Human Serum Albumin Stabilized Gold Nanoclusters as Selective
Luminescent Probes for
Staphylococcus aureus and
Methicillin-Resistant
Staphylococcus aureus
Po-Han Chan and Yu-Chie Chen*
Department of Applied Chemistry, National Chiao Tung University, Hsinchu 300, Taiwan
*
S Supporting InformationABSTRACT: In this work, human serum albumin (HSA) stabilized gold nanoclusters (HSA-AuNCs) with reddish photoluminescence were used as sensing probes for pathogenic bacteria including Enterobacter cloacae, Escherichia coli J96, Pseudomonas aeruginosa, pandrug-resistant Acinetobacter baumannii (PDRAB), Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Streptococcus pyogenes, and vancomycin-resistant Enterococcus faecalis (VRE). We discovered that HSA-AuNCs have unique affinity with S. aureus and MRSA. In addition to demonstrating the selective sensing ability of HSA-AuNCs toward S. aureus and MRSA, the binding peptide
motifs identified from HSA-AuNCs were characterized by mass
spectrometry. The identified binding peptides were further used as the reducing and stabilizing agents for generation of peptide-bound AuNCs (Pep-AuNCs). The generated Pep-AuNCs were demonstrated to have the binding affinities with S. aureus and MRSA.
S
taphylococcus aureus and methicillin-resistant S. aureus (MRSA) are common nosocomial pathogens. MRSA is especially notorious in the history of bacterial infections because of its antibiotic-resistant properties. Bacterial infections may be fatal with improper medical treatment. Conventional biochemical assays for microorganism identification are not efficient; thus, a rapid and sensitive diagnostics assay must be developed for proper medical care. Functional nanoparticles play an important role in the development of rapid bacterial identification methods.1−9 Nanoparticles are used as affinity probes based on their unique properties, including magnetic features,1−9visible colors,10−14 or photoluminescence.15−18Protein-encapsulated gold nanoclusters (AuNCs) (protein-AuNCs) are gaining substantial interest because of their bright photoluminescence and ease of synthesis.19Several applications have been explored within a short period.20−28 We recently used lysozymes as chelating and reducing agents in the synthesis of lysozyme-encapsulated AuNCs. The activity of lysozymes can be retained and the lysozyme-AuNCs can be used to inhibit the cell growth of bacteria, including Gram-positive, Gram-negative, and antibiotic-resistant bacterial strains.20 Prompted by these results, we further investigated the binding activity of the protein-directed synthesis of AuNCs using human serum albumin (HSA) as the reagent. HSA is a carrier protein capable of binding with various species (e.g., ions and small molecules) as chelating and reducing agents. Although serum albumins in different species have similar functions, their sequences slightly differ from one another. We observed that AuNCs directly synthesized from HSA
(HSA-AuNCs) have binding affinities with S. aureus and MRSA,
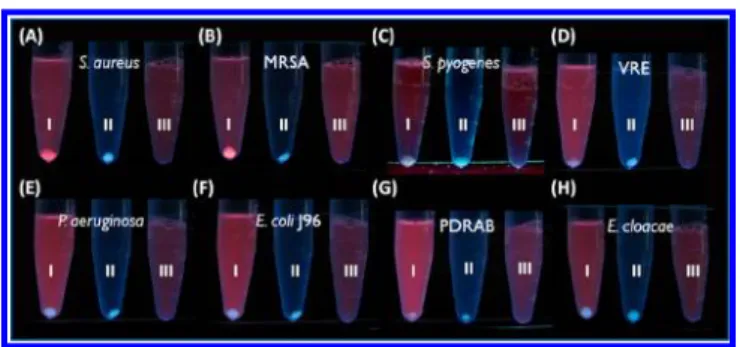
making AuNCs suitable sensing probes for these bacteria. The HSA-AuNCs were produced based on the previously proposed method.20 The properties of the generated HSA-AuNCs were similar to those of BSA-HSA-AuNCs,19including red emission under ultraviolet (UV) light (Figure S1 in the Supporting Information). We then used the HSA-AuNCs as affinity probes for several pathogenic bacteria. Tube I in Figure 1A−H displays the results obtained after using the HSA-AuNCs as the sensing probes to interact with the target bacteria in phosphate buffered saline (PBS) solution (pH 6). The bacteria included S. aureus, MRSA, Streptococcus pyogenes, vancomycin-resistant Enterococcus faecalis (VRE), Escherichia coli J96, Pseudomonas aeruginosa, pandrug-resistant