ISSN: 0731-3837 print / 1525-6057 online DOI: 10.1081/TXR-200046377
CHARACTERIZATION OF TWO MAJOR FAMILIES OF FIBRINOGENOLYTIC PROTEASES FROM THE VENOM OF
TAIWAN HABU WITH SPECIAL REFERENCE TO THEIR MEDICAL APPLICATIONS
SHYH-HORNG CHIOU and YEN-SHAN CHEN
Institute of Biochemical Sciences, College of Life Science, National Taiwan University, Taipei, Taiwan
Venoms from various snake species alter the haemostatic and blood coagulation systems of human victims or experimental animals in a complex manner. Different venoms contain multiple components that behave as pro- or anti-coagulants, which directly (or indirectly) induce or inhibit fibrinogen and/or platelet aggregation and related complex biochemical processes, resulting in common clinical complications of blood clotting or uncontrolled hemorrhage by envenomation of snakebites. Two major families of venom proteases with fibrinogenolytic activity have been isolated and characterized from the venom of Taiwan habu (Trimeresurus mucrosquamatus), one major crotalid snake species in Taiwan. In this account we will review the structures of one venom metalloproteinase and its complex with the endogenous peptide inhibitors as revealed by X-ray crystallography. We will also report another kallikrein-like protease family with strongβ-fibrinogenolytic activities in vitro and hypotensive activity in vivo. It is deemed necessary and essential to isolate these venom enzymes that are responsible for the fibrinogen-degrading activities that destroy the precursor fibrinogen molecules with the result of excluding the formation of fibrin clot in blood plasma. The structural work by crystallographic studies of these proteases may provide some insights into the rational design of thrombolytic or antihypertension drugs that bind to the active sites of these clinically useful venom proteases.1
Keywords: Fibrinogenolytic proteases, Venom metalloproteinase, Kallikrein-like protease, Thrombosis, Hypertension, Crotalidae snakes.
1. Introduction and Historical Background
There are currently more than 200 species of venomous snakes worldwide. They are generally classified into four major families:
Address correspondence to S.-H. Chiou, Institute of Biochemical Sciences, National Taiwan University, Taipei, Taiwan. E-mail: shchiou@gate.sinica.edu.tw
Hydrophidae, Elapidae, Viperidae, and Crotalidae. The venom com-ponents are fairly common and similar to one another within each family of snakes. Small neurotoxins are generally found in the Hydropidae and Elapidae venoms and hemorrhagic and myonecrotic toxins or proteases in the venoms of the Viperidae and Crotalidae snakes (Ouyang et al., 1990; Tu, 1996).
Venom research has a long and distinctive history in the basic medical research of Taiwan. Most notable are the extensive studies both chemically and pharmacologically on the venom toxins of the elapid snakes during the past four decades (Lee, 1979; Karlsson, 1979). The components isolated from crude venoms of the most poisonous Elapidae family generally fall into three major categories based on their structures and activities, i.e., (A) phos-pholipases A2 (B) neurotoxins, and (C) cardiotoxins (or called
cytotoxins). All these biologically active proteins have been used widely as tools in the studies of various biological phenomena of molecular and cell biology. Another less poisonous snake family of Crotalidae has received less attention in Taiwan due to less dramatic envenomation induced by snakebites of this family. Nev-ertheless, the venoms, particularly those obtained from the snake families of Crotalidae and Viperidae, are known to possess many different fibrinogenolytic proteases that may initiate or affect blood coagulation process associated with snakebites (Tu, 1982).
Venoms from various snake species alter the hemostatic and blood coagulation system of human victims or experimental animals in a complex manner. Different venoms contain several components that behave as pro- or anticoagulants, which directly (or indirectly) induce or inhibit platelet aggregation and the related complex biochemical processes, resulting in the clinical complications of blood clotting or uncontrolled hemorrhage induced by envenomation of snakebites (Ouyang and Teng, 1973; Meaume, 1966; Kini and Evans, 1990). These apparently contrasting activities have been attributed to the presence of various fibrinogenolytic or fibrinogen clotting enzymes in snake venoms (Brinkhous and Smith, 1988; Stocker, 1990).
Concerning the action of Formosan snake venoms on blood coagulation, it was reported early in 1921–25 that the crude venoms of two crotalid snake species, Agkistrodon acutus and Trimeresurus gramineus, had a coagulant action on whole blood and plasma, while the venom of another species Trimeresurus
mucrosquamatus of the same family showed an inhibitory action (Ouyang, 1957 and references therein). The inhibitory action on blood coagulation was believed to be caused mostly by destruction of fibrinogen in the case of the venom of Trimeresurus mucrosqua-matus. Ouyang and his associates at the Pharmacological Institute of the Medical College of National Taiwan University was the first group in pointing out the characteristics of fibrinogenolysis and fibrinolysis in crotalid snake venoms (Ouyang and Teng, 1972; Ouyang and Yang, 1974). Although they have made a preliminary characterization of severalα- and β-fibrinogenases from Trimeresu-rus mucrosquamatus (Ouyang and Teng; 1976; Ouyang et al., 1977), the basic identity of these proteases remains elusive in the absence of detailed primary structures of these important enzymes.
