0270-7306/97/$04.0010
Copyrightq 1997, American Society for Microbiology
Identification and Characterization of a Nucleolar Phosphoprotein,
Nopp140, as a Transcription Factor
LUO-HUA MIAU,1CHING-JIN CHANG,2WEN-HAI TSAI,3ANDSHENG-CHUNG LEE2,3* Institute of Biochemical Sciences, College of Science,1and Institute of Molecular Medicine, Dr. H. L. Tsai
Memorial Laboratory, College of Medicine,3National Taiwan University, and Institute of Biological Chemistry, Academia Sinica,2Taipei, Taiwan
Received 15 March 1996/Returned for modification 22 April 1996/Accepted 17 October 1996
Expression of the alpha-1 acid glycoprotein (AGP) gene (agp) is activated by a key transcription factor, AGP/enhancer-binding protein (AGP/EBP, commonly called C/EBPb), in the liver during the acute-phase response. In addition to this positive regulation, agp is negatively regulated by nucleolin (T. H. Yang et al., Mol. Cell. Biol. 14:6068–6074, 1994). Other factors involve in positive regulation of the agp gene are poorly charac-terized. In a systematic search for factors that may interact with AGP/EBP, we have identified Nopp140, a phosphoprotein of 140 kDa, by immunoaffinity chromatography. Nopp140 not only functions as a transcrip-tional activator per se but also interacts with AGP/EBP to synergistically activate the agp gene in an AGP/ EBP-binding motif-dependent manner. In addition to interacting with AGP/EBP, Nopp140 interacts specifi-cally with TFIIB. Distinct regions of Nopp140 that interact with AGP/EBP and TFIIB have been characterized. The sequence of Nopp140 contains several stretches of serine- and acidic amino acid-rich sequences which are also found in ICP4 of herpes simplex virus type 1, a known transcription factor that interacts with TFIIB. The physical interaction between TFIIB and wild-type Nopp140 or several deletion mutants of Nopp140 correlates with the ability of Nopp140 to activate the agp gene synergistically with AGP/EBP. Thus, the molecular mechanism for agp gene activation may involve the interaction of AGP/EBP and TFIIB mediated by coactivator Nopp140.
Alpha-1 acid glycoprotein (AGP) is a liver-derived plasma glycoprotein, the level of which increases during the acute-phase response (3, 17). Both transcriptional and posttranscrip-tional mechanisms are responsible for this induction of AGP (4, 19, 40). We have shown previously that transcriptional regulation of the agp gene is mediated by positive and negative factors (6, 22, 23, 46). Among the positive transcription factors, members of the C/EBP (enhancer-binding protein) family such as AGP/EBP are by far the most important (6). Surprisingly, an RNA-binding nucleolar protein, nucleolin, has been shown to negatively regulate transcription of the agp gene (46). The activities of AGP/EBP and nucleolin were increased and de-creased, respectively, during the acute-phase reaction (23, 46). Besides C/EBP and nucleolin, other positive and negative fac-tors are likely to be involved in regulation of the agp gene.
Members of the C/EBP family include C/EBPa (20), C/EBPb (also known as NF-IL6, LAP, IL6-DBP, and AGP/ EBP) (1, 2, 5, 6, 8, 33), C/EBPg (34), C/EBPd (5, 16, 43), and CHOP (35). These C/EBP family members share a highly conserved carboxyl-terminal basic amino acid-rich region and a flanking leucine zipper (bZIP) domain that are essential for DNA binding and dimer formation (homo- or heterodimers) (21). The activation domains are located at regions proximal to the amino terminus and are completely different among C/EBP family members. CHOP lacks the amino-terminal ac-tivation domain and thus functions as a repressor (35). Fur-thermore, both activator and repressor forms can be expressed from the same C/EBPb mRNA through in-frame translation initiation (9). We have recently identified two activators of
AGP/EBP that differ in the amino-terminal 21 amino acids due to alternative translational initiation (22).
Some transcription factors such as NF-kB and members of the c-Rel family (38, 39), glucocorticoid receptor (29), AP-1 (14, 30), and p53 (24) can interact with members of the C/EBP family. These interactions result in cross talk between tran-scription factors and are responsible for the increased subtle regulation of gene expression through combinatorial mecha-nisms. Other factors that may interact with AGP/EBP have yet to be identified.
To systematically identify for proteins that interact with AGP/EBP, we used an anti-AGP/EBP antibody affinity column to fractionate rat liver nuclear extract. The proteins retained by the column were further separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). By sequenc-ing analysis, we have identified Nopp140 as the specific protein retained by the anti-AGP/EBP affinity column. In this study, we present evidence that Nopp140 functions as a transcrip-tional activator of the agp gene. Furthermore, Nopp140 and AGP/EBP can activate the agp gene synergistically in an AGP/ EBP-binding motif-dependent manner. Biochemical charac-terizations indicate that Nopp140 interacts with AGP/EBP and TFIIB physically. Thus, Nopp140 not only functions as a tran-scriptional activator per se but also mediates the interaction between a specific motif-binding transcription factor, AGP/ EBP, and a general transcription factor, TFIIB, that results in the synergistic activation of the agp gene.
MATERIALS AND METHODS
Plasmids.The AGP-chloramphenicol acetyltransferase (CAT) and cytomega-lovirus (CMV)-AGP/EBP constructs were as described previously (23). Nopp140 cDNA was isolated by reverse transcription-PCR from rat liver RNA and cloned into pRSET vector (Invitrogen, San Diego, Calif.). The primers used for reverse transcription-PCR of the open reading frame of Nopp140 were 59-CGTTTAAC CCGGAGTATGGCGGAT-39 (positions 38 to 61; sense) and 59-AAGATG
* Corresponding author. Mailing address: Institute of Molecular Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan. Phone: 886-2-356-2982. Fax: 886-2-321-0977.
230
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
ACACAGGTCACTCGCTGT-39 (positions 2180 to 2157; antisense). CMV-Nopp140 (correct orientation of CMV-Nopp140) and CMV-Nopp(Rev) (reverse ori-entation of Nopp140) expression vectors were prepared by cloning the full-length Nopp140 cDNA into pCMV, similar to the preparation of CMV-AGP/EBP. Deletion mutants of AGP/EBP were created by NcoI/HindIII (AGP/EBP-N) and
PvuII/HindIII (AGP/EBP-P) digestion of pRSET-AGP/EBP. A deletion mutant
of CMV-Nopp140/P2 was prepared by partial digestion of CMV-Nopp with PstI followed by isolation of the incompletely digested plasmids. CMV-Nopp140/ BS was created by ligation of the 59 fragment of Nopp140 cDNA (obtained by digestion with BamHI and SacI) to a CMV expression vector. Glutathione
S-transferase (GST)–Nopp140, GST-Nopp140/P2, and GST-Nopp140/BS were
prepared by cloning the BamHI/EcoRI fragment of Nopp140 into the pGEX vector (Pharmacia Biotech).
Plasmid p(C2AT)19and a truncated form of plasmid pML(C2AT)19(referred to as AdML200) were obtained from M. Sawadogo (36) through U. Schibler. AGP-C2AT was constructed by inserting the DNA fragment (generated by PstI/
EcoRI digestion) of the mouse agp gene upstream regulatory region (bp2180 to 110) into the SstI site of p(C2AT)19after the ends of both insert and vector had been blunted with T4 DNA polymerase.
Recombinant proteins and antibodies.Recombinant Nopp140 (rNopp140) from pRSET was expressed in Escherichia coli BL21(DE3) and purified by using an Ni column. Recombinant wild-type and mutant AGP/EBPs were prepared as described previously (6). GST-Nopp from pGEX was expressed in E. coli DH5a and purified by glutathione-Sepharose affinity column. Rabbit anti-Nopp140 antibody was prepared by immunizing the rabbit with the purified rNopp140. The specificity of this antibody was assessed by its reactivity with native Nopp140 by immunoprecipitation and its usefulness for recognizing cellular Nopp140 in Western blots. Furthermore, no cross-reactivity of this antibody with nucleolin or nucleoplasmin was detected. Anti-NF-kB antibodies, c-Jun, and c-Rel were ob-tained from Santa Cruz Biotechnology. Monoclonal and polyclonal anti-AGP/ EBP antibodies were as described previously (6, 23). A control, nonspecific antibody was from preimmune rabbit serum.
