Other uses, including reproduction and distribution, or selling or
licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the
article (e.g. in Word or Tex form) to their personal website or
institutional repository. Authors requiring further information
regarding Elsevier’s archiving and manuscript policies are
encouraged to visit:
Immunochemical and molecular characterization of GBC4 as a tanycyte-like cell line
derived from grouper brain
Chiu-Ming Wen
a,⁎
, Jing-Yi Huang
b, Jian-Hao Ciou
a, Yu-Lin Kao
a, Yeong-Hsiang Cheng
c,⁎
aDepartment of Life Sciences, National University of Kaohsiung, Kaohsiung, Taiwan bInstitute of Biotechnology, National University of Kaohsiung, Kaohsiung, Taiwan c
Department of Animal Science, National Ilan University, Ilan, Taiwan
a b s t r a c t
a r t i c l e i n f o
Article history:
Received 2 December 2008
Received in revised form 7 February 2009 Accepted 10 February 2009
Available online 24 February 2009 Keywords:
Astroglia Cell line Ependyma
Neural progenitor cell Oligodendrocyte Radial glial cell
A clonal cell line, GBC4, derived from grouper (Epinephelus coioides) brain is proposed to represent an immature astroglial cell line because it expresses glialfibrillary acidic protein (GFAP), cytokeratin and vimentin. In teleost brain, tanycytes are the most abundant GFAP-expressing cell type, suggesting that GBC4 cells are derived from tanycytes. To test this hypothesis, protein and mRNA expression profiles of GBC4 cells were evaluated. We detected protein and/or mRNA expression of aromatase B, brain lipid binding protein, connexin43 protein, glutamine synthetase, S100 protein and Sox2. These proteins/mRNAs are also expressed in fish tanycytes. GBC4 cells also contained oligodendroglia proteins, including A2B5, galactocerebroside, myelin basic protein, proteolipid protein and platelet-derived growth factor receptorα as well as certain neuronal protein markers such as connexin35 protein and tyrosine hydroxylase. Our results indicate that GBC4 cells may be multipotent neural progenitor cells similar to tanycytes. Because GBC4 expresses several neural-specific genes, this line will be useful for studies on gene expression/regulation and neural development.
© 2009 Elsevier Inc. All rights reserved.
1. Introduction
Glialfibrillary acidic protein (GFAP), cytokeratin and vimentin are co-expressed in goldfish (Carassius auratus) astroglia cells (Sivron et al., 1994) and in the cell line, TB2, derived from tilapia brain (Wen et al., 2008a). The clonal cell line, GBC4, derived from grouper (Epinephelus coioides) brain, also expresses intermediatefilaments of GFAP, cytoker-atin and vimentin (Wen et al., 2008b), which is indicative of an immature astroglial lineage. TB2 cells also express oligodendrocytic proteins such as A2B5, proteolipid protein (PLP), galactocerebroside (GalC) and myelin basic proteins (MBP), as well as neuronal proteins including substance P and tyrosine hydroxylase (TH). However, TB2 is not a clonal cell line and exists as polymorphous cells.
The molecular expression profiles of fish neural progenitor cells remain to be fully determined. GFAP-expressing cells in adult teleost fish brain are mainly distributed in the ependymal and subependymal regions. Radial glial cells (tanycytes) are of ependymal origin, cover the pial surface with endfeet, and are the primary GFAP-expressing cells in fish (Bodega et al., 1993; Kálmán, 1998; Arochena et al., 2004; Lazzari and Franceschini, 2004). Cells of the ependyma co-express GFAP,
cytokeratin and vimentin, whereas GFAP-expressing cells in the subependyma generally do not express vimentin and keratin (Bodega et al., 1993, 1995; Arochena et al., 2004; Lazzari and Franceschini, 2004). In bothfish and mammals, GFAP-expressing radial glial cells express brain lipid binding protein (BLBP) (Adolf et al., 2006; Raymond et al., 2006). In addition, the radial glial cells that express aromatase (AroB) have been recognized as neural stem cells (Pellegrini et al., 2007).
The ependyma lines the ventricles and central canal as a single layer of epithelial cells that separates the cerebrospinalfluid from the central nervous system (CNS). Ependymal cells are derived from neuroepithelial cells of the ventricular zone during embryonic development. Ependymal cells in fish and other vertebrates are heterogeneous and may express one or more of GFAP, glutamine synthetase (GS), vimentin and S100 protein, and these cells may be either ciliated or nonciliated (Bodega et al., 1994; Bruni, 1998; Hernández et al., 1999). GFAP- and vimentin-positive cells in the ependyma of adult teleostfish brain can be either ependymocytes or tanycytes (Bodega et al., 1994), whereas only tanycytes express vimentin in higher vertebrates. Tanycytes have also been observed to express abundant GS, S100 (Chiba, 2000; Germanà et al., 2008) and AroB (Forlano et al., 2001; Menuet et al., 2003; Peterson et al., 2004). In adult teleostfish, ventricular zones (including ependyma) are the main proliferative zones (Ekström et al., 2001; Adolf et al., 2006; Chapouton et al., 2006) where neural stem (progenitor) cells exist. Some stem cells have protein profiles that are similar to AroB-expressing tanycytes (Pellegrini et al., 2007). Because ependymal cells ⁎ Corresponding authors. Wen is to be contacted at the Department of Life Sciences,
National University of Kaohsiung, No. 700, Kaohsiung University Road, Nan-Tzu District, Kaohsiung 81148, Taiwan, ROC. Tel.: +886 7 5919231; fax: +886 7 5919404. Cheng, Department of Animal Science, National Ilan University, Ilan 26041, Taiwan, ROC. Tel.: +886 7 9357400; fax: +886 3 9359005.
E-mail addresses:wenchiumin@nuk.edu.tw(C.-M. Wen),yhcheng@niu.edu.tw
(Y.-H. Cheng).
1095-6433/$– see front matter © 2009 Elsevier Inc. All rights reserved. doi:10.1016/j.cbpa.2009.02.017
Contents lists available atScienceDirect
Comparative Biochemistry and Physiology, Part A
such as tanycytes are the major proliferative cells in adultfish brain, it is reasonable to suggest that cell lines fromfish brain, including GBC4, might be derived from tanycytes or tanycyte-related ependymal cells. Although many markers have been discovered that characterize neural progenitor cells as well as mature neural cells, little is known about ependymal cell profiles.
Here we used a panel of antibodies and RT–PCR to clarify the origin of GBC4 cells and to determine the molecular expression profile of fish neural progenitor cells in vitro. The data show that GBC4 cells express markers of progenitor cells as well as glia and neurons and suggest that GBC4 cells are neural progenitor cells derived from ependyma. 2. Materials and methods
2.1. Cells and antibodies
GBC4 cells were established from brain tissues of a young orange-spotted grouper (Epinephelus coioides) (Wen et al., 2008b). The cells were routinaly cultivated in 25-cm2 standard cell culture
flasks (Nunc, Rosklide, Denmark) in Leibovitz's L-15 media (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) without antiboitics at 25 °C. Cells were passaged every 5–6 days using 0.1% trypsin–EDTA solutionwith a split ratio 1:2, and cells at 80–100 passages were used. The cells appeared not to be transformed because they displayed contact inhibition, required high fetal bovine serum for proliferation, and had a normal karyotype (Wen et al., 2008b).
