver Trial
MATERIALS AND METHODS
Preparation of milk samples.
Bulked whole raw milk obtained from an university dairy farm (Tunghi University, Taichung, Taiwan) and dry milk (Nestle Australia Ltd, Sidney, Australia) without further heat or other manipulation (unless specifically mentioned) were used for the polyacrylamide gel electrophoresis (PAGE), Westernblot analysis, and ELISA.
Immunization of mice. (19).
Production of monoclonal antibody. (20). Enzyme linked immunosorbent assay. (21). Gel electrophoresis. (18) (22).
Native PAGE and SDS PAGE Westernblot analysis.
Trypsin and CNBr treatment on LG and its immunoreactivity for dry milk specific mAb. (23).
Isotyping of monoclonal antibodies. (18, 24, and 25).
RESULTS AND DISCUSSION:
It has been well established that some milk proteins are denatured during the process of dry milk (mostly heating is involved) (22). Identification of such a denatured protein(s) would be a subject of essential in differentiating dry and freshly prepared raw milks. Since the structural changes are subtle, some complicated physical (26-28) and biochemical (39-32) methods have been employed to monitor such changes. Previously, we have shown that mAb are extremely sensitive as a reagent for probing the structural changes of human low-density-lipoproteins (LDL) (24) and for discriminating the patients with and without coronary artery disease (33-36). Monoclonal antibodies prepared against human hepatic lipase can even distinguish between active and inactive forms of lipase (21). Thus, we anticipated that the mAb might allow us to detect the thermal denaturation of proteins as that occurred in heat processed milk. To test this possibility, we immunized the mice with commercially prepared dry milk and produced a panel of mAb. These mAb were than screened, without bias, against both dry and raw milk using an ELISA.
Pr imar y scr eening. As shown in Table 1, from 960 hybridomas in a primary screening,
there were 68 hybridomas reacted equally with dry and raw milk. Remarkably, 8 hybridomas were able to distinguish the dry milk apart from the raw milk. A typical example of the hybridomas displaying the dry milk specificity on ELISA is shown in
Figur e 1. In general, immunoreactivity of clones specific to dry milk was at least 10
times greater than that to raw milk. All of these 76 hybridomas were subjected to limiting dilutions for producing monoclones. Final established monoclonals (n=4) that distinctly recognized the dry milk (designated as DM-1, -2, -3, and -4 or 1B5F2, 1C10F10, 1D8F8, and 2F2D9, respectively) are given in (Table 1).
Dose-r esponsive binding cur ve of mAb specific to dr y milk. A representative
dose-responsive curve for the immunoreactivity of each established mAb specific to dry milk (n=4) or recognizing both dry and raw milk (n=1; 2B4B4) is shown in Figur e 2. Only mAb DM-1 to DM-4 (n=4) possessed the ability in discriminating the dry and raw milk (Figur e 2A). A slight cross-reactivity of these mAb, at high dose, with raw milk was
Zeon PDF Driver Trial
noticed, while the other mAb (n=20) reacted with both. A typical example is shown in
Figur e 2B. Nevertheless, the results indicated that mAb approach was novel in detecting
the structural changes in dry milk proteins.
Effect of heat on the immunor eactivity of r aw milk. Because heating is one of the
major processes in preparation of dry milk, we hypothesized that thermal denaturation might have exposed new antigenic epitopes from the raw milk. To test this hypothesis, in the next experiment we heated the raw milk and determined whether or not there were newly formed epitopes that could be probed by mAb DM-1 to DM-4. Figur e 3 reveals that these new epitopes were indeed exposed upon the heating on raw milk as demonstrated in increasing immunoreactivity on an ELISA. The data suggested that our mAb (DM-1 to DM-4) were direct to heat sensitive proteins in processed dry milk. In addition, we demonstrated that mAb based ELISA could detect the dry milk spiked into the raw milk as low as 5% (V/V) in final concentration (Figur e 4).
