Journal of Organometallic Chemistry, 469 (1994) 169-178 169
Steric effects of bulky diazolylmethane
ligands (N-N)
on syntheses and structures of [Mo(N-N)(CO),]
Kom-Bei Shiu, Sheng-Ting Lin and Chang-Chuan Chou
Department of Chemistry, National Cheng Kung University, Tainan 70101 (Taiwan)Shie-Ming Peng, and Ming-Chu Cheng,
Department of Chemistry, National Taiwan University, Taipei 10764 (Taiwan)
Sue-L&n Wang and Fen-Ling Liao
Department of Chemistry, National Tsing Hua University, Hsinchu, Taiwan, 30043 (Taiwan)
(Received August 11, 1993; in revised form September 1, 1993)
Abstract
The N-N ligands of PhHC(3,5-Me,Pz),, H,C(3,5-Me,;4-BzPz)z, HzC(3-‘BuPzIz and HzC(3(5)-PhPz), were prepared readily from PhHCCl, or CH,Cl, by the same procedure as for H#3,5-Me,Pz), (Pz = pyrazol-1-yl). Subsequent reactions with either [Mo(pip),(CO),] (pip = piperidine) or [Mo(CO),], however, gave only [Mo(PhHC(3,5-Me,Pz)&COIJ (11, MoO-I&W’hPzXS-
PhPz)XCO),] (2) and [Mo(H,C(3,5-Mez;4-BzPz),KCO).,] (3). The structural characteristics of 1-3, [WIH,C(3,4,5-Me,Pz),XCO)J, [W{H,C(3,5-Me,Pz)&CO),] and [Mo(H,C(3,5-Me2Pz)&C0&], reported previously, suggest firstly that the presence of a bulky
substituent such as a phenyl or tert-butyl group at the pyrazolyl skeleton may afford strong nonbonded interaction in [Mo(N- N)(CO),] and inhibit the formation of the stable complexes, [Mo(H,C(3-LBuPz)&CO).J or [Mo(H#3-PhPz)&CO).+] and secondly, that the phenyl substituent at the carbon end of the six-membered boat metallacyle, formed by the N-N ligand and the central metal atom, is cir to the most distorted carbonyl in 1. This explains the facile formation of the $-arene compound, [Mo{PhHC(3,5-MezPz)ZXCO)3] as well as the regiospec$ic formation of [MoIPhHC(3,5-Me,Pz),HC0)2(173-allylXBr)]. 1, mono- clinic, P 2*/n, a = 9.718(2), b = 14.198(4), c = 16.076(4) A, p = 96.62(2), Z = 4, R T 0.034, R, = 0.037, based on 2538 reflections with Z > 30(Z); 2, monoclinic, P 2,/n, a = 18.093(12), b = 13.138(4), c = l&745(6) A, B = 98.79(6), Z = 8, R = 0.040, R, = 0.031, based on 4465 reflections with Z > 2&Z); and 3, orthorhombic, Pbca, a = 12.2387(18), b = 22.525(4), c = 20.028(5) A, Z = 8, R = 0.035, R, = 0.032, based on 2095 reflections with Z > 2u(Z).
Key worak Molybdenum; Diazolylmethane
1. Introduction
Recently Trofimenko et al. have shown that the introduction of various bulky substituent groups on the pyrazole rings can afford unique tris(pyrazolyl)borate ligands [l]. In particular, the ligands with either a phenyl or a tert-butyl (‘Bu) group at the ring-3 position can limit severely the effective space management
Correspondence to: Dr. K-B. Shiu.
