Continuous Cell Line from Pupal Ovary of Perina nuda (Lepidoptera:
Lymantriidae) That Is Permissive to Nuclear Polyhedrosis Virus from
P. nuda
CHUNG-HSIUNGWANG,* CHIH-MINGCHOU,† HWEI-CHUNGLIU,* SHIH-LIANGKAU,‡ GUANG-HSUNGKOU,†AND CHU-FANGLO†
Departments of *Plant Pathology and Entomology and †Zoology, National Taiwan University, Taipei, Taiwan, ROC; and ‡Pathology Department, Veterans-General Hospital, Taipei, Taiwan, ROC
Received February 2, 1995; accepted November 14, 1995
A continuous cell line, designated NTU-PN-HH, was established from the pupal ovary of Perina nuda Fab-ricius (Lepidoptera: Lymantriidae). The cells have been through more than 300 passages during 4 years in TNM-FH medium supplemented with 10% FBS, at a constant temperature of 28°C. The cell line consists of four major morphologic types: polymorphic cells, spindle-shaped cells, round cells, and squamous cells. The characterization of this cell line showed that NTU-PN-HH is a newly established cell line. It is the first cell line that is permissive to PnNPV (P. nuda multiple nuclear polyhedrosis virus). © 1996 Academic Press, Inc.
KEY WORDS: Perina nuda; NTU-PN-HH cell line; PnNPV.
INTRODUCTION
Many Lepidopteran cell lines have been established since 1962 (Grace, 1962; Hink, 1970, 1980), several of which have been successfully used to multiply nuclear polyhedrosis virus (NPV) (Wu et al., 1989). These vi-rus-permissive cell lines have contributed significantly to pest control (Smiths and Vlak, 1988) and to the pro-duction of recombinant proteins (Fraser, 1989; Maeda
et al., 1985; Smith et al., 1983; Wu et al., 1989). Over
600 isolates of baculoviruses have been found, but the study of most of these have been limited by a lack of susceptible cell lines on which to propagate these vi-ruses (Gelernter and Federici, 1986). To facilitate fur-ther study of baculoviruses, it is necessary to establish an insect cell line of high viral susceptibility.
Perina nuda (Fabricius) is a major pest of banyan
(Ficus spp.), an important garden tree in Taiwan and mainland China. The larvae of P. nuda are external feeders on foliage and cause the destruction of tree leaves. The epizootic disease occurs every year from spring to early summer in Taiwan and mainland China. The key pathogen was found to be baculovirus PnNPV (P. nuda multiple nuclear polyhedrosis virus)
(Su et al., 1983; Lo et al., 1990; Wang and Tsai, 1995). Because there were no permissive cell lines for PnNPV, the study of this virus has been limited to morphologi-cal descriptions. We have established a permissive cell line derived from pupal ovary of P. nuda, which repre-sents a great hope for extensive study of PnNPV at the pathologic, cellular, and molecular levels.
MATERIALS AND METHODS Primary Culture and Subculture
Larvae of P. nuda were collected from the campus of National Taiwan University, and fed banyan leaves at 25°C. The larvae were allowed to go through the pupal stage, after which the pupae were collected and steril-ized with a 10% Clorox solution and 70% iodine alcohol. The ovaries were removed with a fine forceps and a pipet, and incubated at 28°C in TNM-FH medium (Hink and Strauss, 1976) containing 100 IU/ml peni-cillin, 100 mg/ml streptomycin, and 1.25 mg/ml fungi-zone. The medium was supplemented with 10% fetal bovine serum (FBS) that had been inactivated at 56°C for 30 min.
The cells were subcultured when approaching con-fluency. During the first 25 passages, the cells adher-ing to the bottom of the flask had to be removed with a rubber policeman, but thereafter could be resuspended by vigorous agitation. When subculturing, 1 ml of sus-pended cells were transferred to a new 25 cm2 flask containing 4 ml of fresh media plus supplements. From the initial subculture to the 40th passage, the interval between subcultures ranged from 1 to 3 weeks, depend-ing on the growth rate of the cells. After the 49th pas-sage, the cells propagated rapidly, and thereafter the interval between passages was 4 days. The resulting cell line has been designated NTU-PN-HH.