Acinetobacter baumannii (PDRAB), and Enterobacter cloacae. Tubes II and III were the control samples that contained bacteria and HSA-AuNCs, respectively, which were also treated with the same steps as for tube I. The HSA-AuNCs cannot be spun down at a low centrifugation speed unless they are bound with their target bacteria. A precipitate with pale bluefluorescence derived from bacterial cells was observed at the bottom of tube II in all panels after low-speed centrifugation, whereas no precipitation was observed in tube III. Apparently, the HSA-AuNCs can be used to selectively bind with S. aureus and MRSA because only tube I in Figure 1A,B shows precipitation with red luminescence under UV light. Additionally, the pH of the sample solution Received: August 28, 2012
Accepted: October 17, 2012 Published: October 22, 2012
also affects the interactions between HSA-AuNCs and bacteria. At pH < 7, the interactions between HSA-AuNCs can be retained (for details see Figure S2 in the Supporting Information). The results suggested that the HSA-AuNCs can be used as selective sensing probes for these two pathogens.
Although HSA-AuNCs cannot be used to distinguish S. aureus from MRSA based on our current sensing approach, these two bacterial strains can be further distinguished by other analytical methods, such as matrix-assisted laser desorption/ ionization (MALDI)-mass spectrometry (MS). MALDI-MS is
useful in the characterization of intact microorganism cells.1−9,29−31 Several MRSA strains can also reportedly be differentiated based on MALDI-MS fingerprinting.31 Data analysis methods are used to differentiate bacterial strains based on the statistical treatment of MS data.7−9,29,30We used a data analysis method, i.e., principal component analysis (PCA), to treat the MALDI-MS results of S. aureus and MRSA. The two different bacterial strains can be roughly distinguished in the PCA plot (Figure S3 in the Supporting Information).
We also examined the limit of detection of this approach for target bacteria. Figure 2A presents the photographs obtained using the HSA-AuNCs as the sensing probes to interact with target bacteria from the samples containing different concen-trations of MRSA. Figure 2B presents the corresponding control experimental results without HSA-AuNCs added to the bacterial samples. The presence of MRSA in the sample solution was visible to the naked eye at cell concentrations >4.2 × 106cells/mL. The red photoluminescence resulted from the
attachment of the HSA-AuNCs onto MRSA at the bottom of the sample vials. However, without HSA-AuNCs added to the bacterial samples, the bacterial cells at the bottom of the tubes after centrifugation cannot be visualized when the bacterial concentrations were lower than 4.2 × 108 cells/mL (Figure 2B). Figure 2C displays the correspondingfluorescence spectra of the samples in Figure 2A, while Figure 2D presents the plot of the change of the fluorescence intensity at the maximum emission wavelength versus the cell concentrations. The change of thefluorescence intensity increased as the concentration of the bacterial cells in the sample increased. The results indicated that HSA-AuNCs can function as sensitive sensing-probes for Figure 1. Photographs obtained after incubation of the following
bacteria: (A) S. aureus, (B) MRSA, (C) S. pyogenes, (D) VRE, (E) P. aeruginosa, (F) E. coli J96, (G) PDRAB, and (H) E. cloacae with HSA-AuNCs (0.12 mg/mL) for 2 h followed by centrifugation at 3500 rpm for 5 min (tube I). All bacterial samples (1 mL) were prepared in PBS solution (pH 6) with an optical density at 600 nm of 0.9 (OD600= 0.9). Tubes II and III (1 mL) in each panel contained bacteria (OD600 = 0.9) and HSA-AuNCs (0.12 mg/mL), respectively. The photographs were taken under ultraviolet light (λmax= 365 nm).