Char acter ization of monoclonals specific to dr y milk. To characterize the denatured
or thermal sensitive antigen(s) that recognized by these 4 monoclonals, Westernblots using dry milk sample in SDS- and native-PAGE were conducted. We identified that these 4 mAb were all direct against LG (Figur es 5 A-C). Meanwhile, Figur es 5 A-C reveals that all 4 mAb (DM-1 to DM-4) reacted with new and high molecular forms of LG in dry milk, but not that in raw milk. Thus, the data would indicate that LG is one of the major sensitive thermal-denatured components in dry milk as judged by the mAb approach that was originally not designed for the preparation of mAb against LG.
On the other hand, none of the tested mAb (n=20) that lacking the specificity to dry milk could recognize LG; a typical example with such mAb 2B4B4 is shown in Figur e
5D. Most of these raw milk mAb reacted with casein proteins as illustrated in
Westernblots (Figur e 5D) and they did not react with LG as judged by ELISA (data not shown). Although these mAb had not been characterized fully thus far, they did not apparently recognize lactalbumin, albumin, and immunoglobulin, which are abundantly present in both bovine milk and serum. This was because any mouse monoclonals that initially produced against serum associated proteins would be automatically neutralized and excluded during the primary screening, when they were routinely cultured in fetal calf serum containing lactalbumin, albumin, and immunoglobulin.
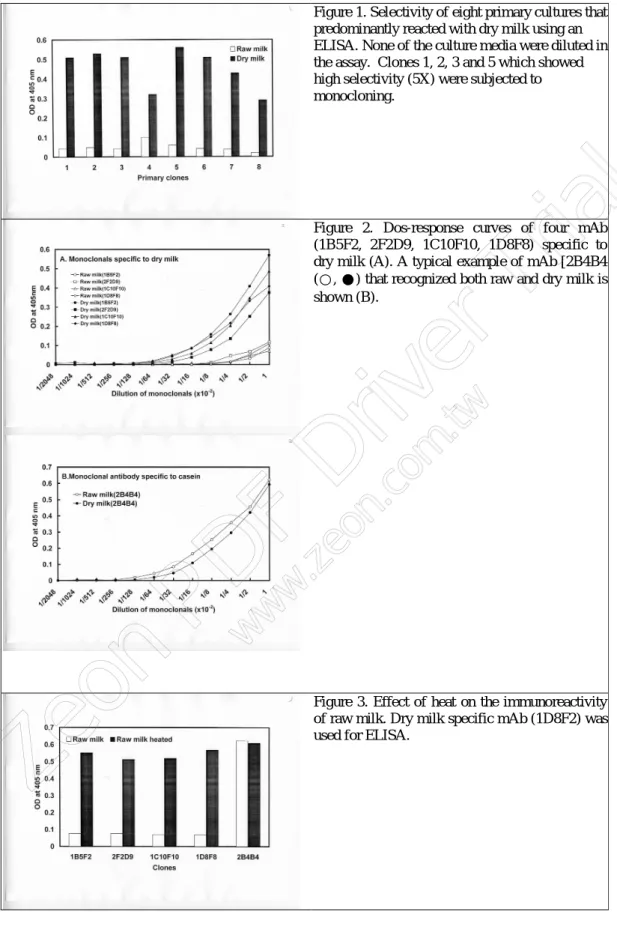
Lack of dr y milk specificity fr om polyclonal antibody pr epar ed against LG. To
address whether or not the polyclonal antibody prepared against dry milk and LG, respectively, could discriminate the dry and raw milk. The same ELISA as mentioned above was probed. Figur e 6 shows that neither dry milk antibody (panel A) nor LG polyclonal (panel B) antibody was able to distinguish between the dry and raw milk. Thus, the result would suggest that the population of polyclonal antibodies prepared against LG reacted with multiple epitopes that were commonly shared in both dry and raw milks.