Knowledge of the molecular characteristics offish neural cells is scarce. Several molecular markers, such as GFAP, GS, S100 and vimentin, have been used to characterize fish astroglial cells, ependymocytes, radial glial cells and tanycytes, but none of these antigens can be used to distinguish between them because none of these proteins is expressed uniquely on a particular cell type. Because no unique marker can define the neural cells, we used several specific antibodies to achieve this goal. The primary antibodies as well as their source, target and dilution are listed in Table 1. Although the antibodies were not raised against telost antigens, their specificites for the respective teleost proteins have been demonstrated (Wen et al., 2008b). The following secondary antibodies were obtained from ICN Biomedicals, Inc. (Costa Mesa, CA, USA): fluorescein (FITC)-conjugated goat anti-rabbit IgG, FITC-(FITC)-conjugated goat anti-mouse IgG, FITC-conjugated goat anti-mouse IgM, and horesradish peroxidase-conjugated goat anti-mouse IgG.
2.2. Electron microscopy
For transmission electron microscopy, cells were suspended using a cell scraper and harvested by centrifugation at 800 g for 10 min. The pellets werefixed for 1 h at 4 °C by immersion in 2.5% glutaraldehyde in PBS and post-fixed in 1% osmium tetroxide for 30 min, dehydrated through a graded series of ethanol, embedded in Spurr's resin, and sectioned for electron microscopy. Ultrathin sections were counter-stained with lead citrate and uranyl acetate and examined with a Hitachi H-7100 electron microscope.
2.3. Indirect immunocytochemistry
A volume containing 5 × 104 GBC4 cells was plated on 12-mm
diameter uncoated coverslips in 4-well plates (Nunc). Cells grown on coverslips at 25 °C for 1–3 days were washed three times with PBS, fixed for 20 min in 10% formalin in PBS, or for 10 min in methanol at room temperature for the intracellular antigens. Cells were then washed three times for 5 min in PBS. Anti-keratin and anti-connexin43 (Cx43) were used to visualize the expression of cytokeratin and gap junctions, respectively, on epithelial cells. Antibodies against BLBP, GS, GFAP, S100 and vimentin were used to identify astroglial cells as well as radial glial cells and tanycytes. A monoclonal antibody against A2B5 was used to label oligodendrocyte precursor cells. Anti-GalC and anti-MBP were used to identify mature oligodendrocytes. Anti-connexin35 (Cx35), anti-HuC/D, anti-MAP2, anti-neurofilament high protein (NF-H) and anti-TH were used to assess neuronal differentiation. Forfluorescence immunocytochem-istry, FITC-conjugated anti-rabbit IgG (1:200), anti-mouse IgG (1:100) or anti-mouse IgM (1:50) were used, and the labeled cells were visualized under an Olympus epifluorescence microscope. In some cases, 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei. A negative control (primary antibody omitted) was included in each experiment, and each experiment was repeated at least three times. Table 1
Primary antibodies used, targets, sources and the dilutions for immunocytochemistry.
Antibodies Targets Species and clones Companies Dilutions
Chicken A2B5 Oligodendrocyte progenitor cells
Mouse 105 Sigma 1:100
Human BLBP Radial glial cells Rabbit polyclone Abcam 1:200
Human Cx36 Neuronal gap junctions Rabbit polyclone Zymed 1:200 Human Cx43 Astroglial and epithelial
gap junctions
Rabbit polyclone Sigma 1:200
Bovine GalC Mature and premature oligodendrocytes
Rabbit polyclone Chemicon 1:100
Porcine GFAP Astroglia Mouse GA5 NeoMarkers 1:200
Sheep GS Astroglia Mouse GS-6 Chemicon 1:200
Human keratin Epithelia Mouse C11 NeoMarkers 1:200
Human HuC/D Immature and mature neurons
Mouse 16A11 Molecular
probe
1:100 Bovine MAP2
(2a + 2b)
Matures neurons Mouse AP20 NeoMarkers 1:200
Human MBP Mature
oligodendrocytes
Rabbit polyclone NeoMarkers 1:200
Porcine NF-H Mature neurons Mouse NF-01 Acris 1:200
Bovine S100 Astroglia Rabbit polyclone NeoMarkers 1:200
Human Sox2 Neural progenitor cells Rabbit polyclone Abcam 1:200
Human TH Dopamine neurons Mouse TH-2 Sigma 1:200
Porcine Vimentin Neural progenitor cells Mouse V9 NeoMarkers 1:200
Table 2
Primer sets used for RT–PCR.
Primer set Sequences (5′-3′) Product size (bp)
Annealing (°C)
Reference
AroB F: tgtattgggatggaaggggacg 868 55 This study
R: tgaactctttgggtttggggaa
BMP4 F: gtttaacctcagcagcatcc 782 55 This study
R: agccctccactaccatttcc
Cx35 F: wgtktgtggtgatcttccg 619 50 Wen et al. (2008a)
R: tgtcttctccgtgggtct
Cx43 F: ggctgctcatccccaactg 325 57 Wen et al. (2008a)
R: gactgctcattctgctgctgg
GFAP-1 F: tggtatcgctcgaagtttgc 290 50 Wen et al. (2008a)
R: aggtcctggtactcctgca
GFAP-2 F: gccagctacatcgagaaggt 537 50 This study
R: cgagcgataccactcctcag
GFAP-3 F: aggacctgctcaatgtcaag 230 55 This study
R: ctccgtagtggactctttaatgat
GS F: aagggctccaacagcgacat 545 55 Wen et al. (2008a)
R: cagccagcaccgttccagtt
Noggin2 F: aggacctctckcsgagargacyc 406 50 This study
R: akgaagccctggcagtaccac
PDGFRα F: gaggacagcgggaactacaccat 735 55 This study
R: tttgagcatctttactgccaccttc
PLP F: caaatgtttccggtcagg 344 50 Wen et al. (2008a)
R: ccgaaggtctgcttgaca
TH F: atcggagacatcgccttcag 324 50 This study
R: gtactgggtgcactggaaca
Sox2 F: tcaacgctgttcctgatgta 941 55 This study
R: ggcacggtgctctggtagtg
F, forward; R, reverse; AroB, aromatase B; BMP, bone morphogenetic protein; Cx, connexin; GFAP, glialfibrillary acidic proteins; GS, glutamine synthetase; PDGFRα, platelet-derived growth factor-α receptor; PLP, proteolipid protein; TH, tyrosine hydro-xylase; degenerate bases, k: g or t; m: a or c; r: a or g; s: c or g; w: a or t; and y: c or t. C.-M. Wen et al. / Comparative Biochemistry and Physiology, Part A 153 (2009) 191–201
Fig. 1. Electron micrograph of GBC4 cells at passage 80 showing distended cisternae of the rough endoplasmic reticulum (ER), Golgi (G) and intermediatefilament bundles (IF) in the cytoplasm. Desmosome-type junctions (arrows) are noted between the cells. Inset shows bundles of IF. Bar = 1μm; in inset=250 nm.