Figure 2.Examination of the limit of detection. The HSA-AuNCs (0.12 mg/mL) were used as sensing probes for MRSA prepared in PBS solution (pH 6). (A) Photograph obtained after vortex mixing the HSA-AuNCs (0.12 mg/mL) with samples containing different concentrations of MRSA for 1 h, followed by centrifugation at 3500 rpm for 5 min. The MRSA concentrations in the tubes (1 mL) from left to right were as follows (in cells/ mL): 4.2× 109, 8.4× 108, 4.2× 108, 8.4× 107, 4.2× 107, 8.4× 106, 4.2× 106, 8.4× 105, 4.2× 105, and 0. (B) Photograph obtained after vortex mixing the samples containing different concentrations of MRSA for 1 h followed by centrifugation at 3500 rpm for 5 min. The concentrations of MRSA in the tubes (1 mL) from left to right were as follows (in cells/mL): 4.2× 109, 8.4× 108, 4.2× 108, 8.4× 107, 4.2× 107, 8.4× 106, 4.2× 106, 8.4× 105, and 4.2× 105. (C) Fluorescence spectra obtained from the supernatant of each sample tube in panel A (λ
ex= 367 nm). (D) Plot of the change of thefluorescence intensity at the wavelength of 640 nm (ΔI640) between the supernatant of the bacterial samples and the blank sample containing only HSA-AuNCs (0.12 mg/mL) as shown in panel A versus cell concentrations. The result from the blank sample (0 cells/mL) was not shown in the plot sinceΔI640was zero.
MRSA. Even though the observations were based only on naked-eye visualizations, the limit of detection using HSA-AuNCs as the sensing probe was ∼100-fold lower than that without the sensing probes in the bacterial samples. We also examined the limit of detection of this approach for target bacteria in urine samples. Figure S4A in the Supporting Information presents the photograph obtained using the HSA-AuNCs as the sensing probes to interact with target bacteria from urine samples containing different concentrations of S. aureus. Figure S4B in the Supporting Information presents the results obtained from control experiments without HSA-AuNCs added to the samples. Apparently, S. aureus sensed by
the HSA-AuNCs with reddish fluorescence can be readily
observed at the bottom of the centrifugation tubes when the S. aureus concentration was higher than ∼4.2 × 106 cells/mL.
These results demonstrated that HSA-AuNCs can interact with traces of target bacteria in complex samples.
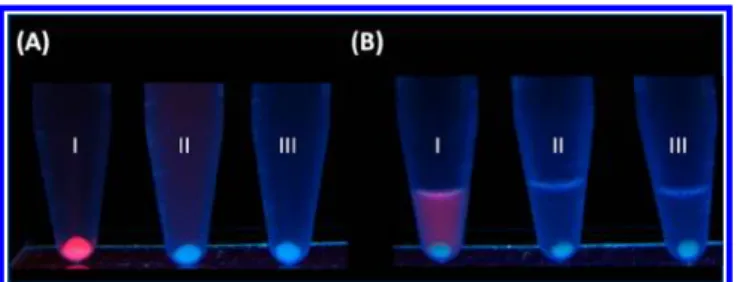
One may suspect that the binding interactions resulted from electrostatic interactions between the HSA-AuNCs and their target bacteria. Thus, we measured theζ potential of the HSA-AuNCs in PBS solution and found it was about−25 mV (pH 6). S. aureus is also known to have a net negative charge in acidic solutions.27Thus, the binding interactions between HSA-AuNCs and S. aureus were unlikely caused by electrostatic interactions. The affinities between the HSA-AuNCs and the two bacterial strains (S. aureus and MRSA) were suspected to result from the interaction of the peptide motifs on the surface of HSA-AuNCs with the cell wall of the bacteria. Thus, we conducted enzymatic digestion of the HSA-AuNCs by trypsin. We assumed that if the enzyme was able to cleave the binding sequences from the surface of HSA-AuNCs, the remaining HSA-AuNCs would lose their binding activity with their target bacteria. The cleaved fragments from the surface of the HSA-AuNCs can be collected and characterized by MS. Accordingly, we digested the HSA-AuNCs using trypsin and explored the binding peptides. We found that trypsin-digested HSA-AuNCs still possessed red fluorescence because the cleaved parts did not affect the linking between HSA and the Au core. Tube I in Figure 3A shows the photograph obtained after using HSA-AuNCs as selective sensing probes for target bacteria from a sample solution containing S. aureus. Tube II in Figure 3A shows the photograph obtained after alternatively using the trypsin-digested HSA-AuNCs product, which contained digested HSA-AuNCs and cleaved peptides, as the sensing