around the coordinated metal. Various structures have further shown that the 3-tert-butyl ligand, HB(3- tBuPz),-, can be described as a “tetrahedral enforcer” with a cone angle of 244” while the 3-phenyl ligand, HB(3-PhPz),-, can satisfy five-coordination geometry. These coordination-limited ligands have been found very useful in preparation of various model compounds for metalloproteins about the activation of dioxygen or ester hydrolysis [2] and for studying the novel reactivity of magnesium [3] and zinc [4] alkyl derivatives. Follow- ing our recent discovery of the q2-arene compound,
0022-328X/94/$7.00
SSDZ 0022-328X(93)24143-8
170 K-B. Shiu et al. / Steric effects of diazolylmethane ligands
Fig. 1. Structural plot of [M(N-NMCO),] (M = MO or W; N-N = H,C(3,5-Me,Pz)2 (R3 = R3’= R5 = R5’= Me, R4 = R4’= H, R6 = R7 = H), H,C(3,4,5-Me3Pz)2 (R3 = R3’= R4 = R4’= R5 = R5’= Me, R6 = R7 = H), PhHC(3,5-Me,Pz), (R3 = R3’= R5 = R”= Me, R4 = R4’ = H, R6 = Ph, R7 = H), Mo(H,C(3-PhPzXS-PhPz)XCO),] (R3 = R5 = Ph, R3’ = R5’ = H, R4 = R4’ = H, R6 = R7 = H), or H&(3,5- Me,;4_BzPz), (R3 = R3’= R5 = R5’= Me, R4 = R4’= Bz, R6 = R7 = H)).
[Mo{PhHC(3,5-Me,Pz)&CO),l[51 and that of the flex- ible boat conformation formed from the metal centre and the dipyrazolylmethane (N-N) ligand (Fig. 1) [6], we have become interested in the steric effects of ligands with a similar bulky substituent on the six- membered boat skeleton in synthesis and structures of the related complexes, [Mo(N-NXCO),]. Is the coordi- nation-limited character still present or is it relaxed partially, due to the flexibility of the boat conforma- tion? We also seek to evaluate the potential of the N-N ligands for preparing model compounds, or zinc and magnesium alkyl compounds with unusual reactivity similar to those reported [2-41). During this investiga- tion, we accidentally prepared the first metal carbonyl derivative with asymmetrical N-N ligand, [Mo{H,C(3- PhPzXS-PhPz)KCO),l, though such asymmetrical N-N ligands as [H&(3-MePzXS-MePz)] were reported more than ten years ago [7].
In order to compare the structural details of various boat conformations including [Mo{PhHC(3,5-Me,- Pz)&CO)~I Cl), which were briefly described previ- ously [5], a typical plot is drawn (Fig. 1) and four angles, (Y, p, y and w, are defined as follows; cy, is defined by the MNN and the CNN planes, p by the two azolyl planes, y by the MNN and the NNNN planes and w by the CNN and NNNN planes.
2. Experimental details
All operations were performed by the usual Schlenk techniques, using deoxygenated, dry solvents and gases. IR spectra, calibrated with polystyrene, were recorded on a Hitachi Model 270-30 instrument. Abbreviations are vs, very strong; s, strong; m, medium and sh, shoulder. NMR spectra were obtained on a Bruker
WP-100 (lH, 200 MHz; r3C, 25.2 MHz), AM-200 (‘H, 200 MHz) or AMC400 (‘H, 400 MHz) FT-NMR spec- trometer. Chemical shifts (6 ppm, J Hz) are positive downfield or negative upfield relative to internal SiMe, (TMS) standard with abbreviations: s, singlet; d, dou- blet; br, unresolved multiplet or two overlapped sin- glets. Elemental analysis results were obtained by the staff of the Microanalytical Service of the Department of Chemistry, National Cheng Kung University. Melt- ing points were determined with a Mel-Temp appara- tus (Laboratory Devices) and are not corrected.