It is also well known in the toxin literature that venoms from crotalid snakes contain complex mixtures of pharmacologically active peptides and proteins. Different groups reported disparate proteases in the crotalid venoms. They included crotalase, a thrombinlike enzyme from the American-Eastern diamondback rattlesnake (Crotalus adamanteus) (Markland and Damus, 1971); hemorrhagic toxins, anticoagulant proteases, and kallikrein-like enzymes from the American-Western diamondback rattlesnake Crotalus atrox (Bjarnason and Tu, 1978; Pandya and Budzynski, 1984; Bjarnason et al., 1983). Recently we have reevaluated the venom components from Crotalus atrox and found that all frac-tions isolated from the anion-exchange chromatography showed varying extents of specific proteolytic activity against α- and/or β-chains of fibrinogen molecules (Chiou et al., 1992, 1992). On the other hand, studies on the toxin components from the Taiwan habu (Trimeresurus mucrosquamatus) (Huang et al., 1993), a major crotalid species in this island, indicated several kinds of stable fibrinogenases present in this phylogenetically more remote species different from those rattlesnake species in the Crotalidae family. We have developed facile methods for the purifi-cation and characterization of these venom proteases from several crotalid snakes with the aim of solving the protein structures by conventional protein sequencing techniques. In the meantime, the recent advent of molecular cloning and sequencing by means of polymerase chain reaction has made the protein structure determination through the determination of their corresponding cDNA less laborious.
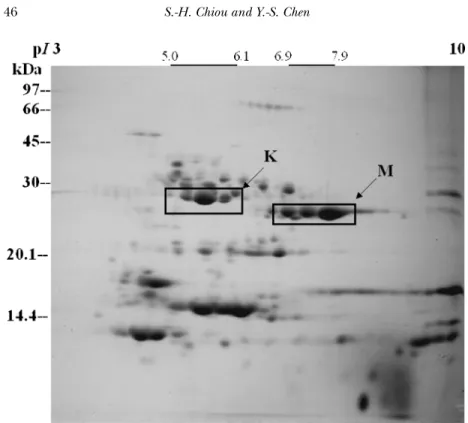
FIGURE 1 Two-dimensional gel analysis of the protein components from Taiwan habu (Trimeresurus mucrosquamatus). The proteins were separated by isoelectric focusing in the first dimension and SDS-PAGE in the second dimension. Blocks K and M indicate two major families of proteinases described in this account, K, Kallikrein-like fibrinogenolytic proteases with pI 5.0-6.1; M, Zinc-containing metalloproteinases with pI 6.9-7.9.
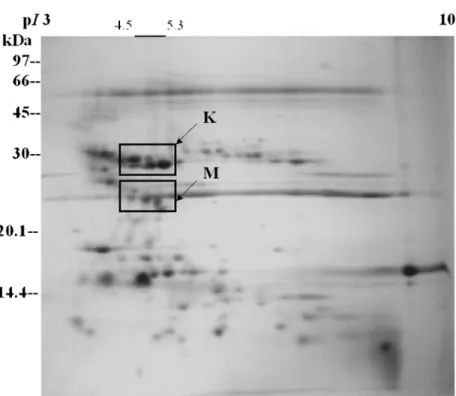
In this account we will review the structures of one venom metalloproteinase and its complex with the endogenous peptide inhibitors as revealed by X-ray crystallography. We will also report another kallikrein-like protease family with strong β-fibrinogenolytic activities in vitro and hypotensive activity in vivo. Figures 1 and 2 show the global distribution of protein compo-nents of total crude venom proteins isolated from Taiwan habu (Trimeresurus mucrosquamatus) and American-Western diamond-back rattlesnake (Crotalus atrox). The two major families of venom proteins reported here are also identified by in-gel digestion of protein components from two-dimensional gel electrophoresis (2-D gels) coupled with MALDI-TOF mass spectrometry and sequence comparison in the protein data bank.
FIGURE 2Two-dimensional gel analysis of the protein components from American-Western diamondback rattlesnake (Crotalus atrox). The proteins were separated by isoelectric focusing in the first dimension and SDS-PAGE in the second dimension. Blocks K and M indicate two major families of proteinases described in this account, K, Kallikrein-like fibrinogenolytic proteases with pI 4.5-5.3; M, Zinc-containing metalloproteinases with pI 4.5-5.6.
2. Characterization of Protein Components from Taiwan Habu
A. Zinc-Containing Fibrinogenolytic Metalloproteinases
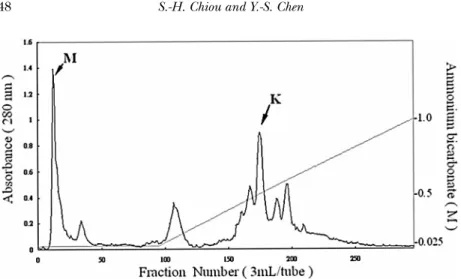
Three fibrinogenolytic proteases were isolated and purified from the venom of Taiwan habu (Trimeresurus mucrosquamatus) (Huang et al., 1995) using anion-exchange (Fig. 3) and gel-filtration chromatographies followed by cation-exchange HPLC. Further characterization of these purified fractions with fibrinogenase activity indicated that they are single-chain proteases of approx-imately 24 kDa, possessing strong cleaving activity mainly on the Aα and less on Bβ and γ chains of fibrinogen subunit chains. Enzyme activities were strongly inhibited by EDTA or
FIGURE 3 Anion-exchange chromatography on TSK DEAE-650 (M) column of crude venom from Taiwan habu, Trimeresurus mucrosquamatus. The lyophilized crude venom dissolved in the starting buffer of 0.025 M ammonium bicarbonate, pH 7.8, was applied to the column equilibrated with the same buffer. Separation was carried out in salt gradient as indicated by the straight line (0.025-1.0 M) in the figure. The column eluates (3.0 ml/tube) were monitored for absorbance at 280 nm. The middle peak (eluted by a salt gradient of 0.40-0.46 M ammonium bicarbonate) indicated by an arrow K is the fraction containingβ-fibrinogenase with kallikrein-like activity. The metalloproteinases were eluted in the first unretarded fraction (arrow M).
