Materials Science and Engineering B 136 (2007) 29–32
Effect of growth conditions on the Al composition
and quality of AlGaN film
G.S. Huang
∗, H.H. Yao, H.C. Kuo, S.C. Wang
Department of Photonics, Institute of Electro-Optical Engineering, National Chiao Tung University, 1001 TA Hsueh Road, Hsinchu, 300 Taiwan, ROC
Received 10 November 2004; received in revised form 26 January 2005; accepted 19 August 2006
Abstract
Effects of growth conditions on Al composition and quality of AlxGa1−xN epilayer grown by low-pressure matelorganic vapor phase epitaxy
(MOVPE) have been investigated. The dependences of Al composition, growth rate and quality on the NH3flow rate, TMAl flow rate and growth
temperature were studied. The Al composition and quality of AlxGa1−xN film depend not only on the TMAl flow rate, but also on the NH3flow rate
and on the growth temperature. The Al composition of AlxGa1−xN film becomes saturated when the gas-phase composition TMAl/(TMAl + TMGa)
increases to 0.4, while quality of AlxGa1−xN film becomes much worse. The Al composition of AlxGa1−xN film increases with increase in the
NH3 flow rate. The quality of AlxGa1−xN epilayer is improved when the growth temperature increases. Possible Al incorporation mechanism is
discussed.
© 2006 Elsevier B.V. All rights reserved. Keywords: MOVPE; AlGaN; Al composition
1. Introduction
The group-III-nitride semiconductors consisting of AlN, GaN, InN and their alloys are recognized as very promising materials for many optoelectronic device applications such as blue-green and UV light-emitting diodes (LEDs), laser diodes (LDs), UV solar blind detectors, and high-temperature/high-power electronic devices[1–3]. AlGaN with the Al composition in the range of 0.1–0.3 is one of the key materials for the opto-electronic devices operating in the UV spectral range. At the same time there are well-known difficulties in growing such layers by MOVPE, associated with low efficiency of aluminum incorporation in AlGaN, caused by parasitic gas-phase reaction. However, the parasitic reaction can be reduced by separating NH3and MO sources [4]. In addition, the growth conditions
affect the Al incorporation and quality of AlxGa1−xN epilayer.
For these devices, one important thing is to obtain high-quality AlxGa1−xN epilayer at an optimal growth conditions. However,
the optimal growth conditions of AlxGa1−xN have not been
investigated in detail. The effects of growth conditions on Al
∗Corresponding author. Tel.: +886 3571 2121x56327; fax: +886 3571 6631.
E-mail address:gshuang@mail.nctu.edu.tw(G.S. Huang).
composition, growth rate and crystal quality are needed to obtain high-quality AlxGa1−xN thin films.
In this paper, the growth conditions for AlxGa1−xN have been
studied systematically by changing the growth parameters such as NH3flow rate, TMAl flow rate and growth temperature.
2. Experimental procedure
All the samples in this work were grown in a low-pressure vertical EMCORE D75 reactor with a gas foil rotation of the susceptor. The reagents were pure ammonia, trimethylgallium (TMGa) and trimethylaluminum (TMAl). Hydrogen and nitro-gen purified by purifier were used as the carrier gas. The reactor pressure and rotating speed were 100 Torr and 900 rpm, respec-tively. C-plane sapphire epi-ready substrates were used for all the growth.
The group III and V precursors were separated in the mani-fold and mixed before they entered the reactor. Prior to material growth, the sapphire substrate was annealed to remove any resid-ual impurities on the surface in H2ambient at 1100◦C for 5 min.
For all samples, a nominal 30 nm thick GaN nucleation layer was deposited at 500◦C. Then before growing a 0.4m thick AlxGa1−xN film, a 2m thick GaN bulk layer was grown on the
substrate. All the growth conditions of GaN bulk layer are same.
0921-5107/$ – see front matter © 2006 Elsevier B.V. All rights reserved. doi:10.1016/j.mseb.2006.08.064
30 G.S. Huang et al. / Materials Science and Engineering B 136 (2007) 29–32
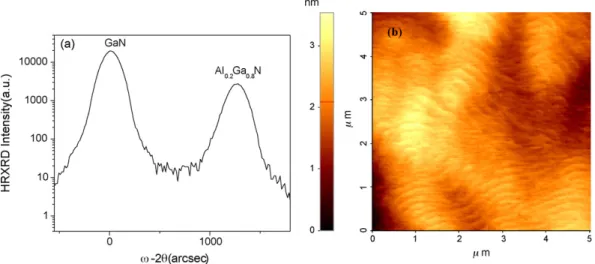
Fig. 1. (a) High-resolution X-ray diffraction rocking curve and (b) AFM image of the surface of Al0.2Ga0.8N film with GaN buffer grown on sapphire.