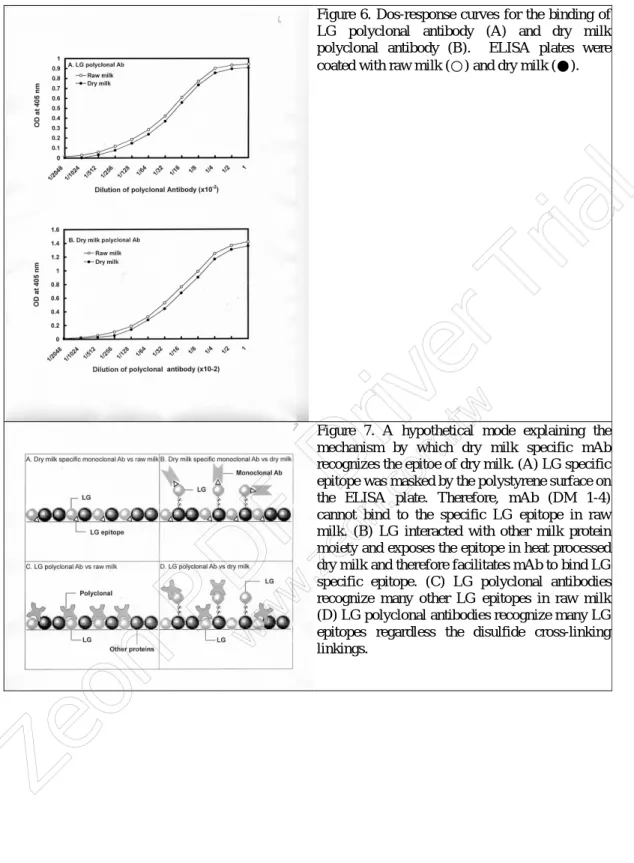
Hypothetical model of immunochemical pr oper ty of LG in dr y milk: we
demonstrated that all of these 4 randomly prepared mAb were LG specific (Figur e 5). They simultaneously recognized both native LG and the denatured larger molecular form of LG in dry milk (Figur e 5). However, on Westernblot we showed that these mAb also
Zeon PDF Driver Trial
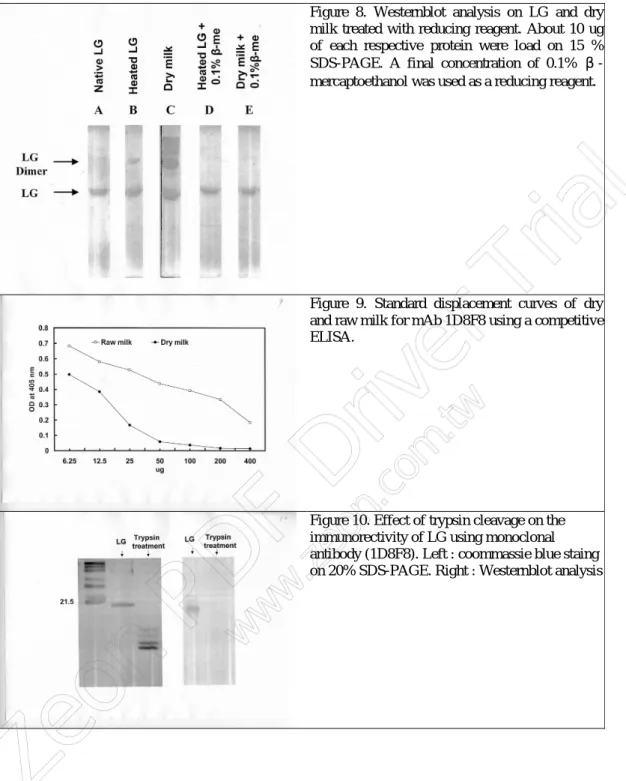
recognized LG in raw milk for some extent (Figur e 5). Therefore, ELISA was a superior method to Westernblot in differentiating dry and raw milk. To explain how these monoclonals effectively bound to dry milk, but not to raw milk; we show a hypothetical drawing (Figur e 7). First, it is probably not so surprising that the ELISA approach was feasible, since the unique mAb were initially identified by the ELISA. One of the possible mechanisms by which the mAb differentially reacted with the dry milk was that the specific LG epitope was either masked by the polystyrene surface on the ELISA plate or being interacting with the other milk proteins during the immobilization of milk antigen (Figur e 7A). Therefore, the mAb could not bind that specific LG epitope in raw milk. In dry milk (Figur e 7B), however, LG was crossly linked to the other milk proteins possibly via disulfide linkages (Figur e 5). With such cross linking the LG epitope was emerged again at the surface, which was accessible for the binding of mAb (Figur e 7B). To prove that LG was crossly linked with other milk proteins in dry milk via disulfide, we treated the dry milk with various dose of reducing reagent (mercaptoethanol) to remove the disulfide linkages (if any). Under this condition, the mAb (DM 1-4) did not react with any cross-linking forms of LG as demonstrated on a Westernblot (Figur e 8). Furthermore LG polyclonal antibodies, which did not recognized the unique LG epitope, would bind both raw (Figur e 7C) and dry milk (Figur e 7D) as mentioned above.
