Crosslinking of Cotton Cellulose in the Presence of Alkyl Di-ally
Ammonium Salts Part II : Reaction Kinetics
Chen, Jui-Chin Yao, Wei-Hua
Department of Materials and Textiles
Key Words
alkyl di-allyl ammonium salts, specific rate constants, enthalpy, entropy, free energy, dimethyloldihydroxy-ethyleneurea (DMDHEU)
SYNOPSIS
We used two kinds of alkyl di-allyl ammonium salts (alkyl groups are methyl and propyl) to combine with dimethyloldihydroxyethy- leneurea (DMDHEU) as crosslinking agents to study the specific rate constants and other activation parameters, and find that the rate constants for the various crosslinking agent systems are ranked as follows : DMDHEU-methyl di-allyl ammonium salt > DMDHEU-propyl di-allyl ammonium salt > DMDHEU. The energies needed for the crosslinking reaction are in the rank of DMDHEU > DMDHEU-propyl di-allyl ammonium salt > DMDHEU-methyl di-allyl ammonium salt. The enthalpies and entropies of activation for DMDHEU are higher than those for DMDHEU-alkyl di-allyl ammonium salts; and enthalpies of activation for DMDHEU are significantly higher than those for DMDHEU-alkyl di-allyl ammonium salts. The data of various activation parameters reveal that the reaction state of DMDHEU-alkyl di-allyl ammonium salts is different from that of DMDHEU.
INTRODUCTION
Our previous study [1] showed that both the
-OH group of the cellulose and dimethyloldihy- droxyethyleneurea (DMDHEU) can react with the vinyl and/or epoxy group of alkyl di-allyl ammonium salts in pad-dry-cure process, and the physical properties and surface migration for DMDHEU -alkyl di-allyl ammonium salts are different from those for DMDHEU. Sumrell et al. [2] mentioned that the vinyl group of N-vinylamide, N-vinyl-sulfonamide, and vinyl ethers could react with cotton cellulose in the presence of acid catalyst. It is well known that reaction rate constants are affected by varying the functional groups on crosslinking agents [3,4,5,6].
In this study, we are interested in the effects of the addition of various alkyl di-allyl ammonium salts in the DMDHEU padding aqueous on the crosslinking reaction. The detailed information of the specific rate constants and other activation parameters for the DMDHEU-alkyl di-allyl ammonium salts and DMDHEU treated fabrics is lacking. Here we examine and discuss those activation parameters calculated with the method described by Ziifle et al.[4,6].
EXPERIMENTAL
In this study, we used desized, scoured, and bleached cotton fabric 20s x 20s end (60) and picks (60).
The crosslinking agents used were DMDHEU ( dimethyloldihydroxyethyleneurea ) and alkyl di-allyl ammonium salts ( Which were synthesized with the method described by Rosemarie TÖpfl [7] .
The alkyl groups were - CH3 and - C3H7,
respectively.)
Ammonium sulfate was reagent grade, as were the other chemicals.
For reaction kinetic studies, the cotton fabric samples were padded twice to about 90% wet pickup with freshly prepared and DMDHEU-alkyl di-allyl ammonium salts ( 0.24M , the mole ratio of DMDHEU:alkyl di-allyl ammonium salts was 2:1 ) in the presence of the aluminum sulfate catalyst ( 0.024M ). Aqueous padding solution of DMDHEU alone was also used for comparison. In order to obtain the bound nitrogen contents (of the heated fabrics)which were changed with the change of heated temperatures(In these heated cases, they are not the fully cured conditions.) . Padded fabric samples were heated at 80, 90, and 100。
Nitrogen determinations were made using the Kjeldahl method.
C for different time intervals, soaped, washed, and dried.