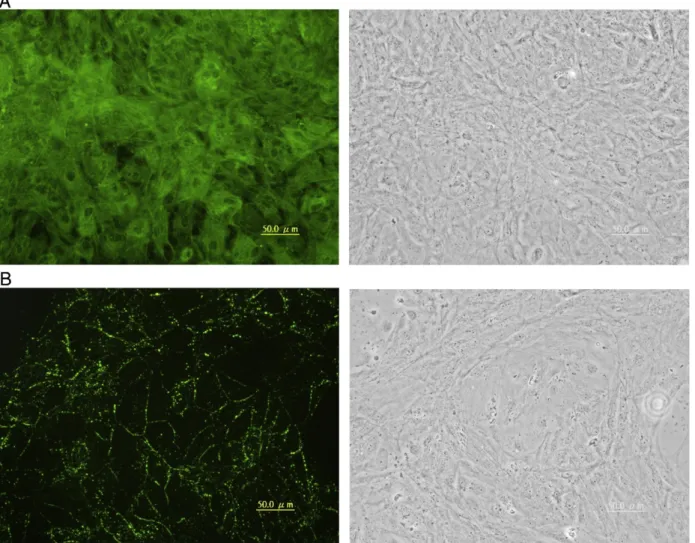
Fig. 2. Expression of epithelial proteins in GBC4 cells. Cells from passages 85 to 90 were labeled with monoclonal antibodies against human keratin (A) or polyclonal antibodies against bovine Cx43 (B). Secondary antibodies were FITC-conjugated goat anti-mouse IgG or rabbit IgG. Each image in the right column represents the corresponding phase contrast micrograph. Bar = 50μm.
2.4. Immunoblotting
Cells were rinsed three times with PBS and collected by scraping into an extraction buffer containing 0.15 M NaCl, 50 mM Tris–HCl (pH 7.4), 2 mM EDTA, 1% SDS and protease inhibitor cocktail (Set I; Merck Ltd., Taipei, Taiwan). Total protein content was measured by a protein assay solution (Bio-Rad Taiwan Branch, Taipei, Taiwan). After
dilution, 10 µg of protein was subjected to SDS–polyacrylamide gel electrophoresis (10% gel), and the protein bands were electro-transferred to a PVDF membrane (Pall Corp., Ann Arbor, MI, USA). Membranes were blocked in 10% BSA in PBS for 1 h and probed with a mouse monoclonal antibody against vimentin, GFAP, GS or TH (each diluted 1:500). Horseradish peroxidase-conjugated goat anti-mouse IgG (1:20,000) was added to bind the primary antibody, and antibody
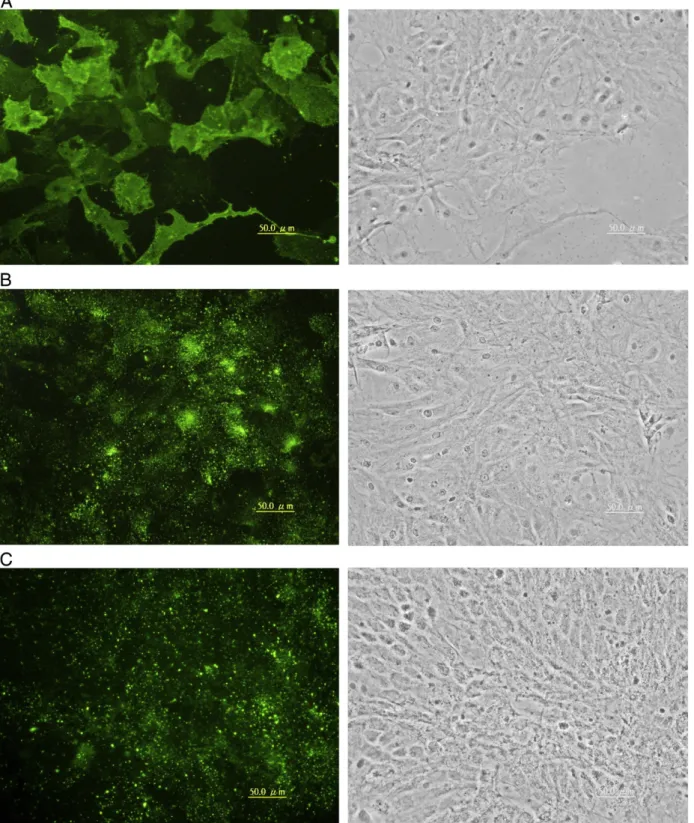
Fig. 3. Expression of astroglial and tanycyte-specific proteins in GBC4 cells. Cells similar to those inFig. 2were labeled with mouse monoclonal antibodies against porcine GFAP (A), porcine vimentin (B), sheep GS (E), and rabbit polyclonal antibodies against bovine S100 (C) or human BLBP (D). FITC-conjugated secondary antibodies were used to label the primary antibodies. In E, nuclei were stained by DAPI. Each image in the right column represents the corresponding phase contrast micrograph. Bar in A and B = 50μm, and in C, D and E = 20μm.
binding was visualized by chemiluminescence (Immobilon Western, Millipore Co., Billerica, MA, USA). Grouper brain extract was used as a positive control.
2.5. RT–PCR analyses
Total RNA from grouper brain tissue and GBC4 cells at passages 90– 100 was isolated and treated with TRIzol (Invitrogen, Taipei, Taiwan) for RT–PCR and DNase (Invitrogen) to prevent amplification of genomic DNA. RNA concentration and purity were determined by measuring the absorbance at 260 nm and 280 nm. RNA (2 µg/sample) was used to generate cDNA using the MMLV-RT kit (Promega, Madison, WI, USA). Primer sets used in this study are listed inTable 2. Primers other than those previously reported were desigened using Primer Premier 5 software (Premier Biosoft International, Palo Alto, CA, USA). Primer sets specific for AroB, BMP4, GFAP-2, GFAP-3, platelet-derived growth factor receptor α (PDGFRα), TH and Sox2 were desigened according the sequence of Epinephelus coioides AroB (GenBank accession no. AY510712), Oreochromis niloticus BMP4 (AB0884664), O. mossambicus GFAP (DQ001950), Danio rerio GFAP (BC164211), Ta-kifugu rubripes PDGFRα (AF456419), Sparus aurata TH (DQ072727) and T. rubripes Sox2 (AY779056), respectively. Degenerate primers for Noggin2 were designed from the homologous sequences of D. rerio (AF159148) and T. rubripes (AY779056). PCR amplifications were performed using the Taq DNA Pol Master Mix (Ampliqon, Copenhagen, Denmark). cDNA (1 µL), 1 µM each of forward and reverse primers, and 12.5 µL of Master Mix were added to each 0.2-mL thin-wall PCR tube
according to the manufacturer's protocol. PCR was carried out under the following conditions: 30 cycles of denaturation at 95 °C for 1 min, annealing (as inTable 2) for 30 s, and extension at 72 °C for 1 min, with afinal extension at 72 °C for 7 min. PCR products were electrophoresed on a 1.5% agarose gel. Fragments were cloned and sequenced as described (Wen et al., 2008b).