1,10-phenanthroline and not by phenylmethanesulfonyl fluoride, suggesting that these fibrinogenases belong to the family of metal-loproteinases and not thrombinlike serine proteases. N-Terminal sequence analysis of these proteases failed to show any free amino-terminal residues, thus hampering the sequence determination by conventional sequencing strategy. Microsequencing on the electroblotted fragments of CNBr-treated proteases separated on SDS-PAGE was then used to determine the partial sequences. Polymerase chain reaction (PCR) was employed to amplify cDNAs constructed from the poly(A)+RNA of fresh venom glands of the same snake species to facilitate cloning and sequencing of these proteases. Sequencing several positive clones containing amplified cDNAs revealed the existence of one fibrinogenase in the Taiwan habu, which was contained within one complete cDNA encoding the preproproteinase precursor of hemorrhagic metalloproteinases.
It is worth noting that the deduced total protein sequence consists of an 18 amino-acid signal peptide, a zymogen propeptide
of 171 amino acids, a mature protein of 203 amino acids (TM= 3) and a disintegrin-like carboxy-terminal segment of 89 amino acids. This uninterrupted precursor sequence corresponding to the purified fibrinogenase of Taiwan habu is found to be very sim-ilar to several characterized proteinase precursors of hemorrhagic proteases such as protrigramin (Neeper and Jacobson, 1990) and hemorrhagic toxin e (Ht-e) (Hite et al., 1992) regarding the overall organization of the above mentioned protein subdo-mains endowed with different functions. The protein sequence homology between the complete precursor of TM-3 (Mpts-8) and precursors of trigramin and Ht-e is 77% and 71%, respectively. We believe that the deduced TM-3 sequence coupled with the sequence determination of several undetermined cDNA clones coding for homologous isoforms of the same fibrinogenolytic metalloproteinase (TM-1, TM-2, and TM-3) would constitute a major multigene family of venom fibrinogenases similar to that of hemorrhagic zinc-containing metalloproteinases (Hite et al., 1994).
It is well known that fibrinogenases are the major venom principles from the crotalid family of snakes. α-Fibrinogenases are usually metalloproteases that are markedly inhibited by EDTA andβ-mercaptoethanol, while β-fibrinogenases are mostly serine proteases that are inhibited by PMSF. Judging by these criteria, TM-1, TM-2, and TM-3 characterized here should be classified as α-type fibrinogenases. Based on pharmacological properties,α-fibrinogenases possess hemorrhagic activity while β-fibrinogenases lack this activity. Detailed sequence analysis and comparison have established the sequence of TM-3 shown in this study as one of the major domains present in an intact precursor encoding pre-proproteinase. TM-3 is supposedly the mature met-alloprotease processed from this much bigger pre-proproteinase, which also encompasses its carboxy-terminal region as an RGD-containing disintegrin peptide with inhibitory activity for platelet aggregation.
B. Characterization of Three Endogenous Peptide Inhibitors for Metalloproteinases
Three small peptide components were isolated and purified from the venom of Taiwan habu (Huang et al., 1998), which
show specific activity to inhibit the strong proteolytic activity of multiple metalloproteinases (TM-1, TM-2, and TM-3) present in the crude venom. Using multiple chromatographies coupled with successive ultrafiltrations, three inhibitors, i.e. pyroglutamate-lysine-tryptophan (pyroGlu-Lys-Trp), pyroglutamate-asparagine-tryptophan (pyroGlu-Asn-Trp) and pyroglutamate-glutamine-tryptophan (pyroGlu-Gln-Trp) were obtained in good yields and high homogeneity. The yields of these peptide fractions were estimated to be about 0.65 mg, 0.55 mg, and 0.42 mg from 250 mg total lyophilized crude venom, which corresponded to the approximate concentrations of 8.4 mM, 7.3 mM, and 5.4 mM, respectively, in venom secretion. Detailed and unambiguous struc-tural determination was established by amino acid analyses, mass spectrometry, and microsequencing of purified peptides. Further functional characterization of these three tripeptides showed that they could weakly inhibit TM-1-3 previously isolated from the same venom. The inhibitory activities were similar among these tripeptides and their IC50 (concentration for 50% inhibition) were estimated in a range of 0.20-0.95 mM, which is much more effective than citrate, another venom protease inhibitor of low molecular-weight component. Since these tripeptides are the endogenous peptide inhibitors present in the lumen of venom glands, it is conceivable that they may act as a self-defensive mechanism against the auto-digestive deleterious effect of the strong metalloproteinases in vivo, particularly several zinc-dependent metalloproteinases present in crotalid and viperid venoms.
C. Crystal Structure of TM-3 Venom Metalloproteinase
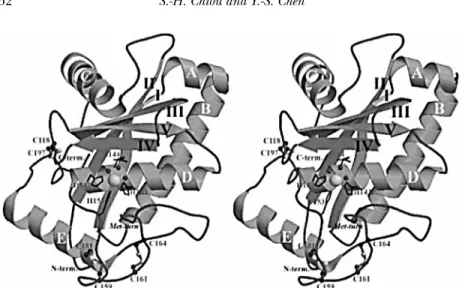
The crystal structure (Fig. 4) of TM-3, a small-size, snake-venom metalloproteinase (SVMP) isolated from Taiwan habu was de-termined at 1.35 ˚A resolution with the resulting R and Rfree values of 0.181 and 0.204, respectively (Huang et al., 2002). The overall structure of TM-3 is an oblate ellipsoid that contains three disulfide cross-links, Cys118-Cys197, Cys159-Cys181 and Cys161-Cys164. It exhibits the typical structural features of SVMP, and is closely related to the structure of the catalytic proteinase domain of TNFα converting enzyme (TACE). In the solved
structure, the essential catalytic zinc ion was found to be replaced by a cadmium ion during crystallization as revealed by atomic absorption analysis and X-ray data. This cadmium ion is bound with six ligands, including three conserved histidines and three water molecules, displaying a coordination geometry of distorted octahedron. One of the water molecules is proposed to play the role of stabilizing the tetrahedral intermediate during catalysis of SVMPs. The putative S-1 specificity pocket of TM-3 is relatively shallow in contrast to the deep pockets of adamalysin II, atrolysin C, and H2-proteinase, but is similar to those in acutolysin A and TACE. The shallow pocket is due to the presence of the nonconserved disulfide bond Cys159-Cys181, and the S-1-bottom residue Gln174. Our results indicate that the active-site structure of TM-3, among the known structures of SVMP examined thus far, has the most similar structure to TACE owing to their close disulfide configurations and the S-1 specificity pocket.