Susceptibility of Virus
The following viruses were used to test the viral sus-ceptibility of NTU-PN-HH cells: AcNPV (Autographa
ARTICLE NO. 0033
199
0022-2011/96 $18.00 Copyright © 1996 by Academic Press, Inc. All rights of reproduction in any form reserved.
JOBNAME: JIP 67#3 96 PAGE: 2 SESS: 12 OUTPUT: Tue May 28 17:28:38 1996 /xypage/worksmart/tsp000/69984c/16pu
californica NPV) and HzNPV (Heliothis zea NPV),
kindly supplied by Dr. M. J. Fraser of Notre Dame University; BmNPV (Bombyx mori NPV) collected from infected Bm-N cells or infected larvae of B. mori and PnNPV collected from infected larvae of P. nuda.
These four viruses were used to examine the viral susceptibility of three other cell lines as a positive con-trol for virus infectivity (IPLB-SF-21AE, Bm-N, and IPLB-HZ-1075 [H. zea cell line]) (Goodwin et al., 1982; Maeda, 1989; Vaughn et al., 1977). AcNPV, HzNPV, and BmNPV were obtained from their permissive cell lines, IPLB-SF-21AE, IPLB-HZ-1075, and Bm-N, re-spectively. The semiconfluent tested cells (log-phase cells) of NTU-PN-HH were inoculated with the filtered homogenate of infected P. nuda larvae or the filtered culture medium of the NPV-infected cells of IPLB-SF-21AE, IPLB-HZ-1075, and Bm-N. After 1 hr of adsorp-tion, the viral solution was discarded and the cells were incubated in fresh TNM-FH medium at 28°C. The cy-topathic effect (CPE) was observed and documented. The virus titer was determined by the end-point dilu-tion method (TCID50 analysis) (Summers and Smith,
1988).
Electron Microscopy
The infected cells were scraped from the surface of the plastic flasks and sedimented at 900 rpm. The me-dium was discarded and the pellets were fixed in 2.5% glutaraldehyde in pH 7.2 phosphate buffer at 4°C for 3 hr and postfixed in 1% OsO4in the same buffer for 2 hr. The pellets were then dehydrated in an alcohol gradi-ent series and embedded in Spurr Epon. Thin sections were cut on a Reichert OMU 3 ultramicrotome and stained with uranyl acetate and lead citrate. The pho-tomicrographs were made with a Hitachi H7100 elec-tron microscope at 100 kV.
Chromosome Number
The NTU-PN-HH chromosome spreads were pre-pared by treating 5 ml of log-phase cultures (2 × 106 cells/ml) with 0.06mg/ml demecolcine (Sigma, D-6279) for 2 hr at 28°C. The cells were dispersed and centri-fuged (900 rpm) for 5 min, resuspended for 10 min in a hypotonic solution of normal saline and distilled water in a 1:4 dilution, and then fixed in 3:1 methanol:glacial acetic acid for 20 min. The fixed cells were dropped vertically onto the slides. After air drying, the cells were stained with Giemsa stain for 30 min and the chromosome number was counted under a microscope.
Growth Rate of NTU-PN-HH Cells
The NTU-PN-HH cells were seeded in 25 cm2flasks,
about 1 × 106cells each, and cultured with 0, 5, 10, and
20% FBS supplementation at 28°C, and at 4, 20, 28, and 37°C with 10% FBS supplementation. The
cul-tured cells were counted with a hemocytometer every 24 hr for 6 days.
Isozyme Analysis
The confluent cells of NTU-PN-HH, IPLB-SF-21AE, and IPLB-LD-652Y (Lymantria dispar cell line) (Good-win et al., 1978) were collected and centrifuged at 900 rpm for 10 min at 4°C. The pellets were washed twice in phosphate-buffered saline (PBS) and then resus-pended in a grinding buffer (0.125 M Tris, 0.046 M citric acid, 10% sucrose, 1% Triton X-100, and 0.02 mM bromophenol blue). The cells were frozen in liquid ni-trogen and thawed (37°C) three times and then cell lysates were centrifuged at 15,000g for 5 min. The su-pernatant liquid was stored at −20°C.