We further substantiated this notion by conducting a “solution phase” ELISA. Figur e
9 demonstrated that raw milk in solution could competitively displace the binding of dry
milk to the mAb, although the immunoreactivity of raw milk is somewhat lower that that of dry milk.
Taking together, we conclude that cross-linking of LG with other milk proteins plays an essential role in our ELISA system by providing the extra epitope for the binding of specific dry milk monoclonals. As to the epitope of LG that interacts with the plate interface or mAb, we have yet identified at the present time. Our preliminary data show that this specific epitope of LG was attenuated to the CNBr and trypsin cleavage. A typical example of the immunoreactivity affected by trypsin treatment on Westernblot is depicted in Figur e 10. Subtyping of IgG class revealed that all the mAb reported in this study were IgG1 without exceptions (Table 1). Further antigenic mapping of LG is now in progress which may provide the insight of the surface property of LG and its interaction with mAb.
With respect to the possible mechanism involved in the cross-linking, one can rationale it as below. LG is a protein consists of 162 amino acids containing 5 cysteines at residues 66, 106, 119, 121, and 160. There are 2 cross-linking disulfide bonds at position Cys 66-160 and Cys 106-119, respectively (37-39). Activation of free Cys 121 by thermal treatment in milk has been proposed to induce the disulfide bond formation between LG (dimerization) and K-casein (40, 41). Our Westernblot (Figur e 5) showing that LG monoclonals reacted with both native LG and LG conjugates (large molecular forms), and mercaptoethanol experiment reversing its immunoreactivity (Figur e 8) support the notion that LG crossly links with other proteins in dry milk (Figur e 7). In the present study, at least three milk proteins were found to be involved in such linkages (Figur e 5); although we have not identified these proteins yet. Nevertheless, the Westernblot technique recognizing the species crossly linked by LG, not reported
Zeon PDF Driver Trial
previously, may help us to further delineate the cross-linking between LG and milk proteins.
LITERATURE CITED
(1) Relkin, P. Thermal unfolding of beta-lactoglobulin, alpha-lactalbumin, and bovine serum albumin. A thermodynamic approach. Crit. Rev. Food Sci. Nutr. 1996, 36,
565-601.
(2) Sanchez, L.; Perez, M. D.; Puyol, P.; Calvo, M.; Brett, G. Determination of vegetal proteins in milk powder by enzyme-linked immunosorbent assay: interlaboratory study.J. AOAC Int. 2002, 85, 1390-1397.
(3) Steffensen, C. L.; Andersen, H. J.; Nielsen, J. H. Aldehyde-Induced Xanthine Oxidase Activity in Raw Milk. J. Agric. Food Chem. 2002, 50, 7392 – 7395.