D. Crystal Structures of the Complex of TM-3 and Endogenous Tripeptide Inhibitors
As described previously, venoms from crotalid and viperid snakes contain several peptide inhibitors that regulate the proteolytic activities of their snake-venom metalloproteinases (SVMPs) in a reversible manner under physiological conditions. We have described the high-resolution crystal structures of TM-3, from Taiwan habu cocrystallized with the endogenous inhibitors Asn-Trp (pENW), Gln-Trp (pEQW), or pyroGlu-Lys-Trp (pEKW) (Huang et al., 2002). The binding of inhibitors causes some of the residues around the inhibitor-binding envi-ronment of TM-3 to slightly move away from the active-site center, and displaces two metal-coordinated water molecules by the C-terminal carboxylic group of the inhibitors. This binding adopts a retro-manner principally stabilized by four possible hydrogen bonds. The Trp indole ring of the inhibitors is stacked against the imidazole of His143 in the S-1 site of the proteinase. Results from the study of synthetic inhibitor analogues showed the primary specificity of Trp residue of the inhibitors at the P-1 site, corrobo-rating the stacking effect observed in our structures. Furthermore, we have made a detailed comparison of our structures with the binding modes of other inhibitors, including batimastat, a
FIGURE 4 A ribbon diagram of the overall structure of TM-3 (Huang et al., 2002). The stereoview faces toward the active-site cleft. Cadmium ion and its coordinated water molecules in the active site are depicted in large and small spheres, respectively. The coordinated histidines and catalytic glutamyl residue are denoted by a stick model. The three disulfide bridges are drawn with a ball-and-stick model. In addition, the locations of α-helices (A-E), β-strands (I-V), methionine-turn as well as the N- and C-terminal residues are also indicated. The figure was produced using MOLSCRIPT. It is noteworthy that the structure exhibits the typical features of SVMPs, i.e., an oblate ellipsoidal molecule composed of a central five-strandedβ–sheet mixed with five α–helices in a unique arrangement.
hydroxamate inhibitor, and a barbiturate derivative. It suggests a close correlation between the inhibitory activity of an inhibitor and its ability to fill the S-1 pocket of the proteinase. Our work may provide insights into the rational design of small molecules that bind to this class of zinc-metalloproteinases.
E. Isolation, Cloning and Expression of Kallikrein-like Proteases from Taiwan Habu
A group of several fibrinogenolytic proteases with acidic pI of 5.0-6.1 (in contrast to metalloproteinases TM1-3 of pI 7.0-7.7) (Fig. 1) were isolated from the venom of Taiwan habu (Hung et al., 1994). In vitro, these proteases cleaved efficiently β-chain of fibrinogen molecules and showed relatively lower activity on α-chain, with almost no activity on γ -chain even after a long
period of incubation. Further characterization showed that they are single-chain proteins with two of them possessing a molecular weight of about 28,000 and the other four of 33,000, the higher molecular-weight species being shown to be glycoproteins. All these venom enzymes are inhibited by the serine protease in-hibitors phenylmethanesulfonyl fluoride and leupeptin but not by ethylenediaminetetraacetate, attesting to their not being in the class of metalloproteases described above. They all exhibit strong N-p-tosyl-L-arginine methyl esterase and N-benzoyl-Pro-Phe-Arg-p-nitroanilide amidase activities. Their stability at high temperatures was examined and the cleavage specificity studied using oxidized insulin B-chains as substrates. The specific cleavage sites in insulinβ chain can be determined from the amino acid compositions of the peptide fragments isolated by HPLC on the incubation mixtures of insulin substrates with these proteases. The proteases share some common scission sites on insulin β chain, especially Tyr16-Leu17 bond is cleaved by all fibrino-genases. His5-Leu6 and Tyr16-Leu17 bonds are also cleaved by all these fibrinogenases. N-Terminal sequence analysis revealed that they are similar to batroxobin and ancrod, which were shown to possess either fibrinogen-clotting or antithrombotic effect. Comparison of amino-terminal sequences among these venom proteases revealed two major isoformic forms, one starting with an N-terminal sequence of Val-Ile-Gly (Tm-VIG) and the other with Ile-Ile-Gly (Tm-IIG), both exhibiting a high sequence homology with other thrombinlike enzymes. Immunoblotting analyses show that these venom proteases can cross-react with several throm-binlike enzymes isolated from various snake venoms. Polymerase chain reaction was employed to amplify cDNAs constructed from the poly(A)+RNA of fresh venom glands of the same snake species in order to facilitate the cloning and sequencing of these fibrinogenases. Sequencing several positive clones revealed the deduced sequences of 257 amino acids, each corresponding to a precursor form of venom fibrinogenase containing a mature pro-tein of 233 amino acids and a 24-amino-acid segment composed of a signal peptide plus a zymogen peptide. It is concluded that there exists a family of novel thrombinlike proteases with strong β-fibrinogenolytic activities in the Taiwan habu. The catalytic triad characteristic of the serine protease family is well conserved in these fibrinogenases.