Following electrophoresis on 5% polyacrylamide gels, the cell lysates were tested for isozymes, esterase, lactate dehydrogenase (LDH), and malate dehydroge-nase (MDH). The upper tank buffer consisted of 0.005
M Tris and 0.036 M glycine (pH 8.3), and the lower
tank buffer consisted of 0.0375 M Tris (pH 8.9). Verti-cal slab gels were run at a constant current of 20 mA for 3 hr and then stained following the protocol of Har-ris and Hopkinson (1977).
Restriction and Electrophoresis of DNA
The isolation and purification of polyhedra and virus particles, and DNA preparation of PnNPV from mori-bund P. nuda larvae and infected NTU-PN-HH cells were achieved as described by Summers and Smith (1988). Both DNAs were digested with EcoRI, Hind III and PstI (Promega Corporation), according to the us-er’s manual, and analyzed in 0.7% agarose gels.
RESULTS
The cell line derived from pupal ovary of P. nuda was established in vitro and designated NTU-PN-HH. Four major morphologically different cell types (polymor-phic, spindle-shaped, round, and squamous) were ob-served in the primary cell culture. These four cell types were still present after 50 passages, though the poly-morphic cells and squamous cells became dominant. The cells have been passaged for more than 4 years in TNM-FH medium containing 10% FBS under a con-stant temperature of 28°C.
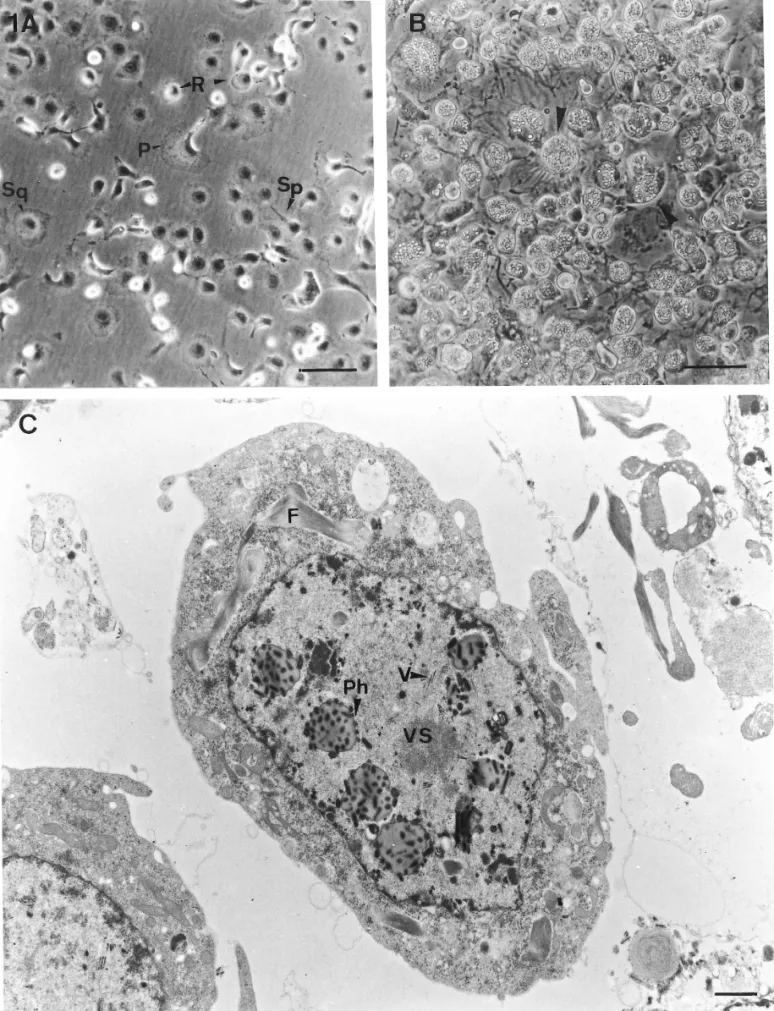
The four major types of NTU-PN-HH cell are easily distinguished after plating for 1 hr. The characteristics of these four cells are as follows (Fig. 1A).