RESULTS AND DISCUSSION
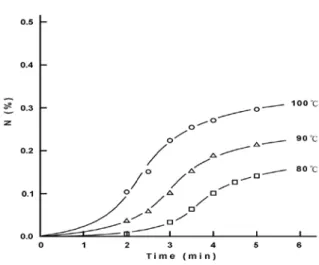
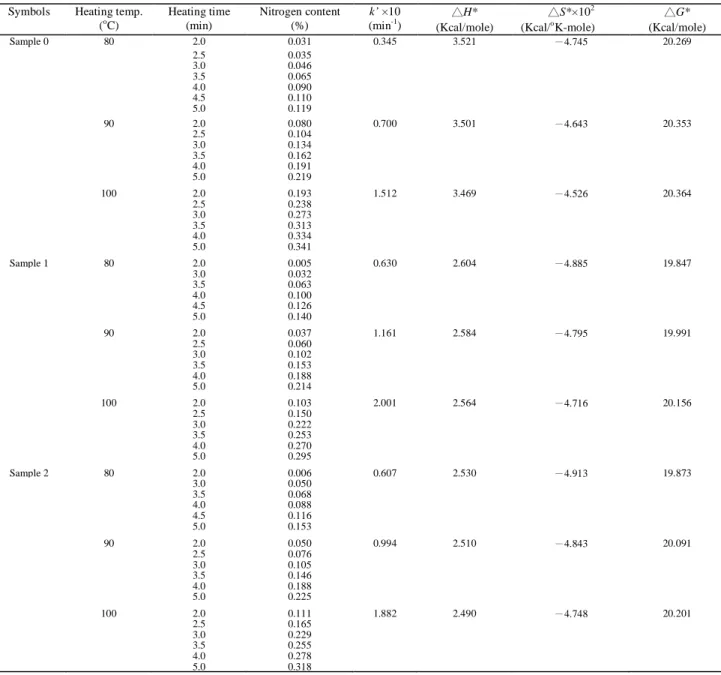
The data in Table 1 shows the changes in nitrogen content of cotton fabrics treated with the DMDHEU-methyl di-allyl ammonium salt ( sample 1 ), DMDHEU-propyl di-allyl ammonium salt ( sample 2 ), and DMDHEU ( sample 0 ),
respectively. All samples were catalyzed by aluminum sulfate with heated time ( minutes ). The table reveals that the nitrogen contents of the treated fabrics are increasing with the increase of heated time for all cases. Figures 1, 2, and 3 show relationships between the nitrogen contents and heated time intervals at different heated temperatures for sample 0, 1, and 2, respectively. Here we can see the initial slightly concave upward shape of the relationships between nitrogen content and the initial curing time in all cases, which we attribute to the time needed to raise the temperature of the fabric in the oven [4,8].
Semilogarithmic plots of ( %NO-%N)/%NO
against heated time in minutes are shown in Figure 4~6 for sample 0, 1, and 2, respectively. Where %NO is the nitrogen content of the treated fabric
after a pad-dry-cure process. ( That is, 90% wet pick-up, 80。C×5 minutes, 135。C×3 minutes; the samples are thought to be fully cured at this condition [9,10].) . %N is the bound nitrogen after a given time interval at the temperature indicated ( 80。C, 90。C and 100。
Table I also contains data calculated from the semilogarithmic plots using least square analysis for each data set at 80, 90, and 100
C ). We obtained straight lines for all cases, the linearity indicating that the reaction is pseudo-first-order for the time shown.
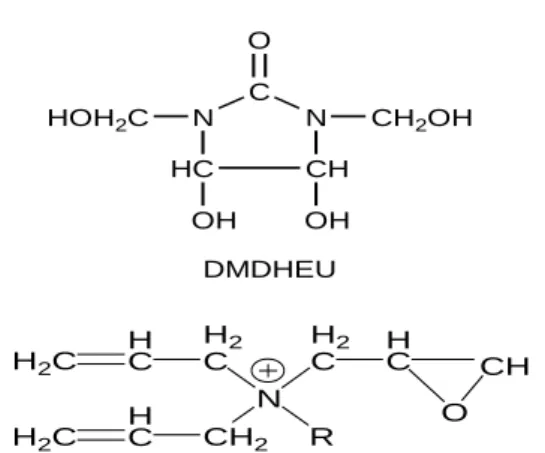
。 N HC C N CH O CH2OH HOH2C OH OH DMDHEU C. Based on pseudo-first-order reaction kinetics, the specific reaction rate constants ( k’ ) were calculated from the slopes of lines. The k’ values shown in Table I reveal that the rate constants for DMDHEU-alkyl di-allyl ammonium salts are higher than those for DMDHEU, and that for DMDHEU-methyl di-allyl ammonium salt is higher than DMDHEU-propyl di-allyl ammonium salt at all heated temperatures. The higher specific reaction rate constant may be caused by the reaction between the vinyl and epoxy
H2C H C H2 C N H2 C HC O CH CH2 H C H2C R
groups of alkyl di-allyl ammonium salts and the hydroxyl group of cellulose. Our previous study [1] revealed the crosslinking reaction between vinyl and epoxy groups of alkyl di-allyl ammonium salts and cellulose. Additionally, the longer propyl group may retard the reaction to lower the specific reaction rate constants of DMDHEU-propyl di-allyl ammonium salt.