In vitro protein interactions.Glutathione beads containing rGST or GST-Nopp140 were incubated with rTFIIB or rAGP/EBP in phosphate-buffered sa-line (PBS)–0.1% Triton X-100 at 48C for 2 h. The beads were washed with PBS–1% Triton X-100 and subjected to SDS-PAGE and Western blot analysis.
Nuclear extract preparations.Nuclear extract from rat liver was prepared as detailed elsewhere (6). Nuclear extracts from 129p, BHK, and HTC cells were prepared as described previously (23). Briefly, subconfluent cells (cultured in Dulbecco modified Eagle medium containing 10% fetal calf serum and antibi-otics) were used. The quality of these nuclear extract preparations was routinely assessed by electrophoretic mobility shift assay using the AGP/EBP-binding motif and by Western blot analysis for AGP/EBP.
Immunoprecipitation and Western blotting.For immunoprecipitation exper-iments, 100mg of nuclear extract was routinely used for reacting with properly titrated antiserum. The primary antibody-nuclear extract reaction was performed at 48C overnight by constant mixing. Protein A-Sepharose was then added, and the mixture was incubated for 2 h at 48C. The protein A-Sepharose was washed four times with PBS–1% Triton X-100 and incubated with SDS sample buffer followed by SDS-PAGE. The separated polypeptides were blotted onto a Hy-bond-C membrane (Amersham). The blots were probed with antibodies and detected by using an enhanced chemiluminescence kit (Amersham).
Gel mobility shift assay.The gel mobility shift assay was performed as de-scribed previously (23). An end-labeled oligonucleotide probe (upper strand, 59-GATCATTTTGTGTAAGAC-39; 1 ng, 10,000 to 20,000 cpm/ng) was incu-bated together with samples in a 20-ml reaction mixture containing 50 mM NaCl, 20 mM Tris-HCl (pH 7.6), 0.2 mM EDTA, 10% glycerol, 5 mM dithiothreitol, and 1mg of poly(dI-dC) for 20 min at room temperature. The mixture was loaded onto a 5% polyacrylamide gel containing glycerol in Tris-glycine buffer. Electro-phoresis was performed at 150 V at room temperature. For the antibody super-shift assay, 1ml of antibody was added, and the reaction mixture was incubated for an additional 10 min before being loaded onto the gel.
Transfections and CAT assay.BHK cells were cultured in Dulbecco modified Eagle medium containing 10% fetal calf serum to 50% confluency. DNA trans-fections were performed by the calcium phosphate precipitation method. For each 6-cm-diameter petri dish, the calcium phosphate-DNA precipitate con-tained the amount of plasmid appropriate for each experiment. pGEM plasmid DNA (Promega) was used to bring the final amount of DNA for transfection to 3mg. The culture was replaced with fresh medium 24 h posttransfection. Cells were harvested 48 h posttransfection. CAT activity was determined by densito-metric scanning of the autoradiogram or by scanning the thin-layer chromatog-raphy plate with the Bio-Rad Image analysis system. Normally, duplicate or triplicate experiments were conducted. The relative CAT activity was determined by using the lowest level of CAT conversion (controls) as the basal value. pCMV-GFP (green fluorescent protein) was included in each transfection as an internal control for transfection efficiency.
Affinity column chromatography.A monoclonal anti-AGP/EBP antibody was purified on a protein G affinity column. The purified immunoglobulin G (IgG) was then immobilized to protein A-Sepharose CL6B (Pharmacia Biotech) and cross-linked by the cross-linking agent DSP (Pierce). IgG of other monoclonal antibodies (e.g., hepatitis B virus surface antigen) was coupled to protein A-Sepharose and used as a nonspecific immunoaffinity column control. The column
was equilibrated with buffer A (50 mM HEPES [pH 7.9], 20% glycerol, 0.5 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) containing 0.1 M NaCl. Rat liver nuclear extract (50 mg) was loaded onto a 1-ml column with constant recirculation of the flowthrough fraction for 30 min at 48C. The column was then washed with buffer A containing 0.1 M NaCl. Stepwise elution was performed with 3 ml of buffer A containing 0.2, 0.5, and 1 M NaCl followed by 0.1 M glycine buffer (pH 2.7). For AGP/EBP affinity column chromatography, the purified recombinant AGP/EBP was immobilized to Affi-Gel-10. One micro-gram of recombinant Nopp140 was applied onto the column equilibrated with buffer A. The elution conditions were the same as for the immunoaffinity column except without the pH 2.7 glycine buffer step.
LC/MS/MS analysis of the purified protein.Rat liver nuclear extracts were purified by anti-AGP/EBP antibody affinity column chromatography and SDS-PAGE. The major polypeptide detected by silver staining after SDS-PAGE was recovered by extraction with buffer (10 mM Tris-HCl [pH 8.0], 0.15 M NaCl, 1 mM EDTA). The extracted protein was precipitated with acetone, washed with 70% ethanol, dissolved in 20ml of 10 mM Tris-HCl–0.5 ml of 1 M CaCl2–0.2mg of trypsin, and digested for 18 h at 378C. Liquid chromatography-mass spectrom-etry (LC/MS) analysis was performed by John Yates’s lab at the Department of Molecular Biotechnology, University of Washington, Seattle.
In vitro transcription assay.In vitro transcription was done essentially as described by Gorski et al. (12). Briefly, transcription reaction mixtures (20ml) contained 1mg of circular DNA template and 40 mg of HeLa cell nuclear extract (Promega Biotech) in a buffer containing 25 mM HEPES (pH 7.6), 50 mM KCl, 6 mM MgCl2, 0.6 mM each ATP and CTP, 35mM UTP, 7 mCi of [a-32P]UTP (400 Ci/mmol; Amersham), 0.1 mM 39-O-methyl-GTP (P-L Biochemicals), 12% glycerol, and 1 ml of RNasin (;30 U; Promega Biotech). After 45 min of incubation at 308C, the reactions were terminated by the addition of 380 ml of stop buffer (50 mM Tris-HCl [pH 7.5], 1% SDS, 5 mM EDTA, 25mg of tRNA per ml) and were extracted three times with phenol-chloroform. The RNA was precipitated by the addition of 40ml of 3 M sodium acetate (pH 5.2) and 880 ml of ethanol. The RNA pellets were rinsed with 70% ethanol, air dried, resus-pended in 10ml of loading mix (80% formamide, 0.01% xylene cyanol, and 0.01% bromophenol blue in 13 Tris-borate-EDTA), and loaded on a 4% poly-acrylamide–7 M urea sequencing gel. The signal was analyzed with an image analyzer (Fuji).