The polymorphic cells (P cells) are irregular in shape and varied in size (20–50mm wide and 50–90mm long). The plasma membrane of one side is generally smooth, but the opposite side spreads on the substratum with an irregular margin containing many processes. The nucleus is round or ellipsoid, varied in size (10–25mm wide and 20–30 mm long), and excentrically located near the smooth margin of the cell.
WANG ET AL. 200
FIG. 1. The characteristic of the NTU-PN-HH cell line established from pupae of Perina nuda, showing: (A) Four major cell types in the NTU-PN-HH cell line, polymorphic cells (P), spindle-shaped cells (Sp), squamous cells (Sq), and round cells (R). Bar, 50mm. (B) The infected cells, 7 days after infection with PnNPV. Note that more than 98% of the cells are susceptible to PnNPV and the hypertrophied nuclei of infected cells fill with polyhedra (arrows). Bar, 50mm. (C) The electron micrograph of a NTU-PN-HH cell infected with MP strain of PnNPV at 3 days after viral inoculation. Note the virion (V) adjacent to virogenic stroma (VS). The developing polyhedra (Ph) in the hypertrophied nucleus are surrounding virogenic stroma and the fibrillar materials (F) located in cytoplasm and nucleus. Bar, 0.1mm.
JOBNAME: JIP 67#3 96 PAGE: 4 SESS: 12 OUTPUT: Tue May 28 17:28:38 1996 /xypage/worksmart/tsp000/69984c/16pu
The spindle-shaped cells (Sp cells) are predomi-nantly ellipsoidal, with two extensions (on opposite sides) and varied in size (8–16 mm wide and 65–110 mm). Both or one of the extensions spread on the sub-stratum, with several processes. The cells are able to continue growing on the confluent monolayer, eventu-ally forming a superimposed layer of cells easily de-tached from the substrate. The nucleus is round or el-liptical, centrally located, and almost fills the cell.
The round cells (R cells) are small cells, have a high nucleo-cytoplasmic ratio, and are 20–28 mm in diam-eter. The nucleus is large, centrally located, and almost fills the cell. The majority of the cells are loosely at-tached to the substratum, occasionally with one or two tiny filipodia, and the cells form aggregates, especially on confluency.
The squamous cells (Sq cells) are large and varied in size (20–80 mm in diameter). The cells are spreading and adhere firmly to the substrate with processes. The nucleus is round, centrally located, and 13–30 mm in diameter. The cytoplasm may contain granules or vacuoles, especially on confluency.
NTU-PN-HH cells showed a high susceptibility to PnNPV in vitro (Fig. 1B). No cytopathic effect was de-tected in NTU-PN-HH cells after inoculation with HzNPV and BmNPV, whereas a few NTU-PN-HH cells showed a lysis effect after inoculation with AcNPV, es-pecially at lower osmotic pressures (less than 315 mil-liosmol), but the cells recovered after 3 days of inocu-lation. The MP strain of PnNPV was isolated in vitro and the average yield of PIB per NTU-PN-HH cell was 35. After inoculation with PnNPV MP strain, 98% of NTU-PN-HH cells produced the polyhedral inclusion bodies (PIBs) (Fig. 1B) and the virus titer increased from TCID50/ml4 10−5.61 ± 0.81to 10−8.72 ± 0.72. The
mor-phogenesis and infectivity of PnNPV in vitro was simi-lar to that in vivo. After 5 days of infection, nucleocap-sids, multiple nucleocapsid virions, virogenic stroma,
and developing polyhedra were observed in the hyper-trophied nuclei, and fibrillar materials were found in the cytoplasm and nucleus. The plasma membrane and nuclear envelope remained intact and showed no sign of destruction. The ultrastructural morphogenesis of PnNPV in vitro revealed that PnNPV is a typical NPV (Fig. 1C).