3. Results
3.1. Expression of epithelial proteins
Confluent GBC4 cells formed a contact-inhibited monolayer typical of a simple epithelium. Transmission electron microscopy showed a well-developed Golgi, distended rough endoplasmic reticulum with numerous bundles of intermediatefilaments in the cytoplasm, and desmosomal junctions between the cells (Fig. 1). Immuno fluores-cence microscopy showed numerous cytokeratinfilaments distribu-ted throughout the cytoplasm with the exception of the nuclear region (Fig. 2A). The keratin antibody used in this study recognizes keratins 4, 5, 6, 8, 10, 13 and 18, but only keratins 8 and 18, the simple epithelial keratins, have been detected in thefish nervous system (Giordano et al., 1989; Holder et al., 1990; Druger et al., 1994; Chua and Lim, 2000). Thus, the keratins visualized in our analysis of GBC4 cells probably were keratins 8 and 18. Indeed, expression of keratin 8 mRNA in GBC4 cells was demonstrated by RT–PCR (data not shown).
Immunolabeling of Cx43 revealed a typical punctate pattern between GBC4 cells (Fig. 2B). Cx43 expression has been reported in Fig. 3 (continued).
radial glial cells, astrocytes, ependymal cells, leptomeningeal cells and endothelial cells in mammalian brain tissues (Dermietzel et al., 1989) in addition to epithelial and cardiac cells. Nevertheless, GBC4 cells contained GFAP (Fig. 3A) and vimentin, the mesenchymal intermedi-atefilament protein (Fig. 3B;Wen et al., 2008b), suggesting that GBC4 cells are neither endothelial cells nor meningeal cells. Combined with the morphology, GBC4 cells showed characteristics most similar to ependymal astroglial cells.
3.2. Expression of astroglial and tanycyte-specific proteins
GBC4 cells contain GFAP as demonstrated by immunoperoxidase staining (Wen et al., 2008b), and our immunofluorescence micro-scopy confirmed that both vimentin and GFAP are expressed as bundles in the cytoplasm and cell processes (Fig. 3A, B). S100 protein was observed both in the nucleus and cytoplasm (Fig. 3C). In agreement with a previous report (Nakazato et al., 1982), the nuclear
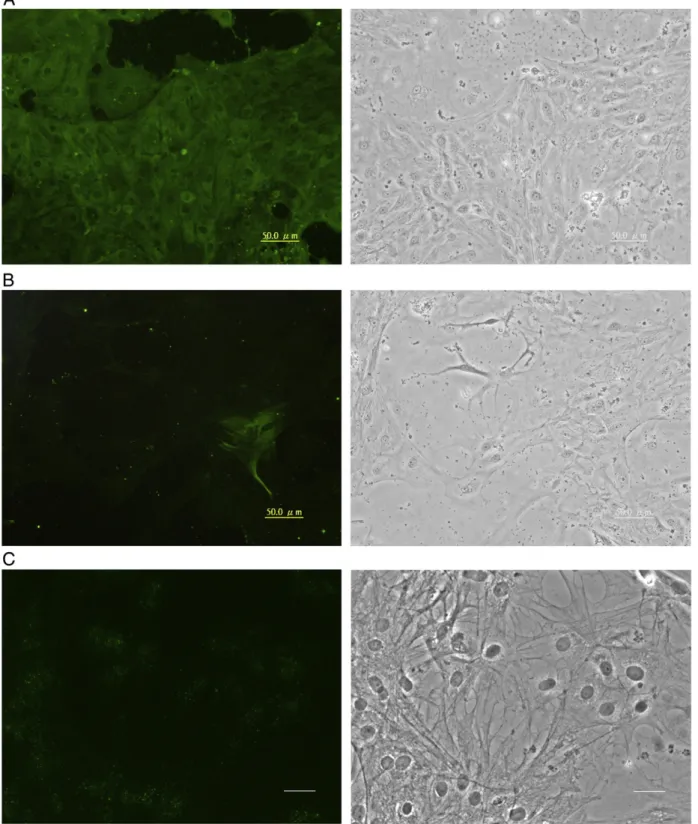
Fig. 4. Expression of oligodendrocytic proteins in GBC4 cells. Cells similar to those inFig. 2were stained with a mouse monoclonal antibody against chicken A2B5 (A), or rabbit polyclonal antibodies against GalC (B) or MBP (C). FITC-conjugated secondary antibodies were used. Each image in the right column represents the corresponding phase contrast micrograph. Bar = 50μm.
staining was more intense than that of the cytoplasm. BLBP, a radial glial marker, was visualized within the nucleus as well as the cytoplasm (Fig. 3D), a localization pattern similar to that observed in radial glia in both the embryonic ventricular zone and postnatal cerebellum of mouse CNS (Feng et al., 1994). GBC4 cells also contained GS (Fig. 3E), as has been reported for non-ependymal astroglial cells and tanycytes (Wicht et al., 1994; Chiba, 2000; Germanà et al., 2008). GS is distributed mainly in the mitochondrial matrix (Vorhaben and
Campbell, 1977), consistent with our observation of GS in cytoplasmic granules (Fig. 3E).
3.3. Expression of oligodendrocytic proteins
In addition to astroglia, some oligodendrocyte progenitor cells (OPCs) also express GFAP (Jeserich and Stratmann, 1992; Jeserich et al., 1993; Sivron et al., 1994). Therefore, GBC4 cells were examined to
Fig. 5. Expression of neuronal proteins in GBC4 cells. Cells similar to those inFig. 2were stained with mouse monoclonal antibodies against TH (A), MAP2 (B) or Cx35 (C). Secondary antibodies were FITC-conjugated goat anti-mouse IgG. Each image in the right column represents the corresponding phase contrast micrograph. Bar in A and B = 50μm; in C=20 μm.
determine whether they contained the OPC protein A2B5. A2B5 was spread homogeneously on the surface of GBC4 cells (Fig. 4A). To verify if GBC4 cells expressed mature oligodendrocyte proteins, the cells were stained with antibodies against GalC and MBP. As shown in
Fig. 4B (GalC) and 4C (MBP), we surprisingly observed punctate labeling of both proteins on the plasma membrane. In some cells GalC was concentrated in nuclear regions, whereas MBP labeling was more diffuse across all cells.