The first group of samples was grown by varying the NH3flow
rate from 0.3 to 1.2 slm with other parameters kept constant. The second group of samples was grown by varying the TMAl flow rate from 3 to 10 sccm with other parameters kept constant. The growth temperature was at 1110◦C for the first and second group of samples. The third group of samples was grown at dif-ferent growth temperatures with other parameters kept constant. The growth process was in situ monitored by using filmetrics F-series thin-film measurement system. The composition and crystal quality of AlxGa1−xN films on GaN were measured by
using a Bede Scientific D1 double-rystal X-ray diffraction. The surface morphology was observed by atomic force microscope (AFM).
3. Results and discussion
Fig. 1(a) shows the high-resolution X-ray diffraction rock-ing curve (0 0 0 4) of AlGaN epilayer grown on the GaN buffer/sapphire substrate. The ratio of TMAl/(TMGa + TMAl) is 0.21. The Al composition is calculated to be 0.2. The NH3flow
rate is 0.6 slm. The full width of high maximum (FWHM) of AlGaN and GaN are 164 and 157 arcsec, respectively.Fig. 1(b) shows an AFM image of the same Al0.2Ga0.8N film. We clearly
observe steps and terraces. The roughness average measured by AFM is less than 1 nm over a 5m × 5 m surface area, which is comparable to that of high-quality GaN layers.
3.1. Dependence of Al incorporation and quality on the NH3flow rate
Fig. 2 shows dependence of Al composition in the solid on the NH3 flow rate with all other parameters kept
con-stants. Al composition in the solid increases with the NH3
flow rate. The reactor pressure was 100 Torr with a total H2
and N2flow of 4.3 slm. The TMGa and TMAl flow rates are
fixed at 16.40 and 4.34mol/min, respectively. The ratio of TMAl/(TMGa + TMAl) is 0.21. The parasitic reaction between NH3 and fixed TMAl is very weak because the lower
met-alorganic source flow rate of group III and lower pressure[4].
However, the Al incorporation in the solid increases with the NH3flow rate at our case. Even at lower NH3flow rate, the Al
composition is solid kept at 0.2. In this experiment, the LT-buffer layer deposited under a high V/III (∼14,000) had N-face polarity
[5]. For the high V/III growth conditions, there are a large num-ber of nitrogen species at surface to pin the aluminum species in place thereby increasing the Al incorporation of AlxGa1−xN.
It is different from the Al incorporation of AlxGa1−xN grown
on the GaN buffer/sapphire substrate, which shows decreasing Al composition in the solid because of the parasitic reaction[6]. Consider the case of a low V/III conditions, there are fewer nitro-gen species at the surface to pin the Al species and Ga species. The Al incorporation is nearly same as the Ga incorporation, so the Al composition in solid remains at 0.2 which is nearly same as the ratio of TMAl/III.Fig. 2 also shows the growth rates of AlxGa1−xN epilayers grown with increasing the NH3
flow rate. Because the TMGa and TMAl concentrations in gas phase decrease when the NH3 flow rate increases, the growth
rates of AlxGa1−xN epilayers decrease.
Structure defects such as strain, bending and dislocations can all contribute to the measured width of a rocking curve[7]. In
Fig. 2. Dependences of Al composition and growth rate on NH3flow rate with
G.S. Huang et al. / Materials Science and Engineering B 136 (2007) 29–32 31
Fig. 3. The difference of dislocation density of AlGaN and underlying GaN on Al composition by changing the NH3flow rate with other parameters kept
constant.