The 3,5-dimethyl-4-benzylpyrazole [H(3,5-Me,;4- BzPz)] was prepared from 3-benzylpentane-2,4-dione by following Vogel’s procedure 181. [H(3,5-Me,;4- BzPz)], a white solid with a typical yield of 28%, mp. 136-138°C Anal. Found: C, 77.40; H, 7.62; N, 15.05. Cr2H,,N2 talc.: C, 77.38; H, 7.58; N, 15.04%. ‘H NMR (23°C CDCl,, 200 MHz); 3- and 5-methyl groups, 6 2.15 (3H, s), 2.13 (3H, s); 4-benzyl group, 3.72 (2H, br), 7.12 (5H, m); N-H, unobserved). 3(5)-tert-butyl- pyrazole [H(3(5)-‘BuPz)], 3(5)-phenylpyrazole [H(3(5)- PhPz)] and [Mo(pip),(CO),] (pip = piperidine) were prepared by following the published procedure [lb,9]. ;ffTzjligands, PhHC(3,5-Me,Pz),, (H&(3,5-Me,;4-
*, H zC(3-t BuPz), and H&(3(5)-PhPz), were then prepared from PhHCCl, or CH,Cl, by the same procedure as for H&(3,5-Me,Pz), [71 and obtained as an oily solid. Recrystallization from hexane gave much purer products, as a white solid which was washed with cold hexane and dried in air.
These solid compounds were characterized as fol- lows. PhHCPz’,: white, 42% yield, mp. 73-74°C. Anal. Found: C 72.99; H, 7.18; N, 19.99. Calcd. for C,,H,N,: C, 72.82; H, 7.19; N, 19.99%. rH NMR (23°C acetone- d,, 100 MHz): PhHC, 7.71 (s, 1H); phenyl protons, 7.35 (m, 3H1, 7.00 (m, 2H); protons at the pyrazolyl ring-4 position, 5.88 (s, 2H); protons of the methyl groups at the ring-3 and -5 positions, 2.19 (s, 6H), 2.12 (s, 6H). 13C{1H) NMR (23°C CD,OD, 25 MHz): car- bon nuclei at the ring-3 and -5 positions, S 140.5 (20, 133.4 (2C); ipso-carbon nucleus of the phenyl group, 129.5 (1C); carbon nuclei of the Ph group, 120.4 (2C), 120.3 (10, 119.8 (2C); carbon nuclei at the ring-4 position, 99.6 (2C); PhHC, 65.3 (10; carbon nuclei of the methyl groups at the ring-3 and -5 positions, 4.1 (20, 2.3 (2C), H,C(3(51-‘BuPzl,: yield 40%, mp. lOl- 102 “C. Anal. Found: C, 69.22; H, 9.23; N, 21.62. Calcd. for C,,H,N,: C, 69.19; H, 9.29; N, 21.52%. ‘H NMR (23°C CD,CN, 100 MHz): proton nuclei at 3- or 5- position, 6 7.55 (2H, d, J = 2.4); proton nuclei at 4- position, 6.15 (2H, d, J = 2.4); tert-butyl group, 1.24 (18H, s); CH,, 6.09 (2H, br). 13C{1HJ NMR (23°C CD,CN, 25.2 MHz): carbon nuclei at 5-position, 6 131.1 (2C); carbon nuclei at 4-position, 103.9 (20;
K-B. Shiu et al. / Steric effects of diazolylmethane ligands 171 carbon nuclei at 3-position, 163.4 (20; tert-butyl group,
32.7 (20, 30.8 (60; CH,, 65.7 (10. H&(3(5)-PhPz),: yield 80%, mp. 89-92”C, Anal. Found: C, 75.86; H, 5.36; N, 18.73. Calcd. for C,,H,,N,: C, 75.98; H, 5.36; N, 18.65%. ‘H NMR (23”C, acetone-d,, 200 MHz): 6 CH,, 6.39 (s), 6.48 (s); proton nuclei at 3-, 4-, or 5- position, 6.70 (d, J= 2.451, 6.71 (d, J= 2.44), 7.82 (d, J = 2.451, 7.98 (d, J = 2.44); phenyl groups, 7.35 cm>; 7.56 (ml; 7.85 (ml. 13C{1H) NMR (23”C, acetone-d,, 50 MHz): carbon nuclei at 4-position, 6 104.0, 104.5, 107.6; ipso-carbon nuclei of the phenyl group, 130.9, 134.2; ipso-carbon nuclei of the pyrazolyl group, 145.1, 152.5, 152.9; other carbon nuclei at 3- or 5- position of the pyrazolyl group or those of the phenyl group, 126.2, 126.3, 128.5, 129.3, 129.6, 130.2, 132.5, 132.8, 140.9; CH,, 63.6, 66.1. H&(3,5-Me,;4-BzPz),: yield 85%, mp. 155-157°C. Anal. Found: C, 78.16; H, 7.46; N, 14.63. Calcd. for C,H,N,: C, 78.09; H, 7.34; N, 14.57%. ‘H NMR (23°C acetone-d,, 200 MHz): 3- and 5-methyl groups, 6 2.84 (6H, br), 2.41 (6H, br); benzyl group, 3.68 (2H, br), 7.17 (lOH, m>; CH,, 6.10 (2H, br).