(4) Recio, I.; Olieman, C. Determination of denatured serum proteins in the casein fraction of heat-treated milk by capillary zone electrophoresis. Electrophoresis 1996, 17, 1228-1233.
(5) Tsonev, L. I.; Hirsh, A. G. Fluorescence ratio intrinsic basis states analysis: a novel approach to monitor and analyze protein unfolding by fluorescence. J. Biochem. Biophys. Methods 2000, 45, 1-21.
(6) Venien, A.; Levieux, D.; Astier, C.; Briand, L.; Chobert, J. M.; Haertle, T. Production and epitopic characterization of monoclonal antibodies against bovine beta-lactoglobulin.J. Dairy Sci. 1997, 80, 1977-1987.
(7) Clement, G.; Boquet, D.; Frobert, Y.; Bernard, H.; Negroni, L.; Chatel, J. M.; Adel-Patient, K.; Creminon, C.; Wal, J. M.; Grassi, J. Epitopic characterization of native bovine beta-lactoglobulin.J. Immunol. Methods 2002, 266, 67-78.
(8) Selo, I.; Creminon, C.; Grassi, J.; Couraud, J. Y. Anti-allergen antibodies can be neutralized by antibodies obtained against a peptide complementary to the allergen: towards a new peptide therapy for allergy.Immunol. Lett. 2002, 80, 133-138.
(9) Kobayashi, K.; Hirano, A. Ohta, A.; Yoshida, T.; Takahashi, K.; Hattori, M. Reduced immunogenicity of beta-lactoglobulin by conjugation with carboxymethyl dextran differing in molecular weight.J. Agric. Food Chem. 2001, 49, 823-831.
(10) Restani, P.; Gaiaschi, A.; Plebani, A.; Beretta, B.; Cavagni, G.; Fiocchi, A.; Poiesi, C; Velona, T.; Ugazio, A. G.; Galli, C. L. Cross-reactivity between milk proteins from different animal species.Clin. Exp. Allergy 1999, 29, 997-1004.
(11) Morgan, F.; Venien, A.; Bouhallab, S.; Molle, D.; Leonil, J.; Peltre, G.; Levieux, D. Modification of bovine beta-lactoglobulin by glycation in a powdered state or in an aqueous solution: immunochemical characterization.J. Agric. Food Chem. 1999, 47,
4543-4548.
(12) Negroni, L.; Bernard, H.; Clement, G.; Chatel, J. M.; Brune, P.; Frobert, Y.; Wal, J. M.; Grassi, J. Two-site enzyme immunometric assays for determination of native and denatured beta-lactoglobulin. J. Immunol. Methods 1998, 220, 25-37.
(13) Duchateau, J.; Michils, A.; Lambert, J.; Gossart, B.; Casimir, G. Anti-betalactoglobulin IgG antibodies bind to a specific profile of epitopes when patients are allergic to cow's milk proteins.Clin. Exp. Allergy 1998, 28, 824-833.
(14) Venien, A.; Levieux, D.; Astier, C.; Briand, L.; Chobert, J. M.; Haertle, T. Production and epitopic characterization of monoclonal antibodies against bovine beta-lactoglobulin.J. Dairy Sci. 1997, 80, 1977-1987.
Zeon PDF Driver Trial
(15) Kamata, N.; Enomoto, A.; Ishida, S.; Nakamura, K.; Kurisaki, J.; Kaminogawa, S. Comparison of pH and ionic strength dependence of interactions between monoclonal antibodies and bovine beta-lactoglobulin. Biosci. Biotechnol. Biochem.
1996, 60, 25-29.
(16) Hattori, M.; Ametani, A.; Katakura, Y.; Shimizu, M.; Kaminogawa, S. Unfolding/refolding studies on bovine beta-lactoglobulin with monoclonal antibodies as probes. Does a renatured protein completely refold? J. Biol. Chem. 1993, 268,
22414-22419.
(17) Kuzmanoff, K. M.; Beattie, C. W. Isolation of monoclonal antibodies monospecific for bovine beta-lactoglobulin. J. Dairy Sci. 1991, 74, 3731-3740.