solution for the same bacterial sample used in tube I in Figure 3A. The precipitate at the bottom of the tube maintained its pale blue fluorescence derived from bacterial cells only. The digested HSA-AuNCs with redfluorescence lost their ability to bind with S. aureus, which implied that the binding peptides on the HSA-AuNCs were cleaved by trypsin. The cleaved peptides capable of binding with S. aureus may have also attached onto the surface of S. aureus at the bottom of the tube. Further characterization of the binding species on the bacteria was conducted. We collected the digested residues byfiltering the trypsin-digested HSA-AuNCs product with a membrane of 3 kDa cutoff mass under centrifugation. The filtered solution containing cleaved peptides was used to interact with the target bacteria in the same bacterial sample used to obtain the results in tubes I and II. After incubation and centrifugation, the white precipitate was spun down at the bottom of the centrifuge tube (tube III in Figure 3A). Given that the peptides are invisible, MS was used to determine if any cleaved peptide was bound to the bacteria. The precipitates at the bottom of tubes I, II, and III were rinsed with PBS solution (pH 6) several times to remove nonspecific binding species. The precipitates were then resuspended in a basic solution (pH 8, 0.2 mL) to reduce the binding interactions between the peptide residues and S. aureus. These three tubes were centrifuged again at a low centrifugation speed. Figure 3B shows the resultant tubes I, II, and III corresponding to the rinsed precipitate from the samples in tubes I, II, and III in Figure 3A, respectively. The precipitate in tube I in Figure 3B no longer showed reddish luminescence because the HSA-AuNCs were released from the bacterial precipitate at a high pH, whereas the supernatant showed redfluorescence derived from HSA-AuNCs that were released from the precipitate (cf. tube I in Figure 3A). Taking this result in tube I as a control, we believed that if there were any peptides bound with the bacterial precipitate in tubes II and III in Figure 3A, they must also be released and redissolved in the supernatant.
Figure S5A in the Supporting Information displays the resultant MALDI mass spectrum of the supernatant from tube I in Figure 3B. No peak was observed in the mass spectrum, which was not surprising because only intact HSA-AuNCs were resuspended in the supernatant. Direct acquisition of any ions
from the intact HSA-AuNCs was difficult without further
sample treatment. Figure S5B,C in the Supporting Information displays the MALDI mass spectra of the supernatant of tubes II and III in Figure 3B, respectively. Two peaks at m/z 1311.77 and 1467.89 appeared in Figure S5B in the Supporting Information, whereas two peaks at m/z 1467.95 and 1623.90 appeared in Figure S5C in the Supporting Information. We searched these observed peaks from the protein database and discovered that the identities of these peaks were H P D Y S V V L L L R ( n o . 3 6 2 - 3 7 2 , m / z 1 3 1 1 . 7 7 ) , RHPDYSVVLLLR (no. 361-372, m/z 1467.95/1467.89; theoretical value/1467.84), and DVFLGMFLYEYAR (no.