3.4. Expression of neuronal proteins
Because some OPCs and ependymal cells in adult mammalian brain have been reported to be stem cells that can generate neurons (Johansson et al., 1999; Kondo and Raff, 2000), GBC4 cells were evaluated for neuron-specific proteins. Staining for TH was observed in the cytoplasm (Fig. 5A). Although most GBC4 cells stained positively for TH, they did not have a typical neuronal morphology. Staining for the neuronal protein HuC/D (data not shown) and microtubule-associated protein 2 (MAP2) (Fig. 5B) was observed in only a few GBC4 cells (HuC/D about 25%, and MAP2 less than 5%), whereas neurofilament protein staining was weak or absent (data not shown). Cx35 labeling appeared in the perinuclear region, but no gap-junction plaques were observed (Fig. 5C), and the labeling was weaker compared with TH. The expression pattern of TH as well as Cx35 suggested that GBC4 cells may be progenitors of dopaminergic neurons, as supported by that fact that a few HuC/D- and MAP2-expressing cells appeared spontaneously in the culture.
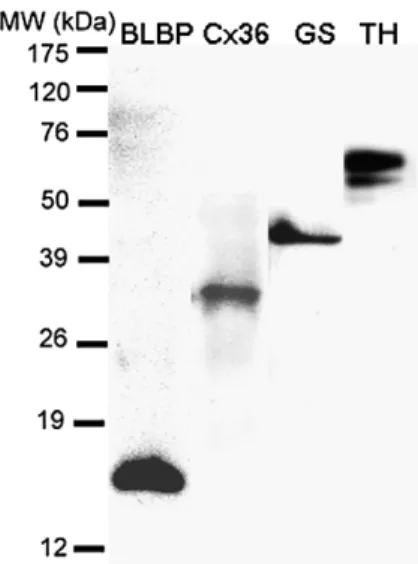
3.5. Confirmation of specific protein expression by immunoblotting Immunoblotting was used to confirm the identity of proteins that were detected by immunofluorescence microscopy. Major bands with molecular weights of about 15, 35 and 45 kDa were identified as BLBP, Cx35 and GS, respectively, in GBC4 cell extracts (Fig. 6). These molecular masses are similar to those of the homologous proteins of otherfish species (Smith et al., 1987; Peterson et al., 2001; Wen et al., 2008a). TH-immunoreactive bands in GBC4 extracts ranged from 52 to 65 kDa (Fig. 6), with the major band at ~ 65 kDa, which is within the range described for TH from otherfish species (Linard et al., 1998; Wen et al., 2008a). GBC4 cells contained abundant TH as compared with brain tissues (data not shown). By contrast, several anti-GFAP-reactive bands were observed in brain extracts, but only one GFAP
isoform was found in GBC4 extract (Fig. 4). The molecular masses of GFAPs from grouper brain ranged from 42 to 50 kDa, with the major band at 42 kDa (Fig. 7). However, GFAP from GBC4 extract was about 54 kDa. In some GBC4 extracts, a 50-kDa GFAP band also was observed (data not shown).
3.6. Confirmation of specific gene expression
Agarose gel electrophoresis of the RT–PCR products revealed bands for AroB, BMP4, Cx35, Cx43, GFAP, GS, PLP, PDGFRα, Sox2 and TH (Fig. 8), and the observed sizes corresponded to the predicted values (Table 2). All nucleotide sequences were deposited in GenBank (NCBI) with the following accession numbers: EU798289 to EU798295 for AroB, Cx43, GFAP, GS, PDGFRα, PLP and Cx35, respectively, and FJ432695, FJ432696 and F436409 for Sox2, TH and BMP4, respectively. The BLAST program revealed that the nucleotide sequences encoding the GBC4 proteins Cx35, Cx43 and BMP4 had 99, 99 and 91% identity with the respective tilapia proteins Cx35 (EU142909), Cx43 (EU142908) and BMP4 (AB084664). The GBC4 GFAP, PLP, Sox2 and TH nucleotide sequences each had 100% identity with the orange-spotted grouper GFAP (EU798296), PLP (EU798298), Sox2 (FJ432693) and TH (FJ432694), and the AroB, GS and PDGFRα sequences had 99, 98 and 99% identity to the grouper AroB (AY510172), GS (EU798297) and PDGFRα (FJ432692), respectively. The TH sequences correspond exactly to the TH1 gene (AF007942), which is one of two teleost TH genes (Candy and Collet, 2005). Primer sets GFAP-1 and GFAP-2 amplified tilapia GFAP mRNA but failed to amplify GFAP mRNA from GBC4 cells or grouper brain (data not shown). RT–PCR also revealed that GBC4 cells had very little (or no) mRNA encoding the BMP antagonist, Noggin2 (data not shown).
4. Discussion
4.1. GBC4 is a tanycyte-like cell line
The present study demonstrates that GBC4 cells, like the fish neural progenitor cell line TB2 (Wen et al., 2008a), express epithelial, glial and neuronal proteins. GBC4 cells are epithelial like, however, and thus are morphologically distinct from most of thefibroblast-like TB2 cells when grown at low density. Much evidence suggests that CNS development is dissimilar infish and mammals. Radial glial cells, which are observed only during CNS development in mammals, are distributed throughout the CNS of adultfish. Cytokeratin expression in neural cells of the mammalian CNS is restricted in neuroepithelial cells, whereas neural progenitor cells isolated from brains of goldfish (Carassius auratus) (Sivron et al., 1992, 1994), of larval rainbow trout (Oncorhynchus mykiss) (Jeserich and Stratmann, 1992; Jeserich et al., 1993) and of tilapia (Wen et al., 2008a) express keratin. The most commonly expressed keratins in vertebrate CNS and optic nerve are keratin 8 and 18 (Holder et al., 1990; Cohen et al., 1991; Kasper et al., 1991; Kasper, 1992; Sivron et al., 1994; Merrick et al., 1995).
In adultfish brain, tanycytes (also called ependymal radial glial cells) are the most dominant GFAP-expressing cells (Kálmán, 1998; Fig. 6. Reactivity of GBC4 cell extracts with BLBP (lane 1), Cx36 (lane 2),
anti-GS (lane 3), or anti-TH (lane 4). Labeling was visualized by chemiluminescence with horseradish peroxidase substrate. Each lane was loaded with 10μg protein.
Fig. 7. Reaction of monoclonal anti-GFAP with grouper brain extract (BE) and GBC4 cell extract (CE).