2.1. Preparation of [Mo(PhHCPz;)(CO),] (1)
A solution containing PhHCPz; (1.27 g, 4.55 mmol) and [Mo(CO),] (1.18 g, 4.47 mmol) in 1.2-di- methoxyethane (DME) (30 ml) was refluxed for 1.5 h. The solvent was then removed under vacuum. Recrys- tallization of the solid residue from CH,Cl,/hexane produced yellow-green blocks of 1 in typical yields of 85%. Anal. Found: C, 51.66; H, 4.13; N, 11.52. Calcd. for C,,H,MoN,O,: C, 51.64; H, 4.14; N, 11.47%. ‘H NMR (23”C, acetone-d,, 100 MHz): PhHC, 6 7.77 (s, 1H); phenyl protons, 7.35 (m, 3H), 6.39(m, 2H); pro- tons at the ring-4 position, 6.32(s, 2H); protons of the methyl groups at the ring-3 and -5 positions, 2.63 (s, 6H), 2.54 (s, 6H). ‘H NMR (23°C CD&l,, 100 MHz): phenyl protons, S 7.40 (m, 3H), 6.39 (m, 2H); PhHC, 7.35 (br, 1H); protons at the ring-4 position, 6.18 (br, 2H); protons of the methyl groups at the ring-3 and -5 positions, 2.55 (s, 6H), 2.48 (s, 6H). IR (CH,Cl,): v(C0) 2016m, 1898vs, 1868s, 1820s cm-‘. IR (KBr): v(C0) 2016m, 1894sh, 1890sh, 1882vs, 186Os, 1816s cm-‘.
2.2. Attempted preparation of [Mo{H,C(3-‘BuPz),}- (COLJ
2.2.1. Approach I
A mixture of H&(3-‘BuPz), (0.26 g, 1.00 mm011 and [Mo(pip),(CO),] (0.37 g, 1.00 mm00 was added with 15 ml of CH,CI,. The resulting yellow suspension was stirred for 10 min at room temperature and a solution IR was then measured showing four carbonyl stretch-
ing bands of 2016m, 1936s, 1888s and 1818s cm-‘, typical for [Mo(pip),(CO),l 191. The suspension was heated at 40°C for 30 min, the colour changed first to brown and then to dark brown and another solution IR spectrum was taken which displayed the four bands in weaker intensity.
2.2.2. Approach II
A mixture of H,C(~-‘BUPZ)~ (0.26 g, 1.00 mmol), and [Mo(CO),l (0.26 g, 1.00 mmol) in 25 ml of DME was refluxed for 4 h. A dark brown solution resulted. A solution IR showed no carbonyl stretching bands be- tween 2100-1650 cm-‘.
2.2.3. Approach III
[Mo(MeCN),(CO),] was prepared in situ from 1 mmol of [Mo(CO),l in 20 ml of MeCN [lo] and one equivalent of H&(3-‘BuPz), was then added to the solution. A solution IR spectrum was taken after stir- ring the solution for 10 min at room temperature showing carbonyl bands responsible for [Mo(Me- CN),(CO),]. The solution was then heated under re- flux for 2 h. A dark brown solution resulted and no carbonyl bands between 2100-1650 cm-’ were ob- served in a solution IR spectrum.