348−360, m/z 1623.90; theoretical value/1623.78) from
HSA. Hence, we believed that HSA-AuNCs used RHPDYSVVLLLR (no. 361-372) and DVFLGMFEYAR (no. 348-360) to bind with S. aureus. To confirm further whether the peptides can interact with S. aureus, DVFLGRGGGC (Pep10) and RHPDYSVVLLLRGGGC (Pep16), which con-tained the sequences from no. 348 to 352 and no. 361 to 372 as discovered in Figure S5 in the Supporting Information, respectively, were synthesized. These two peptides were designed to link three extra glycines as a spacer and one Figure 3.(A) Photograph obtained after using the HSA-AuNCs (tube
I), the trypsin digest of HSA-AuNCs (tube II), and the cleaved peptides from the trypsin digest of HSA-AuNCs (tube III) as the sensing probes for the samples containing S. aureus (OD600= 0.9, 1 mL). (B) The precipitates in tubes I, II, and III in panel A were rinsed with PBS solution (pH 6) several times, resuspended in aqueous sodium hydroxide (2 N, 0.2 mL), and centrifuged at 3500 rpm for 5 min. The photographs were taken under ultraviolet light (λmax= 365 nm).
extra cysteine in the end of the sequences as a reducing and chelating agent for the synthesis of the peptide-immobilized AuNCs (Pep-AuNCs). Pep10-AuNCs were successfully gen-erated under microwave heating (power = 90 W) for 7 cycles (5 min/cycle) and then used as sensing probes for S. aureus. The sensing steps were similar to those when HSA-AuNCs were used as the sensing probes. Figure S6A,B in the Supporting Information presents the photographs obtained
after using Pep10-AuNCs and Pep16-AuNCs as the affinity
probes to trap target bacteria in PBS solution (pH 6) containing S. aureus, respectively. Tube I contained the Pep-AuNCs and S. aureus, which were vortex mixed for 2 h and centrifuged at 3500 rpm for 5 min. Precipitates with reddish luminescence were observed at the bottom of tube I. Tubes II and III contained S. aureus and Pep-AuNCs, respectively, as control samples. The results indicated that the Pep-AuNCs can interact with S. aureus, suggesting that the two peptide sequences were the binding sites when using HSA-AuNCs as the sensing probes for target bacteria. Peptide aptamers toward specific bacteria have been explored through the studies of protein−bacterium interactions.32−34HSA is commonly used as the model protein when studying the interactions between bacteria and proteins since it is abundant in human plasma.34In our approach, the 3-dimensional (3D) structure of the HSA molecules on the surface of HSA-AuNCs may differ from that of free HSA molecules at the same experimental conditions, leading to differences in binding affinity and binding sites. According to our experimental results, when free HSA molecules were used as the probes to interact with S. aureus, we were unable to observe noteworthy affinity between the proteins and bacteria. Additionally, to the best of our knowledge, we have not found any binding peptide sequences similar to what we explored in this work.
We also hypothesized that BSA-AuNCs may have binding affinity with S. aureus. However, the results showed that the binding affinity was not apparent (Figure S7 in the Supporting Information), which may be due to the slightly different binding sequences in HSA from those in BSA (for details see the Supporting Information). The results confirmed again that the sequences we discovered have selective binding affinity with S. aureus and MRSA.
In conclusion, we have demonstrated that HSA-AuNCs were sensitive and selective sensing probes for S. aureus and MRSA. To the best of our knowledge, this is thefirst report to explore the capability of HSA-AuNCs in selectively sensing S. aureus and MRSA. The possibility of distinguishing S. aureus from MRSA using MALDI-MS combined with PCA was demon-strated. We also proposed a useful strategy to discover peptide aptamers from protein-AuNCs for their target species in a practical manner. The binding peptide motifs of the HSA-AuNCs with their target bacteria were therefore determined. In this study, we do not only discover useful luminescent probes for S. aureus and MRSA but we also explore two peptide aptamers that can bind specifically with these two bacterial strains. Given the danger of these pathogenic bacteria, the newly discovered probes can be used for the rapid screening of these bacteria in complex samples. Although the limit of detection by the naked eye is∼106cells/mL, it can be further improved by observing the target bacteria probed by the HSA-AuNCs under afluorescence microscope. Nevertheless, it will be easier and more practical if further efforts can be made to develop sensing methods with higher sensitivities and eliminating the requirement of expensive instruments.