a first approximation the different contribution on the FWHM is assumed to be Gaussian shapes, so that the measured FWHM can be described as[8]:
W2
AlGaN= w2s + w2b+ w2d (1)
wherews, wb and wd are contributions from strain, bending
and dislocation, respectively. The residual strain is negligible, a result to be expected for layers exceeding the critical thickness, and no evidence of characteristic bending patterns was found
[7,9]. Therefore, we assume that the dominant contribution to the width of the rocking curves observed here is due to lattice distortion caused by dislocation. The density of dislocations can then be calculated from the FWHM by using the formula[10]
ρAlGaN= WAlGaN2 /4.35b2, where WAlGaNis the FWHM and b is
burgers vector which, for the AlxGa1−xN alloy, is expected to be
a√3/2 where a is the in-plane lattice parameter[11]. The dislo-cation densities of AlxGa1−xN epilayer and underlying GaN can
be calculated from their FWHMs, respectively.Fig. 3shows the dependence of difference of dislocation density of AlxGa1−xN
epilayer and underlying GaN on Al composition by varying the NH3flow rate. As Al composition in AlxGa1−xN increases
from 0.2 to 0.22, the dislocation density increases lightly and quality of AlxGa1−xN epilayer becomes worse. However, the
dislocation density increases as the Al composition is same with increase in NH3flow rate from 0.3 to 0.6 slm, which may result
from the desorption of III species. It agrees with that the growth rate also decreases with increasing NH3flow rate shown inFig. 2.
3.2. Dependence of Al incorporation and quality on the TMAl flow rate
Because the Al solid composition of the film is nearly same as the ratio of TMAl to group III sources at NH3flow rate of 0.6 slm,
we chose these growth conditions and changed the TMAl flow rate to check the dependence of Al incorporation on the TMAl flow rate.Fig. 4shows that the Al solid compositions increase with increase in TMAl flow rate. The Al solid composition of
Fig. 4. The Al composition in the solid and growth rate vs. the TMAl source flow composition in a group III gas mixture.
15.5% is more than the Al gas composition of 13.6%. This indi-cates that the Al incorporation is larger than Ga incorporation at lower TMAl flow rate. Then the Al solid composition of 20% is nearly same as the Al gas composition of 21%. Finally, the Al solid composition becomes saturated when the Al gas com-position is 40%. This implies that the nitrogen species as the absorption centers of Al atoms are limited when the NH3and
TMGa flow rates are fixed. If the high Al solid composition is achieved, the NH3flow rate must be increased.Fig. 4also shows
the dependence of growth rate on TMAl flow rate. Al species would depress Ga diffusion, so the grow rate of AlxGa1−xN
epi-layer decreases.
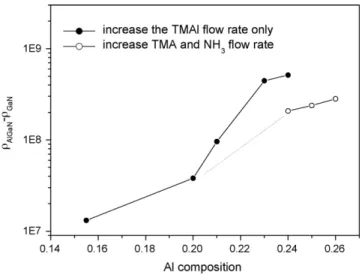
Fig. 5 shows the dependence of the dislocation density of AlxGa1−xN epilayer on the TMAl flow rate. When the Al
solid composition increases from 0.155 to 0.21, the quality of AlxGa1−xN becomes worse lightly. When the Al solid
composi-tion increases with increasing TMAl flow rate further, the quality becomes worse rapidly. The excess TMAl flow will increase the dislocation density of AlxGa1−xN epilayer. The quality of
AlxGa1−xN becomes worse lightly with increase in both TMAl
Fig. 5. The differences of dislocation density of AlGaN and underlying GaN on Al solid composition with increasing TMAl flow rate only or both TMAl and NH3flow rate, respectively.
32 G.S. Huang et al. / Materials Science and Engineering B 136 (2007) 29–32
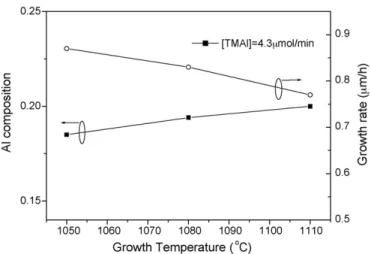
Fig. 6. Dependences of Al composition and growth rate of AlxGa1−xN epilayer
on the growth temperature with other conditions kept constant.
and NH3 flow rate as shown inFig. 5. The excess Al species
would depress diffusion of the Ga species but the Ga species is dominant III species. Dislocations increase only with the increase of TMAl flow rate. However, the III species were diluted by increasing both TMAl and NH3flow rate. The depression of
diffusion of the Ga species decreases, so the dislocations also decrease. AFM image also shows that the surface of the sample is improved by increasing both TMAl and NH3flow rate.