2.3. Preparation of [Mo{H,C(3(5)-PhPz),}(CO),]
2.3.1. Method I
H&(3(5)-PhPz), (0.21 g, 0.69 mm00 and [Mo- (pip),(CO),] (0.26 g, 0.68 mm00 were added to 15 ml of CH,Cl,. The resulted yellow suspension was heated at 40°C for 12 h to give a yellow brown solution. A solution IR spectrum showed the presence of a 1936 cm-’ band in strong intensity, indicating the presence of [Mo(pip),(CO),]. Filtration through a pad of silica gel (230-400 mesh) on a medium frit gave a clear yellow solution. The brown residue was further washed five times with 5 ml of CH,Cl,. 10 ml of MeOH was then added to the combined filtrate and the volume of the resulted solution was reduced to about 10 ml, forming a yellow precipitate, which was collected by filtration and washed thoroughly with Et,O. The yellow solid (0.052 g, 15% yield) was characterized as [Mo{H,C(3- PhPzX5-PhPz)KCO),l. Anal. Found: C, 53.98; H, 3.16; N, 10.85. Calcd. for C,,H,,MoN,O,: C, 54.34; H, 3.17; N, 11.02%. ‘H NMR (25”C, acetone-d,, 400 MHz): phenyl protons, S 7.63 (m, 5H), 7.55 (m, 2H), 7.43 (m, 3H); hydrogen atoms at ring-4 position, 6.60 (d, J = 2.0, lH), 6.54 (d, J = 2.8, 1H); hydrogen atoms at ring-3 or -5 position, 7.99 (d, J= 2.0, lH), 8.27 (d, J = 2.8, 1H); CH,, 6.76 (br, 2H). IR(CH,Cl,): v(CO), 202Om, 1902vs, 1872s, 1836s cm-‘.
172 K-B. Shiu et al. / Steric effects of diazolylmethane ligands TABLE 1. Selected bond lengths and angles in l-3 TABLE 1 (continued)
Compound 1 Bond lengths (A)
MO-N(~) 2.302(4) MO-C(I) 1.930(6) MO-C(~) 1.928(6) N(lbN(2) 1.366(6) N(2bC04) 1.367(6) cxll)-Co21 1.480(9) cx13)-c(14) 1.344(8) N(3)-N(4) 1.368(5) N(3)-C 1.455(5) c(21)-C(22) 1.490(8) c(23)-c(24) 1.360(7) c-C(31) 1.509(6) C(31)-C(36) 1.382(6) C(33)-C(34) 1.361(7) C(35)-C(36) 1.378(7) c(2)-O(2) 1.135(8) c(4)-C(4) 1.155(7)
Bond angles (deg)