Addi-tionally, the peptide aptamers explored in this study has no capability to distinguish these two S. aureus strains. However, it may be possible to further use our current approach to explore peptide aptamers to distinguish different MRSA strains, which do not only resist toβ-lactam antibiotics but also they do not respond to glycopeptide antibiotics. The cell wall of glycopeptide antibiotic-resistant bacterial strains usually differs from that of glycopeptide-antibiotic sensitive bacterial strains. On the basis of this feature, it may be possible to explore different binding peptide motifs for different MRSA strains using our current approach. Thus, we are optimistic about the further applications of the HSA-AuNCs and the strategies proposed in this study in the development of analytical methods and nanomedicine.
■
ASSOCIATED CONTENT*
S Supporting InformationAdditional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
■
AUTHOR INFORMATIONCorresponding Author
*E-mail: yuchie@mail.nctu.edu.tw. Phone: 886-3-5131527. Fax: 886-3-5723764.
Notes
The authors declare no competingfinancial interest.
■
ACKNOWLEDGMENTSThe authors thank the National Science Council of Taiwan for financial support of this research.
■
REFERENCES(1) Gu, H. W.; Ho, P. L.; Tsang, K. W. T.; Yu, C. W.; Xu, B. Chem. Commun. 2003, 1966−1967.
(2) Gu, H. W.; Ho, P. L.; Tsang, K. W. T.; Yu, C. W.; Wang, L.; Xu, B. J. Am. Chem. Soc. 2003, 125, 15702−15703.
(3) Ho, K.-C.; Tsai, P.-J.; Lin, Y.-S.; Chen, Y.-C. Anal. Chem. 2004, 76, 7162−7168.
(4) Lin, Y.-S.; Tsai, P.-J.; Weng, M.-F.; Chen, Y.-C. Anal. Chem. 2005, 77, 1753−1760.
(5) Chen, W.-J.; Tsai, P.-J.; Chen, Y.-C. Anal. Chem. 2008, 80, 9612− 9621.
(6) Liu, J.-C.; Tsai, P.-J.; Lee, Y.;C.; Chen, Y.-C. Anal. Chem. 2008, 80, 5425−5432.
(7) Ho, Y.-P.; Reddy, P. M. Clin. Chem. 2010, 56, 525−536. (8) Ho, Y.-P.; Reddy, P. M. Mass Spectrom. Rev. 2011, 30, 1203− 1224.
(9) Chen, C.-T.; Reddy, P. M.; Ma, Y.-R.; Ho, Y.-P. Int. J. Mass Spectrom. 2012, 312, 45−52.
(10) Hayden, S. C.; Zhao, G.; Saha, K.; Phillips, R. L.; Li, X.; Miranda, O. R.; Rotello, V. M.; EI-Sayed, M. A.; Schmidt-Krey, I.; Bunz, U. H. F. J. Am. Chem. Soc. 2012, 134, 6920−6923.
(11) Ronnie, L. P.; Miranda, O. R.; You, C.-C.; Rotello, V. M.; Bunz, U. H. F. Angew. Chem., Int. Ed. 2008, 47, 2590−2594.
(12) Miranda, O. R.; Li, X.; Garcia-Gonzalez, L.; Zhu, Z.-J.; Yan, B.; Bunz, U. H. F.; Rotello, V. M. J. Am. Chem. Soc. 2011, 133, 9650− 9653.
(13) Li, C.-Z.; Vandenberg, K.; Prabhulkar, S.; Zhu, X.; Schneper, L.; Methee, K.; Rosser, C.-J.; Almeide, E. Biosens. Bioelectron. 2011, 26, 4342−4348.