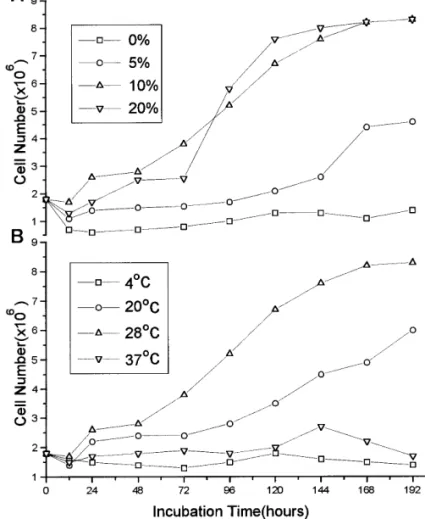
A representative chromosomal spread from NTU-PN-HH cells shows the typical round shape of Lepidop-teran chromosome (Fig. 2A). The distribution of chro-mosome numbers varied widely from 52 to 285, with an average of 117 (Fig. 2B). The influence of various con-centrations of FBS on the growth rate of NTU-PN-HH cells at a constant temperature of 28°C indicated that 10 and 20% FBS stimulated cell growth better than did 0 and 5% (Fig. 3A). The doubling times for cell popu-lation in TNM-FH medium with 10 and 20% FBS at 28°C were 86 and 54 hr, respectively. The doubling times of NTU-PN-HH cells were also recorded after cultivating the cells in TNM-FH medium supple-mented with 10% FBS at different temperatures. The maximum growth rate of the cells was at 28°C (86 hr doubling time). The cells grew very slowly at 4 and 37°C and eventually died, whereas at 20°C, the cells remained viable; cell growth continued slower than at 28°C (94 hr doubling time) (Fig. 3B).
The mobility of esterase, LDH, and MDH from NTU-PN-HH cells was different from that from IPLB-SF-21AE and IPLB-LD-652Y cells (Fig. 4). The restriction endonuclease fragment patterns of the PnNPV DNAs purified from in vivo and in vitro were identical for
EcoRI, HindIII, and PstI (Fig. 5). DISCUSSION
The cell line derived from P. nuda pupal ovary has been designated NTU-PN-HH. NTU-PN-HH cell line is the second viral-susceptible cell line derived from the
FIG. 2. A representative mitotic chromosomal spread obtained from NTU-PN-HH cells (A) and the distribution of chromosome number in NTU-PN-HH cell population (B). Bar, 10mm.
WANG ET AL. 202
species belonging to Lymantriidae (Quiot, 1976; Good-win et al., 1978). Although an excess of 600 isolates of baculoviruses has been estimated, few of them have been shown to replicate in cultured cells—possibly be-cause investigators have concentrated on viruses and cell lines from insects of economic importance (Kelly, 1982). P. nuda is a major garden pest, causing defolia-tion of Ficus spp. The insect is oligophagous, and easy to find from fall to early summer. The serious epizootic disease spread by this insect occurs frequently from late spring. It was considered that the pathogen
PnNPV plays an important role in the natural popula-tion control (Wang and Tsai, 1995). IPLB-PN-HH cells, because of their high susceptibility to PnNPV, can pro-vide a suitable in vitro system for study.
The cell line contains four major morphological cell types, no subclones of NTU-PN-HH were obtained in more than 10 clonal tests. It is believed that there is metabolic coupling among the different morphophic cells. The variability in shape of NTU-PN-HH cells is a result either of the variety of originating cells or the cells being inherently pleiomorphic.
Newly established cell lines should be characterized to distinguish them from all other cell lines, determine the tissue from which the line originated, and assure that is not a contaminant (Hink et al., 1985). Tabach-nick and Knudson (1980) demonstrated that 10 iso-zymes are suitable for distinguishing insect cell lines— three were used in this attempt to identify the PN-HH cells. Isozyme patterns reveal that the NTU-PN-HH cell line is markedly different from other cell lines that are routinely maintained in our lab.
Several established cell lines failed to replicate their homologous viruses or were semipermissive to the ho-mologous virus (Goodwin et al., 1978; Gelernter and Federici, 1986; Quiot, 1976). The homologous virus, PnNPV, successfully propagated in this newly estab-lished cell line (PN-HH). The four types of NTU-PN-HH cells show similar susceptibility to PnNPV, so the cloned NTU-PN-HH cell is not necessary on the basis of viral susceptibility. Appearance of PnNPV vi-ral progeny in the nucleus and polyhedvi-ral formation is
FIG. 3. The influence of cultivation condition on growth rate of NTU-PN-HH cells showing: (A) FBS supplementation (0, 5, 10, and 20%) at 28°C, (B) cultivation temperature (4, 20, 28, and 37°C) with 10% FBS supplement.