N(l)-MO-N(~) 80.10) N(4)-MO-C(~) 98.4(2) N(4)-MO-C(~) 90.5(2) N(l)-MO-C(~) 98.6(2) C(l)-MO-C(~) 83.0(2) NW-MO-C(~) 97.6(2) C(l)-MO-C(~) 82.2(2) C(3)-MO-C(~) 83.4(2) MO-N(l)-C(12) 130.7(4) N(l)-N(2)-C(14) 111.8(4) C(14)-N(2)-C 126.8(4) N(l)-CX12)-C(13) 110.3(5) C(12)-C(13)-CX14) 108.1(5) N(2)-CX14bC(15) 123.5(5) N(4)-N(3)-C(24) 112.1(3) C(24)-N(3)-C 126.1(4) MO-N(4)-C(22) 131.3(3) N(4)-c(22)-C(21) 122.0(4) C(21)-C(22)-C(23) 127.1(4) N(3)-Ct24)-C(23) 105.6(5) C(23)-C(24)-a25) 131.0(5) N(2)-C-C(31) 114.5(3) C-C(31bC(32) 121.4(4) CX32)-C(31)-c(36) 118.9(4) C(32)-C(33)-c(34) 120.5(4) C(34)-C(35)-Ct36) 120.3(4) MO-C(l)-O(1) 175.7(5) MO-C(3)-C(3) 176.2(6) compound 2A Bond lengths (2) MO-NW 2.341(5) MO-N(~) 2.291(5) MO-C(~) 1.935(6) MO-C(~) 2.042(7) MO-C(~) 1.932(7) MO-C(~) 2.033(7) N(lbN(2) 1.370(7) N(lbCX5) 1.3348) N(2)-C 1.433(8) N(2HX7) 1.336(8) N(3)+(4) 1.76(6) N(3)-c(8) 1.308(8) N(4)-C 1.451(8) MO-N(~) 2.309(4) MO-C(~) 2.037(6) MO-C(~) 2.018(5) N(l)-C(12) 1.353(6) N(2)-C 1.441(5) C&2-C/(13) 1.377(9) C(14)-C(15) 1.471(9) N(3)-C(24) 1.354(6) N(4)-C(22) 1.347(5) C(22)-C(23) 1.367(8) C(24HX25) 1.493(8) C(31)-C(32) 1.377(6) C(32)-C(33) 1.382(6) C(34wx35) 1.357(7) C(lbO(1) 1.155(8) C(3)-O(3) 1.167(8) N(l)-MO-C(~) 178.42) NW-MO-C(~) 93.8(3) C(l)-MO-C(~) 86.7(2) N(4)-MO-C(~) 177.0(2) C(2)-MO-C(~) 86.8(2) N(4)-MO-C(~) 99.5(2) C(2)-MO-C(~) 166.0(2) MO-N(l)-N(2) 125.0(2) N(2)-N(l)-C(12) 104.0(4) N(lbN(2bC 121.3(3) N(l)-C(12)-C(11) 121.9(5) C(ll)-CX12)-C(13) 127.8(5) N(2)-C(14)-C(13) 105.8(5) C(13)-c(14)-C(15) 130.7(5) N(4)-N(3)-C 121.4(3) MO-N(4)-N(3) 123.6(2) N(3)-N(4)-C(22) 103.8(4) N(4)-C(22)-C(23) 110.9(4) C(22)-c(23)-C(24) 107.6(4) N(3)-C(24)-C(25) 123.4(5) N(2)-C-N(3) 111.1(3) N(3)-C-C(31) 113.6(3) C-C(31bC(36) 119.4(4) C(31)-C(32)-C(33) 119.9(4) C(33)-C(34)-C(35)120.1(5) C(31)-Ct36)-C(35) 120.3(4) MO-C(2)-O(2) 171.5(5) MO-C(4)-0(4) 166.7(5) C(17)-c(18) C(17)-c(22) CX18HX19) c(19)-c(20) C(2Ob-Ct21) c(21AbCc221 c(5)-C(6) C(5)-cc171 C(6)-C(7) C(8)-C(9) C(9bC(lO) c(10bCt11) c(llb-c(12) 1.35700) 1.378(9) 1.386(10) 1.372(H) 1.351(13) 1.400(10) 1.382(9) 1.471(9) 1.357(10) 1.385(10) 1.373(9) 1.475(9) 1.373(10) N(4)-C(10) 1.341(8) CwO(1) 1.165(7) C(2)-O(2) 1.139(8) C(3)-O(3) 1.160(8) C(4)-O(4) 1.129(8)
Bond angles (deg)