RESULTS
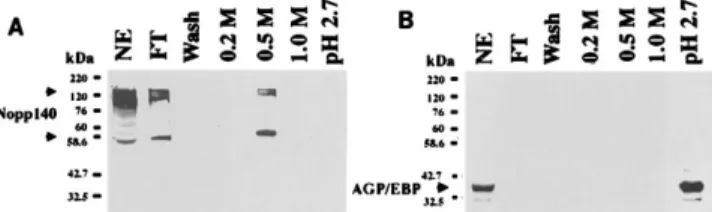
Association of Nopp140 and AGP/EBP.To investigate pro-teins that may interact with AGP/EBP, we performed anti-AGP/EBP immunoaffinity column chromatography and subse-quent SDS-PAGE analysis of rat liver nuclear extracts. One of the specific polypeptides retained by the anti-AGP/EBP im-munoaffinity column and eluted at 0.5 M NaCl has a molecular mass of approximately 55 kDa in SDS-PAGE (Fig. 1A). This polypeptide could not be retained by a control immunoaffinity column containing a nonspecific antibody (Fig. 1B). The 55-kDa polypeptide was eluted from the SDS-gel and subjected to trypsin digestion followed by LC/MS/MS analysis. One of the tryptic peptides showed a mass of 1,709, and the sequence matched that of the rat nucleolar phosphoprotein Nopp140 (amino acids 9 to 23, VVPSDLYPLVLGFLR). Other tryptic peptides identified were assigned to the heavy chain of the
FIG. 1. Purification of AGP/EBP-interacting proteins in rat liver nuclear extract on an anti-AGP/EBP antibody affinity column. For details, see Materials and Methods. Fractions were analyzed by SDS-PAGE (10% gel) and silver staining. (A) Anti-AGP/EBP antibody affinity column chromatography. NE, 15 mg of nuclear extract; FT, flowthrough fraction; 0.2 M, 0.5 M, and 1.0 M, NaCl elution fractions. (B) Nonspecific antibody affinity column chromatography frac-tions. A polypeptide with an apparent molecular mass of 55 kDa specifically retained by the anti-AGP/EBP antibody was eluted at 0.5 M NaCl (indicated by an arrowhead).
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
antibody molecule. With these data, we decided to clone the Nopp140 cDNA and prepare the recombinant protein and rabbit anti-Nopp140 antibodies. Western blot analysis using rabbit Nopp140 was performed on fractions from the anti-AGP/EBP affinity column. Two major bands of 55 and 140 kDa were detected (Fig. 2A). These results confirmed that Nopp140 or its derivative(s) was retained by the anti-AGP/ EBP affinity column. To rule out that anti-AGP/EBP antibody recognizes Nopp140, we applied rNopp140 to an anti-AGP/ EBP antibody immunoaffinity column. rNopp140 could not bind to the anti-AGP/EBP affinity column (data not shown). In contrast, not only Nopp140 but also AGP/EBP from the nu-clear extract could bind to the anti-AGP/EBP affinity column (Fig. 2B). These results indicate that Nopp140 does not react with the anti-AGP/EBP antibody but interacts with AGP/EBP in the nuclear extract. To investigate the biochemical basis for the specific retention of Nopp140 by the AGP/EBP anti-body affinity column, we performed immunoprecipitation ex-periments. Nuclear extracts from rat liver were immunopre-cipitated with anti-AGP/EBP, anti-Nopp140, or a control antibody and subjected to Western blotting using the anti-AGP/EBP antibody. Anti-anti-AGP/EBP and anti-Nopp140, but not the control antibody, could bring down AGP/EBP from liver nuclear extracts (Fig. 3A). To further demonstrate that AGP/EBP and Nopp140 could be immunoprecipitated by an-tibodies to AGP/EBP and Nopp140, we conducted several immunoprecipitation experiments using nuclear extracts from a number of cell lines. Immunoprecipitation of AGP/EBP by the anti-AGP/EBP or anti-Nopp140 antibody was achieved in
every nuclear extract that we examined, e.g., BHK, 129p (a hepatoma cell line from C3H mice), and HTC (a rat hepatoma cell line) (Fig. 3B). These results suggest that AGP/EBP and Nopp140 exist as a complex in nuclear extracts of normal liver tissue as well as in a number of cell lines.
To demonstrate unequivocally that Nopp140 and AGP/EBP can interact directly, recombinant Nopp140 was applied to an immobilized recombinant AGP/EBP affinity column and sub-sequently eluted with increasing NaCl concentrations. Specific retention of rNopp140 by the AGP/EBP Affi-Gel column was observed (Fig. 3C).
Taken together, these results demonstrate that there is di-rect physical interaction between Nopp140 and AGP/EBP.
Nopp140 functions as a transcriptional activator. Having demonstrated that Nopp140 can interact with AGP/EBP, we decided to examine the possible involvement of Nopp140 in transcription of the agp gene. Transfection experiments were performed with expression vectors of Nopp140 in both correct (CMV-Nopp140) and reverse [CMV-Nopp140(Rev)] orienta-tions. CMV-Nopp140, but not CMV-Nopp140(Rev), can acti-vate AGP-CAT in a dose-dependent manner (Fig. 4A, 0.5 and 1.0mg). These results suggest that Nopp140 may function as a transcription factor for the agp gene. To further demonstrate that Nopp140 functions as a transcriptional activator, we per-formed an in vitro transcription assay using HeLa cell nuclear extract and an AGP promoter-ligated G-free cassette (AGP-C2AT) as a reporter (12, 36). Purified rNopp140 was tested for its effect on the in vitro transcription of AGP-C2AT (AGP400). AdML200 was included as an internal control, while recombi-nant TFIIB was used as a positive control. The results show that both Nopp140 and TFIIB can stimulate in vitro transcrip-tion of AGP400 and AdML200 in a dose-dependent manner (Fig. 4B, 20 and 50 ng). The extents of stimulation by recom-binant Nopp140 and TFIIB on the AGP400 reporter were comparable (Fig. 4C). However, stimulation of AdML200 is better by TFIIB than by Nopp140 (Fig. 4C).
To further investigate the functional outcomes of the phys-ical interactions between Nopp140 and AGP/EBP, we per-formed cotransfection experiments using CMV-Nopp140 and CMV-AGP/EBP expression vectors and the AGP-CAT re-porter. Nopp140 and AGP/EBP can activate AGP-CAT syn-ergistically (Fig. 5A). A suboptimal dosage of the CMV-AGP/ EBP expression vector (i.e., 50 ng per transfection) was used. To assess the specificity of this synergistic interaction, expres-sion vectors of other transcription factors were used for
co-FIG. 2. Western blot analysis of fractions eluted from an anti-AGP/EBP antibody affinity column by using anti-Nopp140 and anti-AGP/EBP antibodies. Rat liver nuclear extract (15mg) and 15 ml from each fraction were separated by SDS-PAGE (10% gel), blotted to a Hybond-C membrane, and probed with an anti-Nopp140 (A) or anti-AGP/EBP (B) antibody. Nopp140 (both 55-kDa and a high-molecular-mass [presumably 140-kDa] species) were eluted at 0.5 M NaCl, while AGP/EBP was eluted at pH 2.7 glycine buffer.
FIG. 3. Immunoprecipitation followed by Western blot analysis demonstrating the existence of a AGP/EBP-Nopp140 complex in different cells. (A) Rat liver nuclear extracts (100mg) were immunoprecipitated with anti-Nopp140 (Nopp140), anti-AGP/EBP antibody (AGP/EBP), or control antibodies and subjected to SDS-PAGE and Western blot analysis using an anti-AGP/EBP antibody. Control antibodies used: rabbit preimmune serum (PI), IgG from rabbit anti-NF-kB-p50 and -p65, anti-c-Rel, and anti-c-Jun. (B) Nuclear extracts (100 mg) derived from 129p, HTC, and BHK cells were precipitated with anti-AGP/EBP (AGP/EBP), anti-Nopp140 (Nopp140), or preimmune serum (PI). NE denotes a direct loading of 15mg of nuclear extract onto an SDS-polyacrylamide gel. The membrane blot was probed with an anti-AGP/EBP antibody. (C) Recombinant Nopp140 (1mg) was chromatographed in an AGP/EBP affinity column. Western blot analysis was performed as described in Materials and Methods. Nopp140 was eluted at 0.5 M NaCl. rNopp140 was used as a control.
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
transfection experiments with CMV-Nopp140 with AGP-CAT as a reporter. Construct CMV-ATF2, CMV-YY1, or RSV-c-jun fail to cooperate with CMV-Nopp140 to activate AGP-CAT (Fig. 5B). The transcription factor AGP/EBP gene (agp/ ebp [c/ebpb]) can also be stimulated by CMV-Nopp140. Although there are motifs for both ATF2 and c-Jun in the agp/ebp promoter region, CMV-Nopp140 fails to interact with these transcription factors synergistically for activating agp/ebp expression (unpublished observations). Taken together, these results indicate that Nopp140 and AGP/EBP not only form a specific complex but also function in activation of the agp gene synergistically.