Arochena et al., 2004). Tanycytes can be distinguished from other astroglial lineage cells because they express BLBP and have long processes. Although it is unclear whether all tanycytes are stem cells, AroB-expressing tanycytes have been demonstrated to be stem cells (Pellegrini et al., 2007). Tanycytes are heterogeneous in mammals and other higher vertebrates, and thus fish tanycytes also may be heterogeneous. Park et al. (2007) reported that Olig2-expressing radial glial cells in the CNS of zebrafish larvae and adults are OPCs that generate only oligodendrocytes, although Olig2-expressing neuroe-pithelial precursors in the embryo are multipotent (Park et al., 2004). Because GBC4 cells share molecular characteristics with tanycytes (e.g., they express BLBP, GFAP, GS, S100 and vimentin) and the cytokeratin-expressing cells infish CNS localize primarily to epen-dyma (Bodega et al., 1995), we propose that GBC4 cells are derived either from tanycytes or ependymal neural progenitor cells. Never-theless, other types of neural progenitor cells exist in adultfish brain (Jeserich and Stratmann, 1992; Jeserich et al., 1993; Wen et al., 2008a). 4.2. Different GFAP forms may corresponding to heterogeneous astroglial cells
Heterogeneous GFAP in addition to diverse astroglial cells have been observed in the CNS of lower vertebrates and mammals. Two GFAPs (51 kDa and 54 kDa) (Quitschke et al., 1985) and two GFAP mRNAs (Blaugrund et al., 1991) were discovered in the in goldfish spinal cord.Dahl et al. (1985)showed that GFAPs from optic nerve of sea bass, tautog, trout and scup have higher molecular mass than GFAPs from brain and spinal cord. They also showed that GFAPs obtained from spinal cord extracts of human, rat and turtle are of varying size.Gheuens et al. (1984) noted that differences in GFAP forms relate to different CNS regions as well as species. Most GFAPs are ≥50 kDa, butGheuens et al. (1984)found a 42-kDa GFAP in human CNS, particularly in the brainstem. The lower-molecular mass GFAP forms have been suggested to be degradation products (Dahl et al., 1985). However, GFAPs of 42–44 kDa also have been found in the brainstem of spiny dogfish (Squalus acanthias) and rat (Gould et al., 1995). Because we included the brainstem when sampling the entire brain, the 42-kDa GFAP we observed probably was from brainstem. TB2 extract as well as brain extract also showed lower-molecular mass GFAPs (Wen et al., 2008a). Nevertheless, the specificity of particular GFAP forms for neural progenitor cells requires further study. 4.3. GBC4 cells may constitute multipotent OPCs
The best-characterized neural progenitor cells are OPCs. Indeed, fish OPCs are analogous to mammalian OPCs because they express A2B5 on their surface. Maturation of OPCs into oligodendrocytes has been demonstrated in vitro, but it is doubtful that astrocytes develop from A2B5- and GFAP-positive oligodendrocyte–type 2 astrocyte-like (O2A-like) OPCs. Our present results demonstrate that GBC4 cells express both A2B5 and GFAP but fail to generate stellate astrocytes in serum-rich medium. We observed that both GBC4 cells and TB2 neural progenitor cells expressed the astrocytic proteins GFAP, GS and S100, the oligodendrocytic proteins GalC, MBP and PLP, the neuronal protein TH, the gap-junction proteins Cx35 and Cx43, and the stem cell-specific transcription factor Sox2. GBC4 cells also expressed AroB, BLBP and PDGFRα; AroB and BLBP are expressed specifically in radial glial cells. Although PDGFRα is abundant on neuroepithelial cells during early development of the mammalian CNS, manyfish cell lines derived from non-brain tissues also express PDGFRα mRNA (by RT– PCR). Therefore, PDGFRα is not considered a specific marker for fish OPCs.
In the present study, GBC4 cells expressed AroB and BLBP, suggesting that the cells may be similar to stem cell-like radial glial cells that have the potential to generate neurons (Pellegrini et al., 2007). Given that a few MAP2-positive cells and HuC/D-positive cells
appeared spontaneously in this study, we suggest that more neurons or neuron-like cells might be produced if GBC4 cells are provided with a suitable milieu, such as a poly-lysine-coated substrate and ciliary neurotrophic factor and transforming growth factorβ, which promote neurogenesis (Bhattacharya et al., 2008; Misumi et al., 2008; Wen et al., 2008a). Using neurosphere culture method, neural stem cells fromfish as well as from mammalian have been demonstrated that generation of neurons (Hinsch and Zupanc, 2006; Servili et al., 2009).
4.4. In the absence of a suitable milieu, GBC4 cells co-expresses astroglial, oligodendrocytic and neuronal proteins
The molecular expression profiles of fish neural progenitor cells in vitro is unusual compared with their mammalian counterparts. For example, mammalian progenitor cells express stem cell markers but not mature cell markers. O2A cells mature to astrocytes or oligodendrocytes and do not co-express GFAP and MBP (Raff et al., 1984). Additionally, neural tumor cells isolated from the mammalian CNS may either co-express markers for stem cells and mature cells or show different lineage-specific markers as is the case for fish neural progenitor cells (Tlhyama et al., 1993; Katsetos et al., 2001; Rani et al., 2006). Neural tumor cells have unusual molecular expression profiles, which may be due to the loss of factors controlling growth and/or differentiation (Tlhyama et al., 1993; Kaul and Khosla, 2000; Mishra et al., 2008). The reason for such loss infish neural progenitor cells remains unclear.
The fate and morphogenesis of neural progenitor cells are controlled by BMPs and their antagonist noggin. In mammals, BMP4 inhibits oligodendrocyte maturation. BMP4 decreases the expression of PLP and MBP, maintains GalC expression (See et al., 2004), and stimulates astrocyte differentiation (Weible and Chan-Ling, 2007). BMP4 also limits the generation of neurons from neural progenitors (Bani-Yaghoub et al., 2000; Gulacsi and Lillien, 2003) and decreases cell proliferation, but it promotes Cx43-type gap-junction formation (Gulacsi and Lillien, 2003).Shou et al. (2000) reported that BMPs exert both positive and negative effects on neurogenesis, depending on ligand identity, ligand concentration and the particular cell lineage. In the present study, GBC4 cells expressed markers for mature oligodendrocytes and neurons despite BMP4 mRNA expression. Noggin2, the antagonist of BMPs that enhances neurogenesis, and the transcription factors Olig2 and Sox10 (oligodendrocyte differ-entiation induction factors) were not expressed in GBC4 cells (data not shown). Therefore, factors that affectfish neural progenitor cell differentiation require further study.
GBC4 has demonstrated that expression of a number of specific genes, such as those encoding AroB, BMP4, BLBP and PDGFRα. As a stable neural progenitor cell line, GBC4 is useful for studies assessing gene expression/regulation as well as development.
Fig. 8. RT–PCR analyses of GBC4 cells to detect transcripts for AroB, BMP4, Cx43, GFAP, GS, TH, PDGFRα, PLP, Cx35 and Sox2. Arrows indicate the amplified bands having the predicted size that were cloned and sequenced to verify the specificity of the PCR reaction. M: 100-bp ladder.
References
Adolf, B., Chapouton, P., Lam, C.S., Topp, S., Tannhauser, B., Strahle, U., Gotz, M., Bally-Cuif, L., 2006. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293.
Arochena, M., Anadón, R., Díaz-Regueira, S.M., 2004. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost. J. Comp. Neurol. 469, 413–436.
Bani-Yaghoub, M., Felker, J.M., Sans, C., Naus, C.C.G., 2000. The effects of bone morphogenetic protein 2 and 4 (BMP2 and BMP4) on gap junctions during neurodevelopment. Exp. Neurol. 162, 13–26.