Figure 7 shows the semilogarithmic Arrhenius plots of the specific reaction rate constants. Lines were determined using the least squares method and activation parameters for the various crosslinking agent systems are recorded in Table I, using the method of Ziifle et al. [6]. Free energies of activation (△G*) based on nitrogen for each fabric were positive. The energies needed for the crosslinking for each fabric are ranked as : sample 0 > sample 2 > sample 1. The positive enthalpies (△H*) of activation for each fabric indicate that the transition state complex is endothermic. The enthalpy of activation for DMDHEU is significantly higher than that for DMDHEU-alkyl di-allyl ammonium salts. However, the enthalpy of activation for DMDHEU-methyl di-allyl ammonium salt is only slightly higher than DMDHEU-propyl di-allyl ammonium salt. These results suggest that the transition state complex is obviously different for DMDHEU and DMDHEU-alkyl di-allyl ammonium salts. Entropies (△S*) of activation for each samples are negative, and are ranked as : sample 0 > sample 1 > sample 2. The entropies of activation are slightly affected by the existence of vinyl and epoxy groups in the DMDHEU-alkyl di-allyl ammonium salts.
CONCLUSIONS
In this study, DMDHEU and DMDHEU-alkyl di-allyl ammonium salts were used to treat cotton fabrics for the examination of the specific rate constants and other activation parameters. The rate
constants for DMDHEU-alkyl di-allyl ammonium salts are higher than those for DMDHEU, and that for DMDHEU-methyl di-allyl ammonium salt is higher than DMDHEU-propyl di-allyl ammonium salt. The energies needed for the crosslinking reaction are ranked as : DMDHEU > DMDHEU-propyl di-allyl ammonium salt > DMDHEU-methyl di-allyl ammonium salt. The enthalpies of activation for DMDHEU are significantly higher than those for DMDHEU-alkyl di-allyl ammonium salts, and those values for DMDHEU-methyl di-allyl ammonium salt is only slightly higher than DMDHEU-propyl di-allyl ammonium salt. The data of various activation parameters reveal that the vinyl and epoxy groups of DMDHEU-alkyl di-allyl ammonium salts do affect the reaction state and the energy consumption.
REFERENCES
1. J. C. Chen, W. H. Yao, J. T. Yeh, and C. C. Chen, Crosslinking of Cotton Cellulose in the Presence of Alkyl Di-ally Ammonium Salts, Part I : Physical Properties and Agent Distribution, Journal of Applied Polymer Science, ( accepted to be published at 2002 ).
2. G. Sumrell, M. F. Margavio, and C. M. Welch, The
Acrylation, Methacrylation, and Acrylamidomethylation of Cellulose, Application of
Two Novel Cross-Linking Reactions to Cotton,
Textile Res. J. 39, 78-85(1969).
3. R. J. Berni, R. R. Benerito, E. E. Coll, and E. J. Gonales, Kinetics of Reaction of Cotton with Dimethylolethy-
lcarbamate, Textile Res. J. 44, 47-55 (1974).
4. C. C. Chen, Kinetics of the Crosslinking Reaction of Cotton Catalyzed with Mixtures of Aluminum Salts and Tartaric Acid, Textile Res. J. 59, 337-342(1989).
5. E. J. Gonzales, R. R. Benerito, and R. J. Berni, Kinetics of the Reaction of Ethyleneurea Derivatives
with Cotton Cellulose, Part II: The Cellulose-Dimethyloldihydroxyethyleneurea
Reaction, Textile Res. J. 36, 571-578 (1966).
6. H. M. Ziifle, R. R. Benerito, E. J. Gonzales, and R. J. Berni, Kinetics of the Reactions of Ethyleneurea Derivatives with Cotton Cellulose, Part III: The Cellulose-Dimethylol- dihydroxyethyleneurea Reaction, Textile Res. J. 38, 925-930 (1968).
7. R. TÖpfl, Process for improving the Yield and the Wet Fastness Properties of Dyeing or Prints Produced with Anionic Dyes on Cellulose Fibre Material Using Alkyl Di-allyl or Halo-hydroxypropyl Ammonium Salts, U.S.Pat. 5,147,411, Sep. 15, 1992.
8. T.H. Sheen, S. M. Li, J. H. Wu, and C. C. Chen, Crosslinking of Cotton Fabrics Premercerized with Different Alkalis, Part I : Kinetics of the
Crosslinking, Textile Res. J. 63, 357-361(1993). 9. B. A. Kottes Andrews, and J. G. Jr. Frick, Catalysts
from Aluminum Alums in Crosslink Finishing,
Textile Res. J. 48, 50-56(1978).