Nopp140 and AGP/EBP activate the agp gene synergistically in an AGP/EBP-binding motif-dependent manner. To delin-eate the activation functions of Nopp140 and AGP/EBP, we conducted cotransfection experiments using expression vectors CMV-AGP/EBP and CMV-Nopp140, with AGP-CAT as a reporter. As demonstrated in the previous experiments, AGP/ EBP and Nopp140 are transactivators for AGP-CAT and, when cotransfected, activate AGP-CAT synergistically (Fig. 5A). In previous studies (6, 23), we have established that mul-tiple AGP/EBP-binding motifs exists in the AGP promoter
(2180 to 110) region. These motifs are essential for transcrip-tional activation of the agp gene. To further elucidate the functional interaction between AGP/EBP and Nopp140, re-porters of both the wild type and mutants of three AGP/EBP motifs (i.e., C motif [273 to 283], D motif [294 to 2103], and E motif [2105 to 2115]) in the promoter region of the agp gene were used (23). We have demonstrated that mutation in the C or E motif of the agp promoter has a severe effect on activation of the agp gene by AGP/EBP (23). Evidently, the synergistic effect of AGP/EBP and Nopp140 on an E-motif mutant reporter gene is greatly reduced (Fig. 6A). Moreover, the synergistic effects of AGP/EBP and Nopp140 were abol-ished when both C and E motifs or C, D, and E motifs were mutated (Fig. 6A). However, the activation of AGP-CAT by Nopp140 alone seemed to be independent of any of these AGP/EBP motifs (Fig. 6A). Rather, the synergistic activation of AGP-CAT by AGP/EBP and Nopp140 was affected. These results suggest that the binding of AGP/EBP to its motifs is crucial for its subsequent activation of the agp gene by inter-acting with Nopp140 synergistically. To further examine the existence of a complex of Nopp140, AGP/EBP, and a probe containing the AGP/EBP-binding motif, we performed
elec-FIG. 4. Nopp140 functions as a transcriptional activator. (A) BHK cells were transfected with AGP-CAT (lanes 1 to 5, 2mg), CMV-Nopp (lane 2, 0.5 mg; lane 3, 1.0mg), or CMV-Nopp-Rev (lane 4, 0.5 mg; lane 5, 1.0 mg). Duplicate exper-iments were performed. (B and C) In vitro transcription assay. The reaction mixtures contain HeLa cell nuclear extracts (40mg) and AGP-C2AT (AGP400) and AdML200 reporter plasmids (1mg). (B) Lane 1, control; lanes 2 and 3, 20 and 50 ng of rNopp140; lanes 4 and 5, 20 and 50 ng of rTFIIB used as a positive control. (C) Fold stimulation of both AGP-C2AT and AdML200.
FIG. 5. Functional interaction between AGP/EBP and Nopp140. (A) Nopp140 and AGP/EBP activate the agp gene synergistically. Shown are relative CAT activities of BHK cells transfected with AGP-CAT (2mg) in the absence of any expression vectors and in the presence of CMV-AGP/EBP (0.05mg), CMV-Nopp140 (0.5mg), and CMV-AGP/EBP and CMV-Nopp140 (0.05 and 0.5 mg of each). (B) BHK cells were transfected with 2mg of AGP-CAT and 0.05 mg of CMV-AGP/EBP (AGP/EBP), CMV-ATF2 (ATF2), CMV-YY1 (YY1), or RSV-c-jun (c-Jun) in the absence (filled bars) or presence (hatched bars) of CMV-Nopp140.
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
trophoretic mobility shift experiments. Anti-Nopp140 or anti-AGP/EBP, but not a control antibody, can supershift the spe-cific complex formed by the liver nuclear extracts and the probe (Fig. 6B). Due to the antibody source, the electro-phoretic mobility supershifts resulting from monoclonal anti-AGP/EBP (Fig. 6B, lane 3) and rabbit anti-Nopp140 (Fig. 6B, lane 5) are different. Since Nopp140 is not a DNA-binding protein, anti-Nopp140 antibody supershift data indicate that it
exists in the complex of AGP/EBP and the AGP/EBP-recog-nized motif (probe). These results show that Nopp140 and AGP/EBP exist as a complex when AGP/EBP binds to its cognate motif and that this complex is critical for their syner-gistic activation of the agp gene.
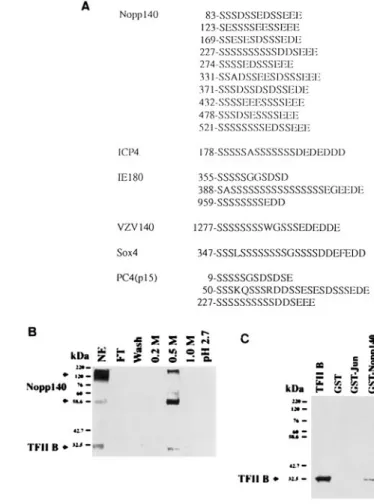
Interaction between Nopp140 and TFIIB.To elucidate the possible mechanisms involved in the activation of agp by Nopp140 per se and the synergistic interaction between Nopp140 and AGP/EBP in induction of the agp gene, we compared the sequences of Nopp140 and other transcription factors. We found that Nopp140 has several stretches of serine-and acidic amino acid-rich sequences (Fig. 7A). These se-quences are similar to those of a number of viral or cellular transcription factors such as ICP4 of herpes simplex virus type 1 (HSV-1) (37), IE180 of pseudorabies virus (7), IE62 of vari-cella-zoster virus (VZV) (32), a coactivator, PC4 (10, 18), and a transcription factor, Sox-4 (41) (Fig. 7A). It has been estab-lished that ICP4 interacts with TFIIB (37), while PC4 could interact with TFIIA and TFIID complexes (10, 15, 18), and mutations in the sequences of these factors result in
abolish-FIG. 6. Activation of agp by Nopp140 and AGP/EBP is dependent on the AGP/EBP-binding motif. (A) Upper panel, schematic representation of the agp promoter indicating mutations in the C, D, and E motifs: mutant C (273 to 275, ACA3GTG), mutant D (295 to 297, CAA3TGG); mutant E (2105 to 2107, AGA3GAG); mutant CE (mutations in C and E motifs); mutant CDE (muta-tions in all three motifs). Lower panel, BHK cells were cotransfected with 2mg of the wild type, mutant E, mutant CE, and mutant CDE with or without 0.5mg of CMV-Nopp140 or with 0.05mg of CMV-AGP/EBP or the combination of CMV-Nopp140 and CMV-AGP/EBP. (B) Electrophoretic mobility shift assay indicating the existence of Nopp140 in the complex of AGP/EBP and oligonu-cleotide probe E. Liver nuclear extracts (2mg) were incubated with 1 ng of probe (10,000 to 20,000 cpm/ng) and subjected to a gel mobility shift assay. Both anti-AGP/EBP (lane 3) and anti-Nopp140 (lane 5) antibodies can supershift the complex formed. Lane 1, probe alone; lanes 2 and 6, in the absence of antibody; lane 4, preimmune serum.
FIG. 7. Nopp140 interacts with TFIIB. (A) Several stretches of serine and acidic amino acid-rich sequences in Nopp140 were similar to the sequences of a number of transcription factors, including HSV-1 ICP4, VZV IE62, IE180, Sox-4, and PC4 (p15). (B) TFIIB is retained by an anti-AGP/EBP affinity col-umn. Liver nuclear extracts (NE; 30 mg) were fractionated in an anti-AGP/EBP antibody affinity column, and the fractions were analyzed by Western blot anal-ysis using anti-TFIIB and anti-Nopp140 antibodies. TFIIB and Nopp140 were coeluted at 0.5 M NaCl. (C) Recombinant TFIIB and Nopp140 form a complex. rTFIIB (0.1mg) was incubated with 3 mg of glutathione bead-immobilized GST, GST-c-Jun, and GST-Nopp140 at 48C for 1 h. The pellets were then subjected to SDS-PAGE and Western blot analysis using anti-TFIIB antibodies. FT, flow-through.