Bhattacharya, S., Das, A.V., Mallya, K.B., Ahmad, I., 2008. Ciliary neurotrophic factor-mediated signaling regulates neuronal versus glial differentiation of retinal stem cells/progenitors by concentration-dependent recruitment of mitogen-activated protein kinase and Janus kinase-signal transducer and activator of transcription pathways in conjunction with Notch signaling. Stem Cells 26, 2611–2624. Blaugrund, E., Cohen, I., Shani, Y., Schwartz, M., 1991. Glialfibrillary acidic protein in the
fish optic nerve. Glia 4, 393–399.
Bodega, G., Suárez, I., Rubio, M., Villalba, R.M., Fernández, B., 1993. Astroglial pattern in the spinal cord of the adult barbel (Barbus comiza). Anat. Embryol. (Berl) 187, 385–398.
Bodega, G., Suárez, I., Rubio, M., Fernández, B., 1994. Ependyma: phylogenetic evolution of glialfibrillary acidic protein (GFAP) and vimentin expression in vertebrate spinal cord. Histochemistry 102, 113–122.
Bodega, G., Suárez, I., Rubio, M., Fernández, B., 1995. Type II cytokeratin expression in adult vertebrate spinal cord. Tissue Cell 27, 555–559.
Bruni, J.E., 1998. Ependymal development, proliferation, and functions: a review. Microsc. Res. Tech. 41, 2–13.
Candy, J., Collet, C., 2005. Two tyrosine hydroxylase genes in teleosts. Biochim. Biophys. Acta 1727, 35–44.
Chapouton, P., Adolf, B., Leucht, C., Tannhauser, B., Ryu, S., Driever, W., Bally-Cuif, L., 2006. Her5 expression reveals a pool of neural stem cells in the adult zebrafish midbrain. Development 133, 4293–4303.
Chiba, A., 2000. S-100 protein-immunoreactive structures in the brains of the elasmobranchs Scyliorhinus torazame and Mustelus manazo. Neurosci. Lett. 279, 65–68.
Chua, K.L., Lim, T.M., 2000. Type I and Type II cytokeratin cDNAs from the zebrafish (Danio rerio) and expression patterns during early development. Differentiation 66, 31–41.
Cohen, I., Yael, S., Blaugrund, E., Schwartz, M., 1991. Isolation and sequence analysis of two intermediatefilament cDNA clones from fish optic nerve. Mol. Brain Res. 11, 181–185.
Dahl, D., Crosby, C.J., Sethi, J.S., Bignami, A., 1985. Glialfibrillary acidic (GFA) protein in vertebrates: immunofluorescence and immunoblotting study with monoclonal and polyclonal antibodies. J. Comp. Neurol. 239, 75–88.
Dermietzel, R., Traub, O., Hwang, T.K., Beyer, E., Bennett, M.V., Spray, D.C., Willecke, K., 1989. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc. Natl. Acad. Sci. U.S.A. 86, 10,148–10,152.
Druger, R.K., Glasgow, E., Fuchs, C., Levine, E.M., Matthews, J.P., Park, C.Y., Schechter, N., 1994. Complex expression of keratins in goldfish optic nerve. J. Comp. Neurol. 340, 269–280.
Ekström, P., Johnsson, C.M., Ohlin, L.M., 2001. Ventricular proliferation zones in the brain of an adult teleostfish and their relation to neuromeres and migration (secondary matrix) zones. J. Comp. Neurol. 436, 92–110.
Feng, L., Hatten, M.E., Heintz, N., 1994. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron 12, 895–908. Forlano, P.M., Deitcher, D.L., Myers, D.A., Bass, A.H., 2001. Anatomical distribution and cellular
basis for high levels of aromatase activity in the brain of teleostfish: aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 21, 8943–8955.
Germanà, A., Marino, F., Guerrera, M.C., Campo, S., de Girolamo, P., Montalbano, G., German, G.P., Ochoa-Erena, F.J., Ciriaco, E., Vega, J.A., 2008. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 71, 248–255.
Gheuens, J., de Schutter, E., Noppe, M., Lowenthal, A., 1984. Identification of several forms of the glial fibrillary acidic protein, or alpha-albumin, by a specific monoclonal antibody. J. Neurochem. 43, 964–970.
Giordano, S., Glasgow, E., Tesser, P., Schechter, N., 1989. A type II keratin is expressed in glial cells of the goldfish visual pathway. Neuron 2, 1507–1516.
Gould, R.M., Fannon, A.M., Moorman, S.J., 1995. Neural cells from dogfish embryos express the same subtype-specific antigens as mammalian neural cells in vivo and in vitro. Glia 15, 401–418.
Gulacsi, A., Lillien, L., 2003. Sonic Hedgehog and bone morphogenetic protein regulate interneuron development from dorsal telencephalic progenitors in vitro. J. Neurosci. 23, 9862–9872.
Herná n dez, C., Martín, M., Bodega, G., Suáez, I., Pérez, J., Fernández, B., 1999. Response of carp central nervous system to hyperammonemic conditions: an immunocyto-chemical study of glutamine synthetase (GS), glialfibrillary acidic protein (GFAP) and 70 kDa heat-shock protein (HSP70). Aquat. Toxicol. 45, 195–207.
Hinsch, K., Zupanc, G.K.H., 2006. Isolation, cultivation, and differentiation of neural stem cells from adultfish brain. J. Neurosci. Methods 158, 75–88.
Holder, N., Clarke, J.D., Kamalati, T., Lane, E.B., 1990. Heterogeneity in spinal radial glia demonstrated by intermediatefilament expression and HRP labelling. J. Neurocytol. 19, 915–928.
Jeserich, G., Stratmann, A., 1992. In vitro differentiation of trout oligodendrocytes: evidence for an A2B5-positive origin. Brain Res. Dev. Brain Res. 67, 27–35.
Jeserich, G., Strelau, J., Tönnies, R., 1993. An immunomagnetic cell separation technique for isolating oligodendroglial progenitor cells of trout CNS. Neuroprotocols 2, 219–224.
Johansson, C.B., Momma, S., Clarke, D.L., Risling, M., Lendahl, U., Frisen, J., 1999. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96, 25–34.
Kálmán, M., 1998. Astroglial architecture of the carp (Cyprinus carpio) brain as revealed by immunohistochemical staining against glialfibrillary acidic protein (GFAP). Anat. Embryol. (Berl) 198, 409–433.
Kasper, M., 1992. Cytokeratins in intracranial and intraspinal tissues. Adv. Anat. Embryol. Cell Biol. 126, 1–82.
Kasper, M., Perry, G., Stosiek, P., 1991. Cytokeratin expression in human spinal meninges and ependymal cells. J. Hirnforsch. 32, 19–25.