FIG. 4. The isozyme patterns of NTU-PN-HH cells ((A) esterase, (B) LDH, and (C) MDH) (lane 1) compared with IPLB-SF-21AE (lane 2) and IPLB-LD-652Y cells (lane 3).
FIG. 5. Restriction endonuclease patterns, EcoRI (lanes 1,2), HindIII (lanes 3,4) and PstI (lanes 5,6), for PnNPV DNAs purified in vivo (lanes 1,3,5) from infected larvae of Perina nuda and in vitro from infected NTU-PN-HH cells (lanes 2,4,6). M indicates thelDNA/ HindIII marker.
JOBNAME: JIP 67#3 96 PAGE: 6 SESS: 12 OUTPUT: Tue May 28 17:28:38 1996 /xypage/worksmart/tsp000/69984c/16pu
similar to other NPVs (Fraser and Hink, 1982; Hink and Vail, 1973; Potter et al., 1976). NTU-PN-HH cells also presented a problem with respect to the formation of polyhedra. Continued serial passage of PnNPV in this cell line resulted in an increase in the FP (few polyhedra) strain in PnNPV viral progeny. In regard to viral susceptibility of NTU-PN-HH cells, the MP strain of PnNPV within three passages after end-point dilu-tion purificadilu-tion was selected for this assay. In contrast to the wide host range of AcNPV (Danyluk and Maru-niak, 1987), PnNPV was found to be host-specific in
vivo and in vitro. It is suggested that the specificity of
NPVs is not the result of nutritional factors, but of virus–host relationships concerning receptors or of rep-lication factors (Maeda, 1993) in the virus or cells. The results of comparing the restriction enzyme profiles of PnNPV DNAs purified from two different sources re-veals that PnNPV isolated in vitro and in vivo origi-nated from the same NPV.
ACKNOWLEDGMENTS
We thank Dr. M. J. Fraser, professor at Notre Dame University, for his valuable technical advice throughout our research. This study was supported by the National Science Council, Republic of China.
REFERENCES
Danyluk, G. M., and Maruniak, J. E. 1987. In vivo and in vitro host range of Autographa californica nuclear polyhedrosis virus and Spodoptera frugiperda nuclear polyhedrosis virus. J. Invertebr. Pathol. 50, 207–212.
Fraser, M. J. 1989. Expression of eucaryotic genes in insect cell cul-tures. In Vitro Cell. Dev. Biol. 25 (3), 225–235.
Fraser, M. J., and Hink, W. F. 1982. The isolation and characteriza-tion of the MP and FP plaque variants Galleria mellonella nuclear polyhedrosis virus. Virology 117, 366–378.
Gelernter, W. D., and Federici, B. A. 1986. Continuous cell line from Spodopera exigua (Lepidoptera: Noctuidae) that supports replica-tion of nuclear polyhedrosis viruses from Spodoptera exigua and Autographa californica. J. Invertebr. Pathol. 48, 199–207. Goodwin, R. H., Topkins, G. J., Gettig, R. T., and Adams, J. R. 1982.
Characterization and culture of virus replicating continuous insect cell lines from the bollworm, Heliothis zea (Boddie). In Vitro 18, 843–850.
Goodwin, R. H., Topkins, G. J., and McCawley, P. 1978. Gypsy moth cell lines divergent in viral susceptibility. In Vitro 14(6), 485–494. Grace, T. D. C. 1962. Establishment of four strains of cells from
in-sect tissues grown in vitro. Nature 195, 788–789.
Harris, H. D., and Hopkinson, A. 1977. “Handbook of Enzyme Elec-trophoresis in Human Genetics,” pp. 297. North-Holland, Amster-dam.
Hink, W. F. 1970. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature 226, 466–467.
Hink, W. F. 1980. The 1979 compilation of invertebrate cell lines and culture media. In “Invertebrate System in vitro” (E. Kurstak, K. Maramojrosch, and A. Dubendorfer, Eds.), pp. 553–578. Elsevier North-Holland, New York.