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
ment of their transcriptional activities. Thus, Nopp140 may interact with TFIIB in a fashion similar to these transcription factors. To test the possible existence of a Nopp140-TFIIB complex in the nuclear extracts, we performed anti-AGP/EBP antibody affinity column chromatography followed by Western blot analysis. Both TFIIB and Nopp140 were retained in the column and could be eluted at 0.5 M NaCl (Fig. 7B). To confirm this possibility, recombinant TFIIB was incubated with GST-Nopp140. The complex was isolated with glutathione beads and subjected to Western blot analysis. Our results dem-onstrated that Nopp140 can interact with TFIIB specifically (Fig. 7C). These results together with those shown above (i.e., Nopp140 was retained by the AGP/EBP affinity column) sug-gest that TFIIB and Nopp140 formed a complex.
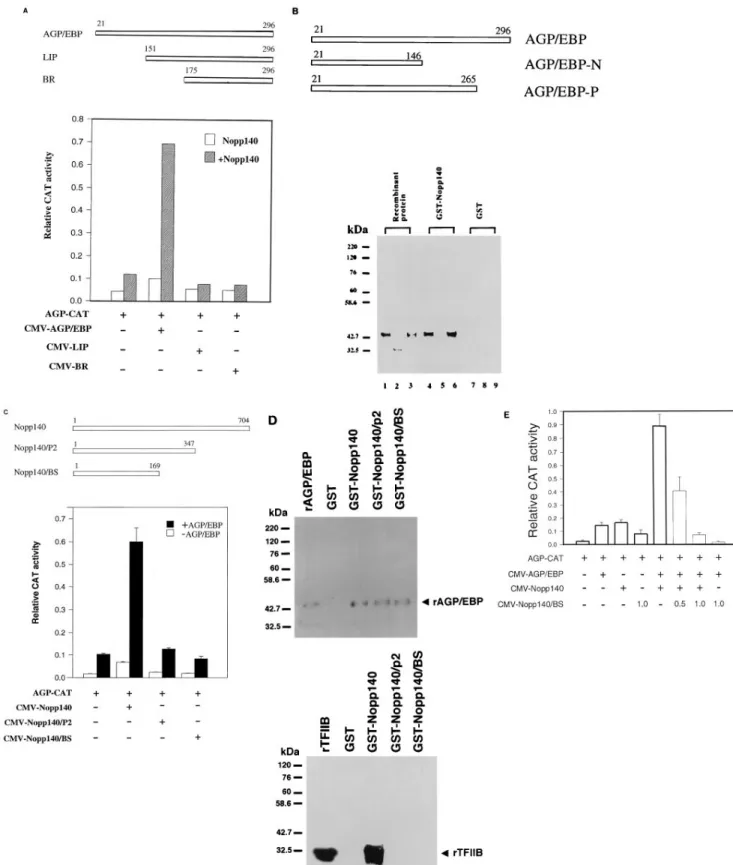
Physical and functional interactions between AGP/EBP and Nopp140 and between Nopp140 and TFIIB. To determine whether the intact molecules of AGP/EBP and Nopp140 are necessary for the synergistic activation of AGP-CAT, we per-formed transfection experiments using expression vectors for the deletion mutants of AGP/EBP and Nopp140. Expression vectors derived from serial amino-terminal deletion AGP/EBP cDNAs (wild-type AGP/EBP [amino acids 21 to 296], LIP [amino acids 151 to 296], and BR [amino acids 175 to 296] [Fig. 8A, upper panel]) were used for cotransfection with CMV-Nopp140. Expression vectors derived from NH2-terminal de-letion of AGP/EBP failed to activate the agp gene by cooper-ating with wild-type Nopp140. Only wild-type AGP/EBP (which contains intact activation domains) can cooperate with Nopp140 synergistically (Fig. 8A, lower panel). These results indicate that the transcriptional activation domains of AGP/ EBP (both LIP and BR mutants are lacking) are essential for its synergistic interaction with Nopp140. To elucidate the do-main of AGP/EBP critical for its physical interaction with Nopp140, we constructed deletion mutants lacking the leucine zipper or the entire bZIP domain (Fig. 8B, upper panel). Recombinant proteins of wild-type AGP/EBP, AGP/EBP-P (leucine zipper deletion mutant), and AGP/EBP-N (bZIP de-letion mutant) were used for binding experiments with GST-Nopp140 or a GST control. Both wild-type AGP/EBP and AGP/EBP-P could specifically bind GST-Nopp140 (Fig. 8B, lanes 4 and 6). However, AGP/EBP-N failed to interact with Nopp140 (Fig. 8B, lane 5). Thus, the basic region of AGP/EBP is critical for the interaction between AGP/EBP and Nopp140, whereas the bZIP domain is required for the dimerization and specific motif recognition of AGP/EBP.
To define the regions of Nopp140 that may interact with AGP/EBP, we constructed expression vectors of various dele-tion mutants of Nopp140 (Fig. 8C, upper panel). Expression vectors derived from mutants of Nopp140 failed to activate the AGP-CAT reporter by themselves or in combination with wild-type AGP/EBP expression vector (Fig. 8C, lower panel).
To further investigate the physical interaction between wild-type and mutant Nopp140 and AGP/EBP or TFIIB, we expressed these GST-Nopp140/P2 and GST-Nopp140/BS recombinant proteins and used them for biochemical char-acterizations. Both the wild-type construct (GST-Nopp140) and deletion mutants of Nopp140 (i.e., GST-Nopp140/P2 and GST-Nopp140/BS) can interact with AGP/EBP (Fig. 8D, upper panel). However, only wild-type GST-Nopp140, not deletion mutants GST-Nopp140/P2 and GST-Nopp140/ BS, can interact with TFIIB (Fig. 8D, lower panel). These re-sults suggest that the carboxyl-terminal portion of Nopp140 is crucial for its interaction with TFIIB, while the amino-terminal region is sufficient for its interaction with AGP/EBP.
To further address the functional interaction between AGP/ EBP and TFIIB through Nopp140, cotransfection assays using
CMV-AGP/EBP and wild-type CMV-Nopp140 and increasing amounts of CMV-Nopp140/BS were performed. Nopp140/BS represses the synergistic activation of AGP-CAT by AGP/EBP and wild-type Nopp140 in a dose-dependent manner (Fig. 8E). Since mutant Nopp140/BS can interact with AGP/EBP but cannot interact with TFIIB physically, the dominant negative effect of Nopp140/BS most likely resulted in disruption of the interaction between AGP/EBP and TFIIB through Nopp140.
Taken together, these results of physical interactions be-tween Nopp140 and AGP/EBP or TFIIB correlate nicely with the results of functional synergistic interaction between Nopp140 and AGP/EBP. Furthermore, these data suggest that Nopp140 may function as a coactivator for transcriptional activation of the agp gene by AGP/EBP.