Further functional characterization of these stable fibrino-genases (Hung and Chiou, 2001) revealed that they possessed strong kallikrein-like activity in vitro, releasing bradykinin from kininogen. The purified enzymes did not coagulate human plasma, yet decreasing fibrinogen levels in plasma and pro-longing bleeding without formation of fibrin clots, indicating that both proteases have specificities different from thrombin and thrombinlike proteases of snake venom reported previously. They also exhibit amidase activity against N -benzoyl-Pro-Phe-Arg-p-nitroanilide, which is a specific synthetic substrate for kallikrein-like proteases. Their stability at high temperatures was examined and found to be more stable when compared with ancrod and thrombin. Intravenous injection of either protease was shown to lower blood pressure in experimental rats. Most noteworthy is the observation that the proteases can cleave angiotensin I and release bradykinin from plasma kininogen in vitro, which is a strong vasodilator and probably responsible for the in vivo hypotensive effect of these venom proteases.
3. Medical Applications and Future Perspectives
A. Zinc-Containing Fibrinogenolytic Metalloproteinases
Many of the venom fibrinolytic enzymes that have been character-ized in detail are zinc metalloproteinases. They are members of the metzincin family described by St¨ocker et al., (1995). In various metzincins, the typical features of the structure are aβ-turn with a Met residue (Met-turn) near the zinc ion, as well as the metal-binding consensus HExxHxxGxxH sequence at the active site. Based on the overall structures or the source of origin, metzincins are also grouped into four subfamilies, including adamalysins (also termed snake-venom metalloproteinases, SVMPs), matrixins (vertebrate collagenases or denoted as matrix metalloproteinases, MMPs), serralysins (large bacterial zinc-endopeptidases), and astacins with the common presence of a similar zinc-dependent catalytic domain (St¨ocker et al., 1995; St¨ocker and Boda, 1995; Bode et al., 1996).
In addition, animal tissues have been reported to produce a large number of multidomain proteins, generally called ADAMs
(A Disintegrin-like And Metalloproteinase proteins), that contain the same central catalytic domain as SVMPs and MMPs (Fox and Long, 1998; Maskos et al., 1998). ADAMs are metalloprotease with conserved disintegrin domain. They constitute a family of transmembrane glycoproteins and serve essential physiological roles in ectodomain shedding, fertilization, myogenesis, neuro-genesis, and inflammation (Wolfsberg and White, 1996; Rooke et al., 1996; Tortorella et al., 1999). The similarity of SVMPs, MMPs, and ADAMs along with the high-degree conservation of their tertiary structures at the active sites suggested that binding of substrates and inhibitors might follow a similar pathway. Most noteworthy is that ADAM17 is the tumor necrosis factor-α (TNFα) convertase, or TACE, responsible for the cleavage of membrane-bound TNFα precursor at the Ala76-Val77 bond, thus, liberating the soluble TNFα into extracellular space (Black et al., 1997; Moss et al., 1997). Recently, the crystal structure of catalytic proteinase domain of TACE was reported and showed similar topology and organization to the structures of SVMP family, with almost identical orientation of α-helices and the central five-stranded β-sheet (Maskos et al., 1998). Since TNFα has been implicated in various deleterious pathological conditions such as rheumatoid arthritis, cachexia, and endotoxic shock, inhibitors that block the proteolytic activity of TACE may be useful as candidates in therapeutics (Barlaam et al., 1999; Xue et al., 2001). As shown in Fig. 5 (Huang et al., 2002), TM-3 and TACE are presumably less susceptible to batimastat, because the S-1 pockets of both structures are too shallow to make proper insertion by the thiophene ring. Conceivably, a good TM-3 inhibitor may be more effective to TACE than human neutrophil collagenase (HNC) or atrolysin C because of the similar depth/dimension of the S-1 pocket between TM-3 and TACE. The structural work along this line may be helpful to form a firm basis for the rational design of inhibitors against TACE-related disorders.
B. Kallikrein-Like Fibrinogenolytic Proteases
The venom fibrinogenolytic serine proteinases (Hung et al., 1994) we cloned and characterized from Taiwan habu revealed extensive sequence homology with the so-called thrombinlike venom serine proteinases (ancrod, batroxobin, and crotalase)
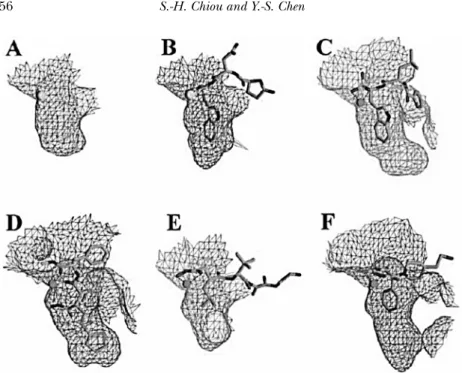
FIGURE 5 Comparison of the S-1 pockets. (A) and (B): The S-1 pocket of TM-3 and its complex bound with pENW, respectively. (C) and (D): The S-1 pockets of adamalysin II and atrolysin C after the binding of a phosphonate inhibitor and the batimastat, respectively. (E) and (F): The S-1 pockets of the catalytic proteinase domain of TNFα converting enzyme and human neutrophil collagenase after the binding of a hydroxamate and a barbiturate inhibitor, respectively. All these diagrams are in the same scale, produced using GRASP and highlight the similarity of the binding pockets between TM-3 and TACE (Huang et al., 2002).