(18) Yang, S. J.; Mao, S. J. T. A simple HPLC Purification Procedure for Porcine Plasma Haptoglobin. J. Chromatagr. B 1999, 731, 395-402.
(19) Huang, G. S.; Wang, S. P.; Chang, W. T.; Sun, T. J.; Wang, S. C.; Chu, R.; Mao, S. J. T. Intracellular processing generated MDA-lys epitope in foam cells. Life Sci. 1999, 65, 285-296.
(20) Mao, S. J. T.; Rechtin, A. E.; Krstenansky, J. L.; Jackson, R. L. Characterization of a monoclonal antibody specific to the amino terminus of the alpha-chain of human fibrin. Thromb. Haemost. 1990, 63, 445-448.
(21) Mao, S. J. T.; Rechtin, A. E.; Jackson, R. L. Monoclonal antibodies that distinguish between active and inactive forms of human postheparin plasma hepatic triglyceride lipase.J. Lipid Res. 1988, 29, 1023-1029.
(22) Oldfield, D. J.; Singh, H.; Taylor, M. W.; Pearce, K. N. Kinetics of denaturation and aggregation of whey protein in skim milk heated in an ultra-high temperature (UHT) plant. Int. Dairy J. 1998a, 8, 311-318.
(23) Selo, I.; Negroni, L.; Creminon, C.; Yvon, M.; Peltre, G.; Wal, J. M. Allergy to bovine beta-lactoglobulin: specificity of human IgE using cyanogen bromide-derived peptides. Int. Arch. Allergy Immunol. 1998, 117, 20-28.
(24) Mao, S. J. T.; Kazmar, R. E.; Silverfield, J. C.; Alley, M. C.; Kluge, K.; Fathman, C. G. Immunochemical properties of human low density lipoproteins as explored by monoclonal antibodies. Binding characteristics distinct from those of conventional serum antibodies.Biochim. Biophys. Acta 1982, 713, 365-374.
(25) Mao, S. J. T.; Kottke, B. A. Tween-20 increases the immunoreactivity of apolipoprotein A-I in plasma. Biochim. Biophys. Acta 1980, 620, 447-453.
(26) Anema, S. G.; Li, Y. Association of denatured whey proteins with casein micelles in heated reconstituted skim milk and its effect on casein micelle size. J. Dairy Res.
2003, 70, 73-83.
(27) Pfeil, W. Is the molten globule a third thermodynamic state of protein? The example of alpha-lactalbumin. Proteins 1998, 30, 43-48.
(28) Dutta, P. K.; Hammons, K.; Willibey, B.; Haney, M. A. Analysis of protein denaturation by high-performance continuous differential viscometry.J. Chromatogr.
1991, 536, 113-121.
(29) Jimenez-Guzman, J.; Cruz-Guerrero, A. E.; Rodriguez-Serrano, G.; Lopez-Munguia, A.; Gomez-Ruiz, L.; Garcia-Garibay, M. Enhancement of lactase activity in milk by reactive sulfhydryl groups induced by heat treatment. J. Dairy Sci. 2002, 85,
2497-2502.
Zeon PDF Driver Trial
(30) Valkonen, K. H.; Marttinen, N.; Alatossava, T. Electrophoretic methods for fractionation of native and heat-denatured bovine beta-lactoglobulin. Bioseparation
2001, 10, 145-152.
(31) Bertrand-Harb, C.; Baday, A.; Dalgalarrondo, M.; Chobert, J. M.; Haertle, T. Thermal modifications of structure and co-denaturation of alpha-lactalbumin and beta-lactoglobulin induce changes of solubility and susceptibility to proteases.
Nahrung 2002, 46, 283-289.
(32) Chang, J. Y.; Li, L. The structure of denatured alpha-lactalbumin elucidated by the technique of disulfide scrambling: fractionation of conformational isomers of alpha-lactalbumin. J. Biol. Chem. 2001, 276, 9705-9712.