DISCUSSION
We have identified and characterized the physical and func-tional interactions between a nucleolar phosphoprotein, Nopp140, and an acute-phase-responsive transcription factor, AGP/EBP. Several lines of evidence indicate that this interac-tion is specific. (i) Nopp140 was identified by LC/MS/MS anal-ysis as the protein retained by the anti-AGP/EBP affinity col-umn. Subsequent Western blot analysis and further serologic evidence indicate that Nopp140 and AGP/EBP exist in a com-plex which can be immunoprecipitated by antibodies to either protein. (ii) Two other nucleolar proteins, nucleolin and nu-cleolar phosphoprotein B23, do not interact with AGP/EBP: we have failed to detect complex formation between these proteins and AGP/EBP in nuclear extracts prepared from liver and other cells. Cotransfection studies also failed to reveal any functional interactions between these proteins and AGP/EBP. (iii) A number of transcription factors, such as YY1, c-Jun, and ATF2, failed to interact with Nopp140 physically and function-ally. We have extended these observations to the agp/ebp gene, which may be activated by either c-Jun/ATF2 or Nopp140, yet there is no synergism between factors (unpublished observa-tions). (iv) The interaction between AGP/EBP and Nopp140 that results in activation of the agp gene depends on the AGP/ EBP-binding motif. There are three AGP/EBP-binding mo-tifs (C, D, and E) in the promoter region of the agp gene. If any one of these motifs is mutated, the synergism between Nopp140 and AGP/EBP is lost. Apparently, multiple AGP/ EBP-binding motifs are necessary for the synergistic interac-tion between AGP/EBP and Nopp140. The multiple AGP/ EBP-binding motifs exists in many acute-phase protein genes. It is likely that these genes may also be subjected to similar regulation by AGP/EBP and Nopp140. (v) There is no obvious RNA- or DNA-binding domain in Nopp140 (27). We have performed DNase I footprinting experiments using a DNA probe derived from the agp gene promoter region (2180 to 110) and recombinant wild-type Nopp140 but failed to un-cover any sequence-specific recognition by Nopp140. Further-more, we performed an electrophoretic mobility shift assay using an AGP/EBP-binding oligonucleotide as a probe and rNopp140 and failed to detect any complex formation. These facts suggest that at least in the agp promoter, there is unlikely to be any DNA binding by Nopp140. The electrophoretic mo-bility supershift of the complex formed between the nuclear extract and the AGP/EBP-E motif probe by anti-AGP/EBP or anti-Nopp140 suggests that AGP/EBP and Nopp140 are present in a complex that binds to the AGP/EBP-binding mo-tif. (vi) When the NH2-terminal portion of AGP/EBP or car-boxy-terminal portion of Nopp140 was deleted, synergistic in-teractions between these two factors were abolished. These
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
FIG. 8. Biochemical and functional characterizations of interactions between AGP/EBP and Nopp140 or Nopp140 and TFIIB. (A) Upper panel, schematic representation of expression vectors constructed from the wild type (amino acids 21 to 296) and deletion mutants of AGP/EBP (LIP, amino acids 151 to 296; BR, amino acids 175 to 296). Lower panel, transfection assay demonstrating that the amino-terminal region is crucial for functional synergism between AGP/EBP and Nopp140. BHK cells were transfected with 2mg of AGP-CAT together with 50 ng of CMV-AGP/EBP, CMV-LIP, or CMV-BR in the absence or presence of 0.5 mg of CMV-Nopp140. (B) Upper panel, schematic representation of the recombinant proteins from wild-type AGP/EBP (AGP/EBP; amino acids 21 to 296) and deletion mutants AGP/EBP-N (amino acids 21 to 146) and AGP/EBP-P (amino acids 21 to 265). Lower panel, aliquots (100 ng) of different AGP/EBP recombinant proteins (lanes 1, 4, and 7, wild-type AGP/EBP; lanes 2, 5, and 8, AGP/EBP-N; lanes 3, 6, and 9, AGP/EBP-P) were incubated with glutathione bead-immobilized recombinant GST-Nopp140 (lanes 4 to 6) or GST (lanes 7 to 9). After extensive washing, the bound proteins were subjected to SDS-PAGE and Western blot analysis and probed
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
results suggest that the complex formation between Nopp140 and specific sequence-bound AGP/EBP is responsible for tran-scriptional activation of the agp gene.
Although Nopp140 has been observed primarily in the dense fibrillar component of the nucleolus, it could also be seen in the nucleoplasm. It was occasionally found in curvilinear tracks that extended for micrometers across the nucleoplasm from the dense fibrillar component of the nucleolus to nuclear pore complexes (25). These observations indicate that Nopp140, when functioning as a nuclear localization signal-binding factor that leads to the nuclear import of proteins, may interact with other proteins in the nuclear pore complex (25, 28). The phys-ical localization of Nopp140 in the nucleoplasm suggests that it may have functions other than its possible roles in nuclear transport. Our present findings indicate that Nopp140 may function as a transcriptional activator.
Apparently, Nopp140 can activate several other genes with minimal promoter elements (i.e., TATA box [28a]). The fact that activation of AGP-CAT by Nopp140 alone (but not by synergistic interaction between AGP/EBP and Nopp140) is independent of the wild type or the mutants of the AGP/EBP-binding motif located in the promoter region of the agp gene suggests that Nopp140 per se is a transcriptional activator. Furthermore, other promoter-linked CAT reporters such as c/ebpb-CAT and simian virus 40 promoter-CAT can also be activated by CMV-Nopp140 in transfection assays (data not shown). These results suggest that Nopp140 is likely to func-tion as a general transcripfunc-tional activator. This conclusion is further supported by the results of stimulation of in vitro tran-scription (using the AGP400 or AdML200 reporter and HeLa cell nuclear extract) by rNopp140. We were unable to demon-strate that rNopp140 and rAGP/EBP stimulate the AGP400 or AdML200 reporter in an in vitro transcription assay. The ma-jor difficulty in correlating the results of in vitro transcription and the transfection assay is that we do not know whether the synergistic interaction between AGP/EBP and Nopp140 de-pends on the heterodimeric or homodimeric form of AGP/ EBP or other factors. Furthermore, posttranslational modifi-cations, including phosphorylation of Nopp140 and/or AGP/ EBP, may also be important.
The most prominent feature of the Nopp140 sequence is that it consists of multiple stretches of serine- and acidic amino acid-rich sequences. Similar sequences in ICP4 of HSV-1 (37), IE180 of pseudorabies virus (7), IE62 of VZV (32), Sox-4 (41), and PC4 (p15) (10, 18) have been identified, and some of them have been shown to be important for transcriptional activation. In the case of ICP4, the serine- and acidic amino acid-rich sequence is also important for its interaction with TFIIB (37). In the present report, we have demonstrated that Nopp140 interacts with TFIIB. Particularly, the carboxyl-terminal por-tion of Nopp140 is crucial for its interacpor-tion with TFIIB. Mu-tants (Nopp140/P2 and Nopp140/BS) that fail to interact with TFIIB also lose their activation function. However, these mu-tants still could interact with AGP/EBP, indicating that the
amino-terminal portion of Nopp140 is sufficient for its physical interaction with the carboxyl-terminal region of AGP/EBP. However, functional interaction requires the amino-terminal part of AGP/EBP, which contains the transcriptional activation domain (which is lacking in either the LIP or BR mutant of AGP/EBP). Furthermore, in the in vitro transcription assay, only wild-type Nopp140, not mutant Nopp140/BS, can stimu-late the AGP400 or AdML200 reporter (data not shown). These results suggest that the inability of TFIIB and Nopp140/ BS to interact may account for the loss of stimulatory activity of Nopp140/BS. When nuclear extract was applied to the mo-noclonal anti-AGP/EBP antibody affinity column, both Nopp140 and TFIIB were retarded and eluted at 0.5 M NaCl, while AGP/EBP was eluted with pH 2.7 glycine buffer. These data suggest that Nopp140, TFIIB, and AGP/EBP (most likely het-erodimeric forms) may form a ternary complex. Nopp140/BS does function as a dominant negative factor for the synergistic activation of the agp promoter by AGP/EBP and wild-type Nopp140 (Fig. 8E). Since Nopp140/BS is unable to interact with TFIIB but remains interactive with AGP/EBP, it may compete for AGP/EBP binding by wild-type Nopp140 and therefore interrupt the interaction between AGP/EBP and TFIIB through Nopp140. Thus, the mechanism of synergistic stimulation of AGP-CAT by Nopp140 and AGP/EBP is likely to involve Nopp140-AGP/EBP-TFIIB ternary complex forma-tion. Together these results suggest that Nopp140 functions as a coactivator in transcriptional activation of the agp gene. The result regarding Nopp140-TFIIB interaction is preliminary, however; much work is needed to demonstrate that TFIIB is a target for Nopp140. As discussed above, Nopp140 functions as a general transcription activator. The fact that Nopp140 also interacts with TFIIB suggests that Nopp140 may serve as a coactivator linking a general transcription factor and an up-stream activator (i.e., AGP/EBP). This interpretation is tenta-tive pending further experiments on the in vivo interactions of Nopp140, TFIIB, and transcriptional activators (i.e., AGP/ EBP). In an attempt to demonstrate a specific effect of AGP/ EBP or AGP/EBP and Nopp140 on the agp promoter, in vitro transcription experiments were performed. rAGP/EBP fails to stimulate the AGP400 or AdML200 reporter by itself or in conjunction with rNopp140 (data not shown). These results suggest that a simple in vitro transcription assay may not reflect the in vivo situations; for example, heterodimeric AGP/EBP or posttranslationally modified forms may exist. Thus, transcrip-tional activation of the agp gene by Nopp140 may be mediated by its linking the motif-bound AGP/EBP-containing activa-tor(s) (e.g., heterodimeric AGP/EBP) and general transcrip-tion factor TFIIB (13).