and with other venom serine proteinases such as the kallikrein-like enzyme from C. atrox (Bjarnason et al., 1983). It is to be noted that D-Val-Leu-Lys p-nitroanilide, which is a specific substrate for plasmin, was demonstrated to be a very poor substrate for these two types of fibrinogenases (Hung and Chiou, 2001), attesting to some distinct features of these fibrinogen-digesting proteases as compared with conventional serine proteases such as thrombin or thrombinlike venom proteinases involved in the process of blood coagulation. Incubation of angiotensin I with these two venom proteases resulted in similar degradation patterns. The four major peptide fragments released by specific cleavage on angiotensin I with Tm-VIG and Tm-IIG as determined from amino acid compositions of these peptide fragments are as follows: His-Leu,
Asp-Arg-Val-Tyr, Ile-His-Pro-Phe, and Asp-Arg-Val-Tyr-Ile-His-Pro-Phe. This would indicate that these two proteases act on the same sites in angiotensin I. In addition, when kininogen was incubated with purified Tm-VIG or Tm-IIG, the disappearance of kininogen coupled with the formation of the major degradation protein fragment of 58 kDa chain is very similar to that observed for human kallikrein. The kinin released by Tm-VIG and Tm-IIG from kininogen was further identified by reverse-phase HPLC. Comparison of the fragmentation profiles by mass spectrometry using synthetic bradykinin as a marker standard identified one of the released peptides as bradykinin, pointing to the fact that these two venom proteases may possess genuine hypotensive effect in vivo through bradykinin.
It is likely that kallikrein-like β-fibrinogenases we charac-terized may be the snake venom enzymes responsible for the generation of bradykinin from endogenous HMW kininogen to lower blood pressure. The in vivo hypotensive effect on rats by these enzymes (Hung and Chiou, 2001) attested to the effec-tiveness of using these fibrinogenases as antihypertensive agents. An additional activity of these fibrinogenases, the degradation of the hypertensive peptide angiotensin I, may also potentiate hypotensive effect exhibited by these venom proteases.
Two reptilian venom proteases, ancrod (Burkhart et al., 1992; Au et al., 1993) and batroxobin (Itoh et al., 1987), thrombinlike venom serine proteinases from Bothrops atrox and Calloselasma rhodostoma of viperid snakes, have been found clinically useful for the treatment of thrombotic diseases during the past three decades. The comparison of activity and thermal stability of habu proteases with ancrod and batroxobin indeed shows great poten-tials in exploiting these novelβ-fibrinogenases with kallikrein-like activity as effective antithrombotic and antihypertensive agents. Moreover, theseβ-fibrinogenases showed no hemorrhagic activity, which is always an important consideration with respect to poten-tial clinical application of these enzymes (Markland, 1998; Lijnen and Collen, 1991). Additional work may need to be performed with these nonhemorrhagic fibrinogenases in different animal models to convincingly demonstrate the antithrombotic efficacy of these enzymes and their relative potency in comparison to the agents presently in clinical use, the tissue plasminogen activators (TPA) (Lijnen and Collen, 1991). We have obtained
a US patent regarding the claims of such purified proteases in treating cardiovascular disorders, including hypertension, stroke, and thrombosis (Chiou, 2003).
Acknowledgments
This work was supported in part by grants from the National Science Council (NSC 91-2311-B-001-121 and 92-2311-B-002-107 to S.-H. Chiou), Taipei, Taiwan. In collaboration with Dr. Andrew H. J. Wang at the Institute of Biological Chemistry, Academia Sinica, the X-ray diffraction study of venom metalloproteinases was carried out by Mr. Kai-Fa Huang and assisted by the staff of the X-ray core facility.
References
Au, L.-C., Lin, S.-B., Chou, J.-S., Teh, G.-W., Chang, K.-J., Shih, C.-M. (1993). Molecular cloning and sequence analysis of the cDNA for ancrod, a thrombin-like enzyme from the venom of Calloselasma rhodostoma. Biochem. J. 294:387– 390.
Barlaam, B., Bird, T. G., Lambert-Van Der Brempt, C., Campbell, D., Foster, S. J., Maciewicz, R. (1999). New a-substituted succinate-based hydroxamic acids as TNFa convertase inhibitors. J. Med. Chem. 42:4890–4908.
Bjarnason, J. B., Tu, A. T. (1978). Hemorrhagic toxins from western diamond-backrattlesnake (Crotalus atrox) venom: Isolation and characterization of five toxins and therole of zinc in hemorrhagic toxin e. Biochemistry 17:3395–3404. Bjarnason, J. B., Barish, A., Direnzo, G. S., Campbell, R., Fox, J. W. (1983). Kallikrein-like enzymes from Crotalus atrox venom. J. Biol. Chem. 258:12566– 12573.
Bjarnason, J. B., Barish, A., Direnzo, G. S., Cambell, R., Fox, J. W. (1983). Kallikrein-like enzyme from Crotalus atrox venom. J. Biol. Chem. 238:12566– 12573.
Black, R. A., Rauch, C. T., Kozlosky, C. J., Peschon, J. J., Slack, J. L., Wolfson, M. F., Castner, B. J., Stocking, K. L., Reddy, P., Srinivasan, S., Nelson, N., Boiani, N., Schooley, K. A., Gerhart, M., Davis, R., Fitzner, J. N., Johnson, R. S., Paxton, R. J., March, C. J., Cerretti, D. P. (1997). A metalloproteinase disintegrin that releases tumour-necrosis factor-a from cells. Nature London 385:729–733. Bode, W., Grams, F., Reinemer, P., Gomis-R ¨uth, F. X., Baumann, U., McKay, D. B.,
St¨ocker, W. (1996). The metzincin-superfamily of zinc-peptidases. Adv. Exp.
Med. Biol. 389:1–11.