(14) Wang, S. G.; Singh, A. K.; Senapati, D.; Neely, A.; Yu, H. T.; Ray, P. C. Chem.Eur. J. 2010, 16, 5600−5606.
(15) Huang, C.-C.; Chen, C.-T.; Shiang, Y.-C.; Lin, Z.-H.; Chang, H.-T. Anal. Chem. 2009, 81, 875−882.
(16) Tseng, Y.-T.; Chang, H.-T.; Chen, C.-T.; Chen, C.-H.; Huang, C.-C. Biosens. Bioelectron. 2011, 27, 95−100.
(17) Wang, L.; Zhao, W. J.; O’Donoghue, M. B.; Tan, W. H. Bioconjugate Chem. 2007, 18, 297−301.
(18) Qin, D. L.; He, X. X.; Wang, K. M.; Zhao, X. J. J.; Tan, W. H.; Chen, J. Y. J. Biomed. Biotechnol. 2007, 89364−89372.
(19) Xie, J.; Zheng, Y.; Ying, J. Y. J. Am. Chem. Soc. 2009, 131, 888− 889.
(20) Chen, W.-Y.; Lin, J.-Y.; Chen, W.-J.; Lo, L.; Diau, E. W.-G.; Chen, Y.-C. Nanomedicine 2010, 5, 755−764.
(21) Tang, K. C.; Chang, H. W.; Chien, Y. C.; Hsiao, J. K.; Cheng, J. T.; Chou, P. T. Angew. Chem., Int. Ed. 2011, 50, 7056−7060.
(22) Chaudhari, K.; Xavier, P. L.; Pradeep, T. ACS Nano 2011, 5, 8816−8827.
(23) Yan, L.; Cai, Y. Q.; Zheng, B. Z.; Yuan, H. Y.; Guo, Y.; Xiao, D.; Choi, M. M. F. J. Mater. Chem. 2012, 22, 1000−1005.
(24) Hu, L.; Han, S.; Parveen, S.; Yuan, Y.; Zhang, L.; Xu, G. Biosens. Bioelectron. 2012, 32, 297−299.
(25) Wen, F.; Dong, Y. H.; Feng, L.; Wang, S.; Zhang, S. C.; Zhang, X. R. Anal. Chem. 2011, 83, 1193−1196.
(26) Xavier, P. L.; Chaudhari, K.; Verma, P. K.; Palc, S. K.; Pradeep, T. Nanoscale 2010, 2, 2769−2776.
(27) Kłodzinska, E.; Szumski, M.; Hrynkiewicz, K.; Dziubakiewicz, E.; Jackowski, M.; Buszewsk, B. Electrophoresis 2009, 30, 3086−3091.
(28) Chen, T.-H.; Tseng, W.-L. Small 2012, 8, 1912−1919. (29) Chen, P.; Lu, Y.; Harrington, P. B. Anal. Chem. 2008, 80, 1474− 1481.
(30) De Bruynea, K.; Slabbinck, B.; Waegeman, W.; Vauterin, P.; Baets, B.; De; Vandammea, P. Syst. Appl. Microbiol. 2011, 34, 20−29. (31) Wolters, M.; Rohde, H.; Maier, T.; Belmar-Campos, C.; Franke, G.; Scherpe, S.; Aepfelbacher, M.; Christner, M. Int. J. Med. Microbiol 2011, 301, 64−68.
(32) Chhatwl, G. S.; Preissner, K. T.; Muller-Beghaus, G.; Blobel, H. Infect. Immun. 1987, 55, 1878−1883.
(33) Lejon, S.; Frick, I. M.; Björck, L.; Wikström, M.; Svensson, S. J . Biol. Chem. 2004, 279, 42924−42928.
(34) Egesten, A.; Frick, I. M.; Mörgelin, M.; Olin, A. I.; Björck, L. J. Biol. Chem. 2011, 286, 2469−2476.