3.3. The influence of growth temperature at same source flow rates
Fig. 6 shows dependence of the Al solid composition on the growth temperature at same source flow rate. The Al solid composition increases with increasing the growth temperature, which shows that the thermal convection of TMAl is higher than that of TMGa during the growth of AlxGa1−xN epilayers
at certain growth conditions[12]. Meanwhile,Fig. 6shows that growth rate decreases with increasing growth temperature. The pyrolysis efficiency of NH3is low when the growth temperature
Fig. 7. The difference of dislocation density between AlxGa1−xN epilayer and GaN on Al solid composition with increasing temperature.
is low. Thus the growth rate increases with decreasing effective V/III ratio discussed in Section3.1.Fig. 7shows the difference of dislocation density between AlxGa1−xN epilayers and GaN
on Al solid composition with increasing growth temperature. The quality of AlxGa1−xN increases with increasing growth
temperature.
4. Conclusions
Al composition and quality of AlxGa1−xN epilayers grown
by low-pressure MOVPE were studied. The dependences of Al composition, growth rate and quality of AlxGa1−xN epilayers
on the NH3flow rate, TMAl flow rate and growth temperature
were investigated in detail. The Al solid composition increases with the NH3flow rate meanwhile the growth rate and quality
of AlxGa1−xN epilayers decreases. The Al composition in the
solid increases with the TMAl flow rate and then becomes sat-urated when the TMAl flow rate reached at 40%. The growth rate decreases with increasing the TMAl flow rate. The qual-ity of AlxGa1−xN becomes worse lightly when the Al solid
composition increases. Then it becomes worse rapidly when the Al composition becomes saturated. The Al composition also increases with increasing the growth temperature but the growth rate of AlxGa1−xN epilayer decreases. The quality of AlxGa1−xN
epilayers increases with increasing the growth temperature.
Acknowledgements
One of authors (G.S. Huang) thanks Mr. Shyh-Horng Shore of Centre for Nano Science of Technology of National Chiao Tung University for XRD measurements. This work was supported in part by the National Science Council of Republic of China (ROC) in Taiwan under contract No. NSC93-2120-M-009-006 and by the Academic Excellence Program of the ROC Ministry of Education under the contract No. NSC93-2752-E-009-008-PAE.
References
[1] F. Omnes, N. Marenco, S. Haffouz, H. Lahreche, P. de Mierry, B. Beaumont, P. Hageman, E. Monroy, F. Calle, E. Munoz, Mater. Sci. Eng. B59 (1999) 401.
[2] J. Han, M.H. Crawford, R.J. Shul, J.J. Figiel, M. Banas, L. Zhang, Y.K. Song, H. Zhou, A.V. Nurmikko, Appl. Phys. Lett. 73 (1998) 1688. [3] T. Someya, Y. Arakawa, Appl. Phys. Lett. 73 (1998) 3653.
[4] I.S. Seo, S.J. Lee, S.H. Jang, J.M. Yeon, J.Y. Leem, Y.J. Park, C.R. Lee, J. Crystal Growth 241 (2002) 297.
[5] M. Sumiya, N. Ogusu, Y. Yotsuda, M. Itoh, S. Fuke, T. Nakamura, S. Mochizuki, T. Sano, S. Kamiyama, H. Amano, I. Akasaki, J. Appl. Phys. 93 (2000) 1311.
[6] S.C. Choi, J.H. Kim, J.Y. Choi, K.J. Lee, K.Y. Lim, G.M. Yang, J. Appl. Phys. 87 (2000) 172.
[7] S.B. Qadri, E.F. Skelton, A.W. Webb, J. Kennedy, Appl. Phys. Lett. 46 (1985) 257.
[8] M.J. Hordon, B.L. Averback, Acta. Metall. 9 (1961) 237. [9] C.L. Kuo, P.E. Vanier, J.C. Bilello, J. Appl. Phys. 55 (1984) 375. [10] P. Gay, P.B. Hirsch, A. Kelly, Acta Mater. 1 (1953) 315.
[11] J.-M. Bethoux, P. Vennegues, F. Natali, E. Feltin, O. Tottereau, G. Nataf, P. De Mierry, F. Semond, J. Appl. Phys. 94 (2003) 6499.