Hink, W. F., Ralph, D. A., and Joplin, K. H. 1985. Metabolism and characterization of insect cell cultures. In “Comprehensive Insect Physiology, Biochemistry, and Pharmacology” (G. A. Kerkut and L. I. Giblet, Eds.), pp. 547–570. Pergamon, New York.
Hink, W. F., and Strauss, E. 1976. Growth of the Trichoplusia ni (TN-368) cell line in suspension culture. In “Invertebrate Tissue Culture, Applications in Medicine, Biology, and Agriculture.” (E. Kurstak and K. Maramorosch, Eds.), pp. 297–300. Academic Press, New York.
Hink, W. F., and Vail, P. V. 1973. A plaque assay for titration of alfalfa looper nuclear polyhedrosis virus in the cabbage looper (TN-368) cell line. J. Invertebr. Pathol. 22, 168–174.
Kelly, D. C. 1982. Baculovirus replication. J. Gen. Virol. 63, 1–13. Lo, C. F., Kou, G. H., Wang, C. H., and Shih, C. J. 1990. Studies on
baculovirus expression vector system. Natl. Sci. Counc. Mon. (Re-public of China) 18, 565–574.
Maeda, S. 1989. Gene transfer vectors of a baculovirus, Bombyx mori nuclear polyhedrosis virus, and their use for expression of foreign genes in insect cells. In “Invertebrate Cell System and Applica-tions” (J. Mitsuhashi, Ed.), pp. 167–181. CRC Press, Boca Raton, FL.
Maeda, S., Kwai, T., Obinata, M., Fujiwara, H., Horiuchi, T., Saeki, Y., and Furusawa, M. 1985. Production of human interferon in silkworm using a baculovilrus vector. Nature 315, 592–594. Maeda, S., Januta, S. G., and Kondo, A. 1993. Host range expansion
of Autographa californica nuclear polyhedrosis virus (NPV) follow-ing recombination of a 0.6-kilobase-pair DNA fragment originatfollow-ing from Bombyx mori NPV. J. Virol. 67 (10), 6234–6238.
Potter, K. N., Faulkner, P., and MacKinnon, E. A. 1976. Strain se-lection during serial passage of Trichoplusia ni nuclear polyhedro-sis virus. J. Virol. 18, 1040–1050.
Quiot, J. M. 1976. Establishment of a cell line (SCLD 135) from Ly-mantria dispar (Lepidoptera) ovaries. C.R. Acad. Sci, Ser. D. 282, 465–467.
Smith, G. E., Summers, M. D., and Fraser, M. J. 1983. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 3, 2156–2165.
Smiths, P. H., and Vlak, J. M. 1988. Selection of nuclear polyhedro-sis viruses as biological control agents of Spodoptera exigua (Lepi-doptera: Noctuidae). Entomophaga 33 (3), 299–308.
Su, X., Tai, K. C., I, H. T., Shih, M. P., and Hsuan, P. J. 1983. Two nuclear polyhedrosis viruses of two forest insect pests. Scientia Silvae Sinicae, Mem. For. Entomol. 114–115. [in Chinese]. Summers, M. D., and Smith, G. E. 1988. A manual of methods for
baculovirus vectors and insect cell culture procedure. Texas Agri-cultural Experiment Station, No. 1555, pp. 57.
Tabachnick, W. J., and Knudson, D. L. 1980. Characterization of in-vertebrate cell lines. II. Isozyme analysis electrophoresis. In Vitro
16, 392–398.
Vaughn, J. L., Goodwin, R. H., Tompkins, G. J., and McCawley, P. 1977. The establishment of two cell lines from the insect Spodop-tera frugiperda (LepidopSpodop-tera: Noctuidae). In Vitro 13, 213–217. Wang, C. H., and Tsai, S. J. 1995. Life history of the Perina nuda
(Fabricius) and virus production of the infected pupae. Chin. J. Entomol. 15, 59–68.
Wu, J., King, G., Daugulis, A. J., Faulkner, P., Bone, D. H., and Goosen, M. F. A. 1989. Engineering aspects of insect cell suspen-sion culture: a review. Microbiol. Biotechnol. 32, 249–255. WANG ET AL.