Nopp140 isolated from rat liver has been shown to exist in multiple forms with different sizes (26, 31). Whether this is due to nonspecific degradation or posttranslational modification by specific proteases is not known (31). The fact that a poly-peptide of 55 kDa interacting with AGP/EBP was isolated and identified as Nopp140 suggests that at least some of the
with an anti-AGP/EBP antibody. Lanes 1 to 3 were loaded with 5 ng of recombinant proteins. (C) Upper panel, schematic representations of wild-type Nopp140 and deletion mutants Nopp140/P2 and Nopp140/BS. Lower panel, transfection assay demonstrating that wild-type but not mutant Nopp140 can interact with AGP/EBP synergistically. (D) Western blot analysis demonstrating the interaction of Nopp140 and AGP/EBP or TFIIB. Upper panel, recombinant GST-Nopp140 (wild-type and deletion mutants are the same as in panel C) proteins (3mg) were incubated with wild-type recombinant AGP/EBP (100 ng). Both wild-type and mutant Nopp140 can interact with AGP/EBP. Lower panel, recombinant GST-Nopp140 (wild-type and deletion mutants are the same as in panel C) proteins (3mg) were incubated with rTFIIB (100 ng). Only wild-type Nopp140 can interact with TFIIB. rAGP/EBP and rTFIIB represent 5 and 20 ng of recombinant proteins loaded onto an SDS-polyacrylamide gel. (E) Mutant Nopp140/BS functions as a dominant negative factor for synergistic activation of the agp promoter by AGP/EBP and wild-type Nopp140 in a cotransfection assay. Transfection conditions were as detailed in Materials and Methods. BHK cells were cotransfected with AGP-CAT (2mg) plasmid (1) and 0.05 mg of CMV-AGP/EBP (1), 0.5 mg of CMV-Nopp140 (1), or 1.0 mg of CMV-Nopp140/BS. To test the effect of CMV-Nopp140/BS on the synergistic activation of AGP-CAT by AGP/EBP and Nopp140, BHK cells were cotransfected with CMV-AGP/EBP (0.05mg), CMV-Nopp140 (0.5 mg), and 0.5 or 1.0 mg of CMV-Nopp140/BS.
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
smaller polypeptides derived from Nopp140 may interact with AGP/EBP. However, the functional relevance of the 55-kDa Nopp140–AGP/EBP interaction is unclear. Results of the trans-fection assay using deletion mutants of Nopp140 and wild-type AGP/EBP suggest that intact Nopp140 is essential for func-tional interaction with AGP/EBP. Furthermore, the amino acid sequence of a tryptic peptide isolated from the AGP/EBP-interacting 55-kDa polypeptide, spanning from positions 9 to 23 of Nopp140, suggests that the 55-kDa polypeptide contains the amino-terminal portion of Nopp140 and that this polypep-tide is sufficient for its physical interaction with AGP/EBP. Nopp140 is one of the most highly phosphorylated proteins: over 10% (82 of 704) of all amino acid residues present in Nopp140 constitute potential phosphorylation sites. Further-more, Nopp140 seems to occur only in a completely phosphor-ylated or dephosphorphosphor-ylated state and not in forms with an intermediate degree of phosphorylation (26). The phosphory-lation state of Nopp140 may be critical for its function. We do not know which state of Nopp140 can interact with AGP/EBP functionally. Although purified rNopp140 (unstable compared with Nopp140 isolated from cell lines) can interact with AGP/ EBP in an in vitro assay, we do not know whether the unphos-phorylated form of Nopp140 can complex with AGP/EBP in vivo. The function of the phosphorylated Nopp140 may be analogous to that of the phosphorylated form ICP4 of HSV1 (44, 45) or PC4 (11); i.e., it may involve in functional interac-tion with general transcripinterac-tion factors.
Does Nopp140 interact with other members of the C/EBP family such as C/EBPa? Although the NH2-terminal regions of C/EBPa and AGP/EBP are completely different, their bZIP domains are highly conserved. It is not surprising that the former factor has also been detected in a complex with Nopp140 in liver nuclear extract (data not shown). In cotrans-fection assays, agp can also be activated by C/EBPa and Nopp140 synergistically. One major difference between AGP/ EBP and C/EBPa is that the former factor responds to the acute-phase reaction positively whereas the latter responds negatively (2, 3). These data suggest that activation of the agp gene by AGP/EBP or C/EBPa and Nopp140 may be depen-dent on physiological conditions. Alternatively, interactions between Nopp140 and homodimers of AGP/EBP or C/EBPa or heterodimers of AGP/EBP and its family members could result in complete different outcomes in activating a specific gene. These possibilities remain to be studied.
ACKNOWLEDGMENTS
We thank J. K. Eng, A. L. McCormack, and J. R. Yates, Department of Molecular Biotechnology, University of Washington, Seattle, for LC/MS/MS analysis to identify Nopp140 as the AGP/EBP-interacting protein, Young-Sun Lin for ATF2 and TFIIB expression vectors, U. Schibler for the G-free cassette and AdML-C2AT reporter plasmids, and Ruey-Hwa Chen for reviewing the manuscript.
This research was supported by a grant from the National Science Council (NSC85-2331-B002-253), Taiwan.
REFERENCES
1. Akira, S., H. Isshiki, T. Sugiya, O. Tanabe, S. Kinoshita, Y. Nishio, T.
Nakajima, T. Hirano, and T. Kishimoto.1990. A nuclear factor for IL-6 expression (NF-IL6) is a member of C/EBP family. EMBO J. 9:1897–1906. 2. Akira, S., and T. Kishimoto. 1992. IL-6 and NF-IL6 in acute-phase response
and viral infection. Immunol. Rev. 127:25–50.
3. Baumann, H., and J. Gauldie. 1994. The acute phase response. Immunol. Today 15:74–80.
4. Birch, H. E., and G. Schreiber. 1986. Transcriptional regulation of plasma protein synthesis during inflammation. J. Biol. Chem. 261:8077–8080. 5. Cao, Z., R. M. Umek, and S. L. McKnight. 1991. Regulated expression of
three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5:1538–1552.
6. Chang, C. J., T. T. Chen, H. Y. Lei, D. S. Chen, and S. C. Lee. 1990.
Molecular cloning of a transcription factor, AGP/EBP, that belongs to mem-bers of the C/EBP family. Mol. Cell. Biol. 10:6642–6653.
7. Cheung, A. K. 1989. DNA nucleotide sequence analysis of the immediate-early gene of pseudorabies virus. Nucleic Acids Res. 17:4637–4646. 8. Descombes, P., M. Chojkier, S. Lichtsteiner, E. Flavey, and U. Schibler.