Brinkhous, K. M., Smith, S. V. (1988). Platelet-aggregating noncoagulant snake venom fractions In: Pirkle H., and Markland Jr., F. S. eds. Hemostasis and
Burkhart, W., Smith, G. F. H., Su, J. L., Parikh, I., LeVine III, H. (1992). Amino acidsequence determination of Ancrod, the thrombin-like alpha-fibrinogenase from the venom of Akistrodon rhodostoma. FEBS Lett. 297:297– 301.
Chiou, S.-H. (2003). Fibrinogenolytic proteases with thrombolytic and antihy-pertensive activities: Medical application and novel process of expression and production. US Patent #6630139, Oct. 7.
Chiou, S.-H., Hung, C.-C., Lin, C.-W. (1992). Isolation of a crotalase-like protease with -fibrinogenase activity from the Western diamondback rattlesnake,
Crotalus atrox. Biochem. International 26:105–112.
Chiou, S.-H., Hung, C.-C., Huang, K.-F. (1992). Characterization of a protease with - and -fibrinogenase activity from the Western diamondback rattlesnake.
Crotalus atrox. Biochem. Biophys. Res. Commun. 187:389–396.
Fox, J. W., Long, C. (1998). The ADAMs/MDC family of proteins and their relationships to the snake venom metalloproteinases In: Bailey, G. S. ed.
Enzymes from Snake Venom Alaken, Inc., Fort Collins, Colorado 151–178.
Hite, L. A., Shannon, J. D., Bjarnason, J. B., Fox, J. W. (1992). Sequence of a cDNAencoding the Zinc metalloproteinase hemorrhagic toxin e from
Crotalus atrox: evidence for signal, zymogen, and disintegrin-like structures. Biochemistry 31:6203–6211.
Hite, L. A., Jia, L.-G, Bjarnason, J. B., Fox, J. W. (1994). cDNA sequences for four snake venom metalloproteinases: structure, classification, and their relationship to mammalian reproductive proteins. Arch. Biochem. Biophys. 308:182–191.
Huang, K.-F., Hung, C.-C., Chiou, S.-H. (1993). Characterization of threefib-rinogenolytic proteases isolated from the venom of Taiwan habu
(Trimeresu-rusmucrosquamatus). Biochem. Mol. Biol. International 31:1041–1050.
Huang, K.-F., Hung, C.-C., Pan, F.-M., Chow, L.-P., Tsugita, A., Chiou, S.-H. (1995). Characterization of multiple metalloproteinases with fibrinogenolytic activity from the venom of Taiwan habu (Trimeresurus mucrosquamatus): protein microsequencing coupled with cDNA sequence analysis. Biochem.
Biophys. Res. Commun. 216:223–233.
Huang, K.-F., Hung, C.-C., Wu, S.-H., Chiou, S.-H. (1998). Characterization of three endogenous peptide inhibitors for multiple metalloproteinases with fibrinogenolytic activityfrom the venom of Taiwan habu (Trimeresurus
mucrosquamatus ). Biochem. Biophys. Res. Commun. 248:562–568.
Huang, K.-F., Chiou, S.-H., Ko, T.-P., Yuann, J.-M., Wang, A. H.-J. (2002). The 1.35 ˚A crystal structure of cadmium-substituted TM-3, a snake venom metal-loproteinase fromTaiwan habu: elucidation of a TNF converting enzyme–like active-site structure with adistorted octahedral geometry of cadmium. Acta
Cryst. D58:1118–1128.
Huang, K.-F., Chiou, S.-H., Ko, T.-P., Wang, A. H.-J. (2002). Determinants oftheinhibition of a Taiwan habu venom metalloproteinase by its endogenous inhibitorsrevealed by X-ray crystallography and synthetic inhibitor analogues.
Eur. J. Biochem. 269:3047–3056.
Hung, C.-C., Huang, K.-F., Chiou, S.-H. (1994). Characterization of one novel venom protease with beta-fibrinogenase activity from the Taiwan habu
(Trimeresurusmucrosquamatus): purification and cDNA sequence analysis.
Biochem. Biophys. Res. Commun. 205:1707–1715.
Hung, C.-C., Chiou, S.-H. (2001). Fibrinogenolytic proteases isolated from the snake venom of Taiwan habu: serine proteases with kallikrein-like and angiotensin-degrading activities. Biochem. Biophys. Res. Commun. 281:1012– 1018.
Itoh, N., Tanaka, N., Mihashi, S., Yamashina, I. (1987). Molecular cloning and sequence analysis of cDNA for Batroxobin, a thrombin-like snake venom enzyme. J. Biol. Chem. 262:3132–3135.
Karlsson, E. (1979). Chemistry of protein toxins in snake venoms In: Lee, C. Y. ed. Snake Venoms, Handbook of Experimental Pharmacology Springer, Berlin 52:159–212.
Kini, R. M., Evans, H. J. (1990). Effects of snake venom proteins on blood platelets. Toxicon 28:1387–1422.
Lee, C. Y. (1979). Recent advances in chemistry and pharmacology of snake toxins In: Ceccarelli, B., and Clementi, F. eds. Advances in Cytopharmacology, Raven Press, New York 3:1–16.
Lijnen, H. R., Collen, D. (1991). Strategies for the improvement of thrombolytic agents. Thrombos. Haemostas. 66:88–110.
Markland, Jr., F. S. (1998). Fibrinogenolytic and fibrinolytic enzymes In: Bailey, G. S. ed. Enzymes from Snake Venom Alaken, Inc., Fort Collins, Colorado
71–149.
Markland, F. S., Damus, P. S. (1971). Purification and properties of a thrombin-likeenzyme from the venom of Crotalus adamanteus. J. Biol. Chem. 246:6460– 6473.