Katsetos, C.D., Del Valle, L., Geddes, J.F., Assimakopoulou, M., Legido, A., Boyd, J.C., Balin, B., Parikh, N.A., Maraziotis, T., de Chadarevian, J.P., Varakis, J.N., Matsas, R., Spano, A., Frankfurter, A., Herman, M.M., Khalili, K., 2001. Aberrant localization of the neuronal class III beta-tubulin in astrocytomas. Arch. Pathol. Lab. Med. 125, 613–624. Kaul, D., Khosla, V.K., 2000. Molecular basis of cholesterol feedback lesion in CNS
tumours. Neurol. India 48, 174–177.
Kondo, T., Raff, M., 2000. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289, 1754–1757.
Lazzari, M., Franceschini, V., 2004. Glial fibrillary acidic protein and vimentin immunoreactivity of astroglial cells in the central nervous system of the African lungfish, Protopterus annectens (Dipnoi: Lepidosirenidae). J. Morphol. 262, 741–749. Linard, B., Pakdel, F., Marmignon, M.H., Saligaut, C., 1998. Cloning of a cDNA coding for active tyrosine hydroxylase in the rainbow trout (Oncorhynchus mykiss): compar-ison with other hydroxylases and enzymatic expression. J. Neurochem. 71, 920–928. Menuet, A., Anglade, I., Guevel, R.L., Pellegrini, E., Pakdel, F., Kah, O., 2003. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: comparison with estrogen receptor alpha. J. Comp. Neurol. 462, 180–193. Merrick, S.E., Pleasure, S.J., Lurie, D.I., Pijak, D.S., Selzer, M.E., Lee, V.M.Y., 1995. Glial cells
of the lamprey nervous system contain keratin-like proteins. J. Comp. Neurol. 355, 199–210.
Mishra, K., Gui, H., Matise, M.P., 2008. Prox1 regulates a transitory state for interneuron neurogenesis in the spin. Dev. Dyn. 237, 393–402.
Misumi, S., Kim, T.-S., Jung, C.-G., Masuda, T., Urakawa, S., Isobe, Y., Furuyama, F., Nishino, H., Hida, H., 2008. Enhanced neurogenesis from neural progenitor cells with G1/S-phase cell cycle arrest is mediated by transforming growth factorβ1. Eur. J. Neurosci. 28, 1049–1059.
Nakazato, Y., Ishizeki, J., Takahashi, K., Yamaguchi, H., 1982. Immunohistochemical localization of S-100 protein in granular cell myoblastoma. Cancer 49, 1624–1628. Park, H.-C., Shin, J., Appel, B., 2004. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development 131, 5959–5969. Park, H.-C., Shin, J., Roberts, R.K., Appel, B., 2007. An olig2 reporter gene marks oligodendrocyte precursors in the postembryonic spinal cord of zebrafish. Dev. Dyn. 236, 3402–3407.
Pellegrini, E., Mouriec, K., Anglade, I., Menuet, A., Page, Y.L., Gueguen, M.-M., Marmignon, M.-H., Brion, F., Pakdel, F., Kah, O., 2007. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 501, 150–167.
Peterson, R.E., Fadool, J.M., Mcclintock, J., Linser, P.J., 2001. Müller cell differentiation in the zebrafish neural retina: evidence of distinct early and late stages in cell maturation. J. Comp. Neurol. 429, 530–540.
Peterson, R.S., Lee, D.W., Fernando, G., Schlinger, B.A., 2004. Radial glia express aromatase in the injured zebrafinch brain. J. Comp. Neurol. 475, 261–269. Quitschke, W., Jones, P.S., Schechter, N., 1985. Survey of intermediatefilament proteins
in optic nerve and spinal cord: evidence for differential expression. J. Neurochem. 44, 1465–1476.
Raff, M.C., Williams, B.P., Miller, R.H., 1984. The in vitro differentiation of a biotential glial progenitor cell. EMBO J. 3, 1857–1864.
Rani, S.B., Mahadevan, A., Anilkumar, S.R., Raju, T.R., Shankar, S.K., 2006. Expression of nestin—a stem cell associated intermediate filament in human CNS tumours. Indian J. Med. Res. 124, 269–280.
Raymond, P., Barthel, L., Bernardos, R., Perkowski, J., 2006. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 6, 36. See, J., Zhang, X., Eraydin, N., Mun, S.-B., Mamontov, P., Golden, J.A., Grinspan, J.B., 2004.
Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol. Cell. Neurosci. 26, 481–492.
Servili, A., Bufalino, M.R., Nishikawa, R., de Melo, I.S., Muñoz-Cueto, J.A., Lee, L.E., 2009. Establishment of long term cultures of neural stem cells from adult sea bass, Di-centrarchus labrax. Comp. Biochem. Physiol. A 152, 245–254.
Shou, J., Murray, R.C., Rim, P.C., Calof, A.L., 2000. Opposing effects of bone morphogenetic proteins on neuron production and survival in the olfactory receptor neuron lineage. Development 127, 5403–5413.
Sivron, T., Jeserich, G., Nona, S., Schwartz, M., 1992. Characteristics offish glial cells in culture: possible implications as to their lineage. Glia 6, 52–66.
Sivron, T., Cohen, I., Schwartz, M., 1994. Intermediatefilaments reminiscent of immature cells expressed by goldfish (Carassius auratus) astrocytes and oligodendrocytes in vitro. Cell Tissue Res. 275, 327–337.
Smith Jr., D.D., Ritter, N.M., Campbell, J.W., 1987. Glutamine synthetase isozymes in elasmobranch brain and liver tissues. J. Biol. Chem. 262, 198–202.
Tlhyama, T., Lee, V.M., Trojanowski, J.Q., 1993. Co-expression of low molecular weight neurofilament protein and glial fibrillary acidic protein in established human glioma cell lines. Am. J. Pathol. 142, 883–892.
Vorhaben, J.E., Campbell, J.W., 1977. Submitochondrial localization and function of enzymes of glutamine metabolism in avian liver. J. Cell Biol. 73, 300–310. C.-M. Wen et al. / Comparative Biochemistry and Physiology, Part A 153 (2009) 191–201
Weible, M.W., Chan-Ling, T., 2007. Phenotypic characterization of neural stem cells from human fetal spinal cord: synergistic effect of LIF and BMP4 to generate astrocytes. Glia 55, 1156–1168.
Wen, C.-M., Cheng, Y.-H., Huang, Y.-F., Wang, C.S., 2008a. Isolation and characterization of a neural progenitor cell line from tilapia brain. Comp. Biochem. Physiol. A 149, 167–180.
Wen, C.M., Lee, C.W., Wang, C.S., Cheng, Y.H., Huang, H.Y., 2008b. Development of two cell lines from Epinephelus coioides brain tissue for characterization of betanoda-virus and megalocytibetanoda-virus infectivity and propagation. Aquaculture 278, 14–21. Wicht, H., Derouiche, A., Korf, H.W., 1994. An immunocytochemical investigation of glial
morphology in the Pacific hagfish: radial and astrocyte-like glia have the same phylogenetic age. J. Neurocytol. 23, 565–576.