(33) Mao, S. J. T.; Patton, J. G.; Badimon, J. J.; Kottke, B. A.; Alley, M. C.; Cardin, A. D. Monoclonal antibodies to human plasma low-density lipoproteins. I. Enhanced binding of 125I-labeled low-density lipoproteins by combined use of two monoclonal antibodies. Clin. Chem. 1983, 29, 1890-1897.
(34) Patton, J.G.; Badimon, J. J.; Mao, S. J. T. Monoclonal antibodies to human plasma low-density lipoproteins. II. Evaluation for use in radioimmunoassay for apolipoprotein B in patients with coronary artery disease. Clin. Chem. 1983, 29,
1898-1903.
(35) Marcovina, S.; France, D.; Phillips, R. A.; Mao, S. J. T. Monoclonal antibodies can precipitate low-density lipoprotein. I. Characterization and use in determining apolipoprotein B. Clin. Chem. 1985, 31, 1654-1658.
(36) Marcovina, S.; Kottke, B. A.; Mao, S. J. T. Monoclonal antibodies can precipitate low-density lipoprotein. II. Radioimmunoassays with single and combined monoclonal antibodies for determining apolipoprotein B in serum of patients with coronary artery disease.Clin. Chem. 1985, 31, 1659-1663.
(37) Papiz, M. Z.; Sawyer, L.; Eliopoulos, E. E.; North, A. C.; Findlay, J. B.; Sivaprasadarao, R.; Jones, T. A.; Newcomer, M. E.; Kraulis, P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. Nature 1986, 324, 383-385.
(38) Cho, Y.; Gu, W.; Watkins, S.; Lee, S. P.; Kim, T. R.; Brady, J. W.; Batt, C. A. Thermostable variants of bovine beta-lactoglobulin.Protein Eng. 1994, 7, 263-270.
(39) Cho, Y.; Singh, H.; Creamer, L. K. Heat-induced interactions of beta-lactoglobulin A and kappa-casein B in a model system.J. Dairy Res. 2003, 70, 61-71.
(40) Kitabatake, N.; Wada, R.; Fujita, Y. Reversible conformational change in beta-lactoglobulin A modified with N-ethylmaleimide and resistance to molecular aggregation on heating.J. Agric. Food Chem. 2001, 49, 4011-4018.
(41) Doi, H.; Hiramatsu, M.; Ibuki, F.; Kanamori, M. Gelation of the heat-induced complex between kappa-casein and alpha-lactalbumin. J. Nutr. Sci. Vitaminol
(Tokyo) 1985, 31, 77-87.
Zeon PDF Driver Trial
Figure 1. Selectivity of eight primary cultures that predominantly reacted with dry milk using an ELISA. None of the culture media were diluted in the assay. Clones 1, 2, 3 and 5 which showed high selectivity (5X) were subjected to monocloning.
Figure 2. Dos-response curves of four mAb (1B5F2, 2F2D9, 1C10F10, 1D8F8) specific to dry milk (A). A typical example of mAb [2B4B4 (○, ●) that recognized both raw and dry milk is shown (B).
Figure 3. Effect of heat on the immunoreactivity of raw milk. Dry milk specific mAb (1D8F2) was used for ELISA.
Zeon PDF Driver Trial
www.zeon.com.tw
Zeon PDF Driver Trial
Figure 4. Immunorectivity of the presence of dry milk in raw milk. Dry milk was spiked into raw milk as a final % and assessed by an ELISA. .
Figure 5. Characterization of the four mAbs (1B5F2, 1D8F8, 2F2D9 and 2B4B4) using a Westernblot analysis. Each lane was loaded with 10 ug of milk protein. Lane A: native LG; lane B: raw milk; lane C: processed milk (from Taiwan); lane D: dry milk; lane E: heated raw milk at 95℃ for 15 min.