Maskos, K., Fernandez-Catalan, C., Huber, R., Bourenkov, G. P., Bartunik, H., Ellestad, G. A., Reddy, P., Wolfson, M. F., Rauch, C. T., Castner, B. J., Davis, R., Clarke, H. R., Petersen, M., Fitzner, J. N., Cerretti, D. P., March, C. J., Paxton, R. J., Black, R. A., Bode, W. (1998). Crystal structure of the catalytic domain of human tumor necrosis factor-a-converting enzyme. Proc. Natl. Acad. Sci.
U S A 95:3408–3412.
Meaume, J. (1966). Snake venoms as modifying agents of blood coagulation.
Toxicon. 4:25–58.
Moss, M. L., Jin, S. L. C., Milla, M. E., Burkhart, W., Carter, H. L., Chen, W. J., Clay, W. C., Didsbury, J. R., Hassler, D., Hoffman, C. R., Kost, T. A., Lambert, M. H., Leesnitzer, M. A., McCauley, P., McGeehan, G., Mitchell, J., Moyer, M., Pahel, G., Rocque, W., Overton, L. K., Schoenen, F., Seaton, T., Su, J. L., Warner, J., Willard, D., Becherer, J. D. (1997). Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-a. Nature London 385:733–736.
Neeper, M. P., Jacobson, M. A. (1990). cDNA sequence of the precursor of trigramin. Nucleic Acids Res. 18:4255.
Ouyang, C., Teng, C.-M., Huang, T.-F. (1990). Characterization of snake venom principles affecting blood coagulation and platelet aggregation. Adv. Exp.
Med. Biol. 281:151–163.
Ouyang, C., Teng, C.-M. (1973). The effect of the purified anticoagulant princi-ple of Agkistrodon acutus venom on blood coagulation. Toxicon. 11:287–292.
Ouyang, C. (1957). The effects of Formosan snake venoms on blood coagulation
in vitro. J. Formosan Med. Assoc. 56:435–448.
Ouyang, C., Teng, C.-M. (1972). Purification and properties of the anticoagu-lantprinciple of Agkistrodon acutus venom. Biochim. Biophys. Acta 278:155–162. Ouyang, C., Yang, F.-Y. (1974). Purification and properties of the thrombin-likeenzyme from Trimeresurus gramineus. Biochim. Biophys. Acta 351:354–363. Ouyang, C., Teng, C.-M. (1976). Fibrinogenolytic enzymes of
Trimeresurusmu-crosquamatus venom. Biochim. Biophys. Acta 420:298–308.
Ouyang, C., Teng, C.-M., Chen, Y.-C. (1977). Physicochemical properties of - and -fibrinogenases of Trimeresurus mucrosquamatus venom. Biochim. Biophys. Acta 481:622–630.
Pandya, B. V., Budzynski, A. Z. (1984). Anticoagulant proteases from westerndi-amondback (Crotalus atrox) venom. Biochemistry 23:460–470.
Rooke, J., Pan, D., Xu, T., Rubin, G. M. (1996). KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis.
Science 273:1227–1231.
Stocker, K. F. (1990). Snake venom protein effects on hemostasis and fibrinolysis In: Stocker, K. F. ed. Medical Use of Snake Venom Proteins CRC Press, Boca Raton, Florida 97–161.
St¨ocker, W., Grams, F., Baumann, U., Reinemer, P., Gomis-Ruth, F. X., Mckay, D. B., Bode, W. (1995). The metzincins–topological and sequential relations between theastacins,adamalysins, serralysins, and matrixins (collagenases).
Protein Sci. 4:823–840.
St¨ocker, W., Bode, W. (1995). Structural features of a superfamily of zinc-endopeptidases: the metzincins. Curr. Opin. Struct. Biol. 5:383–390.
Tortorella, M. D., Burn, T. C., Pratta, M. A., Abbaszade, I., Hollis, J. M., Liu, R., Rosenfeld, S. A., Copeland, R. A., Decicco, C. P., Wynn, R., Rockwell, A., Yang, F., Duke, J. L., Solomon, K., George, H., Bruckner, R., Nagase, H., Itoh, Y., Ellis, D. M., Ross, H., Wiswall, B. H., Murphy, K., Hillman, M. C., Hollis, G. F., Newton, R. C., Magolda, R. L., Trzaskos, J. M., Arner, E. C. (1999). Purification and cloning of aggrecanase-1: amember of the ADAMTS family of proteins. Science 284:1664–1666.
Tu, A. T. (1982). Hemorrhagic factors from rattlesnakes In: Tu, A. T. ed.
Rattlesnake Venoms: Their Actions and Treatment Marcel Dekker, New York
247–312.
Tu, A. T. (1996). Overview of snake venom chemistry. Adv. Exp. Med. Biol. 391:37– 62.
Wolfsberg, T. G., White, J. M. (1996). ADAMs in fertilization and development.
Develop. Biol. 180:389–401.
Xue, C. B., Voss, M. E., Nelson, D. J., Duan, J. J., Cherney, R. J., Jacobson, I. C., He, X., Roderick, J., Chen, L., Corbett, R. L., Wang, L., Meyer, D. T., Kennedy, K., DeGradodagger, W. F., Hardman, K. D., Teleha, C. A., Jaffee, B. D., Liu, R. Q., Copeland, R. A., Covington, M. B., Christ, D. D., Trzaskos, J. M., Newton, R. C., Magolda, R. L., Wexler, R. R., Decicco, C. P. (2001). Design, synthesis, and structure-activity relationships of macrocyclic hydroxamic acids that inhibit tumor necrosis factor a release in vitro and in vivo. J. Med. Chem. 44:2636–2660.