1990. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 4:1541–1551. 9. Descombes, P., and U. Schibler. 1991. A liver-enriched transcription
activa-tor protein, LAP, and a transcriptional inhibiactiva-tor protein, LIP are translated from the same mRNA. Cell 67:569–579.
10. Ge, H., and R. G. Roeder. 1994. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell 78:513–523.
11. Ge, H., Y. Zhao, B. T. Chait, and R. G. Roeder. 1994. Phosphorylation negatively regulates the function of coactivator PC4. Proc. Natl. Acad. Sci. USA 91:12691–12694.
12. Gorski, K., M. Caneiro, and U. Schibler. 1986. Tissue-specific in vitro tran-scription from the mouse albumin promoter. Cell 47:767–776.
13. Guarente, L. 1995. Transcriptional coactivators in yeast and beyond. Trends Biochem. Sci. 20:517–521.
14. Hsu, W., T. K. Kerppola, P. L. Chen, T. Curran, and S. Chen-Kiang. 1994. Fos and Jun repress transcription activation by NF-IL6 through association at the basic zipper region. Mol. Cell. Biol. 14:268–276.
15. Kaiser, K., G. Stelzer, and M. Meisterernst. 1995. The coactivator p15 (PC4) initiates transcriptional activation during TFIIA-TFIID-promoter complex formation. EMBO J. 14:3520–3527.
16. Kinoshita, S., S. Akira, and T. Kishimoto. 1992. A member of the C/EBP family, NF-ILb, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc. Natl. Acad. Sci. USA 89:1473–1476.
17. Koj, A. 1985. Pages 139–144. In A. H. Gordon and A. Koj (ed.), The acute phase response to injury and infection. Elsevier, Amsterdam, The Nether-lands.
18. Kretzschmar, M., K. Kaiser, F. Lottspeich, and M. Meisterernst. 1994. A novel mediator of class II gene transcription with homology to viral imme-diate-early transcriptional regulators. Cell 78:525–534.
19. Kulkarni, A. B., R. Reinke, and P. Feigelson. 1985. Acute phase mediators and glucorticoids elevatea-1 acid glycoprotein gene transcription. J. Biol. Chem. 260:15386–15389.
20. Landschultz, W. H., P. F. Johnson, E. Y. Adashi, B. J. Graves, and S. L.
McKnight.1988. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 2:786–800.
21. Landschultz, W. H., P. F. Johnson, and S. L. McKnight. 1989. The binding domain of the rat liver nuclear protein C/EBP is bipartite. Science 243:1681– 1688.
22. Lee, Y.-M., L.-H. Miau, C.-J. Chang, and S.-C. Lee. 1996. Transcriptional induction of alpha-1 acid glycoprotein (AGP) gene by synergistic interaction of two alternative activator forms of AGP/enhancer-binding protein (C/ EBPb) and NF-kB or Nopp140. Mol. Cell. Biol. 16:4257–4263.
23. Lee, Y. M., W. H. Tsai, M. Y. Lai, D. S. Chen, and S. C. Lee. 1993. Induction of liver alpha-1 acid glycoprotein gene expression involves both positive and negative transcription factors. Mol. Cell. Biol. 13:432–442.
24. Margulies, L., and P. B. Sehgal. 1993. Modulation of the human interleu-kin-6 promoter (IL-6) and transcription factor C/EBPb (NF-IL6) activity by p53 species. J. Biol. Chem. 268:15096–15100.
25. Meier, U. T., and G. Blobel. 1990. A nuclear localization signal binding protein in the nucleolus. J. Cell Biol. 111:2235–2245.
26. Meier, U. T., and G. Blobel. 1992. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell 70:127–138.
27. Meier, U. T., and G. Blobel. 1994. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J. Cell Biol. 127:1505–1514. 28. Melchior, F., and L. Gerace. 1995. Mechanisms of nuclear protein import.
Curr. Opin. Cell Biol. 7:310–318. 28a.Miau, L.-H. Unpublished results.
29. Nishio, Y., H. Isshiki, T. Kishimoto, and S. Akira. 1993. A nuclear factor for interleukin-6 expression (NF-IL6) and the glucocorticoid receptor synergis-tically activate transcription of the rata1-acid glycoprotein gene via direct protein-protein interaction. Mol. Cell. Biol. 13:1854–1862.
30. Nolan, G. P. 1994. NF-AT-AP-1 and Rel-bZIP: hybrid vigor and binding under the influence. Cell 77:795–798.
31. Pai, C. Y., H. K. Chen, H. L. Sheu, and N. H. Yeh. 1995. Cell cycle-dependent alterations of a highly phosphorylated nucleolar protein p130 are associated with nucleogenesis. J. Cell Sci. 108:1911–1920.
32. Perera, L. P., J. D. Mosca, W. T. Ruyechan, G. S. Hayward, S. E. Straus, and
J. Hay.1993. A major transactivator of varicella-zoster virus, the immediate-early protein IE62, contains a potent N-terminal activation domain. J. Virol.
67:4474–4483.
33. Poli, V., F. P. Mancini, and R. Cortese. 1990. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper protein related to C/EBP. Cell 63:643–653.
34. Roman, C., J. S. Platero, J. D. Shuman, and K. Calame. 1990. Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org
similar to C/EBP and heterodimerizes with C/EBP. Genes Dev. 4:1404–1415. 35. Ron, D., and J. F. Habener. 1992. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant negative inhibitor of gene transcription. Genes Dev. 6:439–453.
36. Sawadogo, M., and R. G. Roeder. 1985. Factors involved in specific tran-scription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc. Natl. Acad. Sci. USA 82:4394–4398.
37. Smith, C. A., P. Bates, R. R. Gonzalez, B. Gu, and N. A. DeLuca. 1993. ICP4, the major transcriptional regulator protein of herpes simplex virus type 1, forms a tripartite complex with TATA-binding protein and TFIIB. J. Virol.
67:4676–4687.
38. Stein, B., P. C. Cogswell, and A. S. Baldwin, Jr. 1993. Functional and physical association between NF-kB and C/EBP family members: a Rel domain-bZIP interaction. Mol. Cell. Biol. 13:3964–3974.
39. Thanos, D., and T. Maniatis. 1995. NF-kB: a lesson in family values. Cell
80:529–532.
40. Vannice, J. L., J. M. Taylor, and G. M. Ringold. 1984. Glucorticoid-mediated induction ofa-1 acid glycoprotein: evidence for hormone-regulated RNA
processing. Proc. Natl. Acad. Sci. USA 81:4241–4245.
41. Wetering, M. V. D., M. Oosterwegel, K. Norren, and H. Clevers. 1993. Sox-4, an Sry-like HMG box proteins, is a transcriptional activator in lymphocytes. EMBO J. 12:3847–3854.
42. Williams, P., T. Ratajczak, S. C. Lee, and G. M. Ringold. 1991. AGP/ EBP(LAP) expressed in rat hepatoma cells interacts with multiple promoter sites and its necessary for maximal glucocorticoid induction of the rat alpha-1 acid glycoprotein gene. Mol. Cell. Biol. 10:4959–4965.
43. Williams, S. C., C. A. Cantwell, and P. F. Johnson. 1991. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 5:1553–1567.
44. Xia, K., N. A. DeLuca, and D. M. Knipe. 1996a. Analysis of phosphorylation sites of herpes simplex virus type 1 ICP4. J. Virol. 70:1061–1071. 45. Xia, K., D. M. Knipe, and N. A. DeLuca. 1996b. Role of protein kinase A and
the serine-rich region of herpes simplex virus type 1 ICP4 in viral replication. J. Virol. 70:1050–1060.
46. Yang, T. H., W. H. Tsai, Y. M. Lee, H. Y. Lei, M. Y. Lai, D. S. Chen, N. H.
Yeh, and S. C. Lee.1994. Purification and characterization of nucleolin and its identification as a transcription repressor. Mol. Cell. Biol. 14:6068–6074.
at NATIONAL TAIWAN UNIV MED LIB on June 17, 2009
mcb.asm.org