10. R. M. H. Kullman, and R. M. Reinhardt, Aluminum Salt Catalysts in Durable-Press Finishing Treatments, Textile Res. J. 48, 320-324(1978).
摘要
本研究使用分別具有甲基及丙烯基之含不飽和 二甲基四級銨鹽混合於DMDHEU架橋劑,進行棉織物樹 脂加工反應動力學之比速常數及其他反應參數之探 討。其中發現不同架橋劑系統之速率常數大小順序為 DMDHEU-methyl di-allyl ammonium salt > DMDHEU-propyl di-allyl ammonium salt > DMDHEU。反應能量需求大小順序則相反。
Figure 1. Changes in nitrogen content of fabrics treated with 0.24M DMDHEU ( sample 0 ) and 0.024M aluminum sulfate as catalyst versus curing time in minutes. ( ○:100℃; △:90℃; □:80℃).
Figure 2. Changes in nitrogen content of fabrics treated with 0.24M DMDHEU- methyl di-allyl ammonium salts ( sample 1 ) and 0.024M aluminum sulfate as catalyst versus curing time in minutes. ( ○:100℃; △:90℃;
Figure 3. Changes in nitrogen content of fabrics treated with 0.24M DMDHEU- propyl di-allyl ammonium salts ( sample 2 ) and 0.024M aluminum sulfate as catalyst versus curing time in minutes. ( ○:100℃; △:90℃;
□:80℃).
Figure 4.Semilogarthmic graphs of ( %NO-%N ) / %NO versus curing time in
minutes for fabrics treated with 0.24M DMDHEU ( sample 0 ) and 0.024M aluminum sulfate as catalyst. ( ○:100℃; △:90℃; □:80℃).
Figure 5. Semilogarthmic graphs of ( %NO-%N ) / %NO versus curing time in
minutes for fabrics treated with 0.24M DMDHEU-methyl di-allyl ammonium salts ( sample 1 ) and 0.024M aluminum sulfate as catalyst. ( ○:100℃; △:90℃; □:80℃).
Figure 6. Semilogarthmic graphs of ( %NO-%N ) / %NO versus curing time in
minutes for fabrics treated with 0.24M DMDHEU-propyl di-allyl ammonium salts ( sample 2 ) and 0.024M aluminum sulfate as catalyst. ( ○:100℃; △:90℃; □:80℃).
Figure 7. Plots of logk’ based on nitrogen versus reciprocal of absolute temperature: ○: sample 0; △: sample 1; □: sample 2.
Table I. Reaction rate constants and activation parameters of cotton treated with DMDHEU ( sample 0 ), DMDHEU-methyl di-allyl ammonium salt ( sample 1 ), and DMDHEU-propyl di-allyl ammonium salt ( sample 2 ) at different conditions.
Symbols Heating temp. (o Heating time (min) C) Nitrogen content (%) k’ ×10 (min-1 △H* ) (Kcal/mole) △S*×10 (Kcal/ 2 o △G* K-mole) (Kcal/mole) Sample 0 80 2.0 0.031 0.345 3.521 -4.745 20.269 2.5 0.035 3.0 0.046 3.5 0.065 4.0 0.090 4.5 0.110 5.0 0.119 90 2.0 0.080 0.700 3.501 -4.643 20.353 2.5 0.104 3.0 0.134 3.5 0.162 4.0 0.191 5.0 0.219 100 2.0 0.193 1.512 3.469 -4.526 20.364 2.5 0.238 3.0 0.273 3.5 0.313 4.0 0.334 5.0 0.341 Sample 1 80 2.0 0.005 0.630 2.604 -4.885 19.847 3.0 0.032 3.5 0.063 4.0 0.100 4.5 0.126 5.0 0.140 90 2.0 0.037 1.161 2.584 -4.795 19.991 2.5 0.060 3.0 0.102 3.5 0.153 4.0 0.188 5.0 0.214 100 2.0 0.103 2.001 2.564 -4.716 20.156 2.5 0.150 3.0 0.222 3.5 0.253 4.0 0.270 5.0 0.295 Sample 2 80 2.0 0.006 0.607 2.530 -4.913 19.873 3.0 0.050 3.5 0.068 4.0 0.088 4.5 0.116 5.0 0.153 90 2.0 0.050 0.994 2.510 -4.843 20.091 2.5 0.076 3.0 0.105 3.5 0.146 4.0 0.188 5.0 0.225 100 2.0 0.111 1.882 2.490 -4.748 20.201 2.5 0.165 3.0 0.229 3.5 0.255 4.0 0.278 5.0 0.318