Zeon PDF Driver Trial
www.zeon.com.tw
Zeon PDF Driver Trial
Figure 6. Dos-response curves for the binding of LG polyclonal antibody (A) and dry milk polyclonal antibody (B). ELISA plates were coated with raw milk (○) and dry milk (●).
Figure 7. A hypothetical mode explaining the mechanism by which dry milk specific mAb recognizes the epitoe of dry milk. (A) LG specific epitope was masked by the polystyrene surface on the ELISA plate. Therefore, mAb (DM 1-4) cannot bind to the specific LG epitope in raw milk. (B) LG interacted with other milk protein moiety and exposes the epitope in heat processed dry milk and therefore facilitates mAb to bind LG specific epitope. (C) LG polyclonal antibodies recognize many other LG epitopes in raw milk (D) LG polyclonal antibodies recognize many LG epitopes regardless the disulfide cross-linking linkings.
Zeon PDF Driver Trial
www.zeon.com.tw
Zeon PDF Driver Trial
Figure 8. Westernblot analysis on LG and dry milk treated with reducing reagent. About 10 ug of each respective protein were load on 15 % SDS-PAGE. A final concentration of 0.1% β-mercaptoethanol was used as a reducing reagent.
Figure 9. Standard displacement curves of dry and raw milk for mAb 1D8F8 using a competitive ELISA.
Figure 10. Effect of trypsin cleavage on the immunorectivity of LG using monoclonal antibody (1D8F8). Left : coommassie blue staing on 20% SDS-PAGE. Right : Westernblot analysis
Zeon PDF Driver Trial
www.zeon.com.tw
Zeon PDF Driver Trial
Table 1. Designated monoclonal antibodies specific to dry milk and their
characterizations
ELISA specific to Hybridoma
Raw and Dry milk Dry milk
IgG subclass
Number of primary
hybridoma 960 68 8
Established and
Expand monoclonal 20 selected 4 IgG1
mAb designated DM 1(1B5F2) DM 2(1D8F8) DM 3(2F2D9) DM 4(2B3D11) IgG1 IgG1 IgG1 IgG1 Reacted with LG on
Western blot none all
計畫成果自評 利用抗體偵測乳品品質之計劃,在本年度有重大突破,在區分生乳及還原乳方 面,在本實驗室依現有發展成熟之單株抗體技術,成功的製作出可區分生乳即還原 乳之單株抗體,此單株抗體將可利於學界及業界之利用,並在乳品品質之監控有有 極大之幫助,如可防此不肖業者或酪農戶利用還原乳充當成生乳,欺騙消費者。其 次經本實驗室深入研究此單株抗體在生乳經 75℃加熱 30 秒後才能偵測出,此研究 結果更可替乳品品質訂定一個新的標準,現今的乳品加熱方式有三種:60℃加熱處 理 30min;75℃加熱處理 15s;135℃加熱處理 2s,然而 135℃加熱處理 2s 因高熱 會破壞乳中蛋白,此結果在上次報告已有深入研究報導,因此 75℃加熱處理 15s 為一重要的加熱殺菌的最佳指標,此單株抗體正可擔任此溫度的一檢測依據,如超 過 75℃加熱處理或處理時間超過 15s,則會被檢測出。此可用於規範牛乳品質之新 的標準。這是本實驗室在執行本年度計劃之重大突破,也獲得最好之成果。 本計劃所研究之成果已在國際會議(美國國際生物醫學年會)及台灣生物醫學年 會 發 表 , 並 將 發 表 論 文 在 國 際 期 刊 (SCI paper) Journal of agricultural and food chemistry 上,已送審中,我們更進一步要申請專利,此專利申請案上在整理中。 因此在計劃執行上本實驗室以超越今年度的研究進度。在明年度的計劃中我們將深 入探討乳品在經加熱後與 â-lactoglobulin 之間的關係,並研發更快速的檢測方式來 監控牛乳的品質,用以提供國內快速檢測乳品品質的方式,目前已有初步的結果。