N(l)-MO-N(~) 78.86(17) N(l)-MO-C(~) 171.02(23) N(l)-MO-C(~) 92.12(21) N(l)-MO-C(~) 102.16(U) NW-MO-C(~) 96.10(21) N(3)-MO-C(~) 92.88(23) N(3)-MO-a(2) 98.29(23) N(3)-MO-C(~) 1;;;;:;; N(3)-MO-C(~) C(l)-MO-C(~) 85:5(3) C(l)-MO-C(~) 86.3(3) C(l)-MO-C(~) 88.0(3) C(2)-MO-C(~) 84.X3) C(2)-MO-C(~) 165.7(3) C(3)-MO-C(~) 82.0(3) MO-N(lbN(2) 119.2(3) MO-N(l)-C(5) 133.7(4) N(2)-N(l)-C(5) 105.3(5) N(l)-N(2)-C 121.1(5) N(l)-N(2)-C(7) 110.5(5) C-N(2)-C(7) 128.4(5) MO-N(3bN(4) 122X4) MO-N(3)-C(8) 133.7(4) N(4)-N(3)-C(8) 104.1(5) N(3)-N(4)-C 119.0(5) N(3)-N(4)-C(10) 112.0(5) C-N(4)-c(10) 128.6(5) N(2)-C-N(4) 111.0(5) MO-C(l)-O(1) 178.0(5) Compound 3 Bond lengths (A)
MO-N(~) 2.279(5) MO-N(~) 2.286(5) MO-C(~) 1.954(6) MO-C(~) 2.033(6) MO-C(~) 2.022(6) MO-C(~) 1.938(7) 0(0-C(1) 1.148(8) 0(2)-C(2) 1.13fX8) 0(3)-C(3) 1.150(8) 0(4)-C(4) 1.165(8) NWN(2) 1.376(7) NWC(5) 1.345(8) N(2bC(7) 1.356(8) N(2)-C(8) 1.442(8) N(3bN(4) 1.374(7) N(3)-C(8) 1.438(8) N(3bC(9) 1.360(8) N(4)-C(11) 1.342(7) CXllXX16) Ct12)-c(13) c(13bcx14) c(14)-cx15) Ct15)-c(16) C(lO)-CW-C(16) C(12)-C(llbC(16) C(ll)-C(12)-C(13) C(12)-C(13)-C(14) C(13)-C(14)-C(15) Ct14)-c(15)-C(16) C(ll)-c(16)-C(15) C(5&-Ct17)-C(18) C(5)-c(17bC(22) C(18)-C(17)-C(22) C(17)-C(18)-C(19) C(18bC(19)-C(20) C(19k-CX2Ob-CX21) c(2oxt21bC(22) C(17)-c(22bc(21) MO-C(2)-O(2) MO-C(3)-O(3) MO-C(4)-O(4) N(l)-C(5)-C(6) N(l)-c(5)-C(17) CX6)-CX5bc(17) C(5)-C(6)-c(7) N(2)-C(7)-C(6) N(3)-C(8)-C(9) c(8)-C(9)-C(lO) N(4)-CtlO)-C(9) N(4)-C(lO)-C(11) c(9Pzt1obc(11) c(1o)-c(11)-c(12) C(6bCX13) cx7PxO) C(9)-coo) CWcI21) c(lO)-C(11) Coo)-C(22) C(llbC(29) C(13P-x4) C(14)-co51 C(14)-C(19) C(15)-C(16) C(16)-C(17) C(17)-C(18) C(18)-C(19) C(22)-C(23) CWWS24) C(23)-C(28) C(24bCW Ct5)-C(6) 1.392(9) C(25)-CX26) c(5WX12) 1.494(10) C(26)-c(27) c(6)-C(7) 1.355(9) CX27HX28)
Bond angles (deg)
NW-MO-N(~) 80.70(17) CX5)-c(6)-c(7) NW-MO-C(~) 175.10(22) CX5)-CX6)-C(13) N(l)-MO-C(~) 94.42(22) C(7)-c(6bCX13) N(l)-MO-C(~) 94.94(22) N(2)-CU-c(6) 1.371(9) 1.38301) 1.35703) 1.350(12) 1.376(10) 120.5(6) 120.0(6) 118.9(7) 120.0(7) 121.6(7) 118.8(7) 120.6(7) 120.9(6) 119.9(6) 119.2(6) 121.3(6) 119.3(7) 120.3(7) 120.3(7) 119.7(7) 170.2(6) 175.1(6) 171.1(6) 110.2(6) 122.6(5) 127.2(6) 106.3(6) 107.7(6) 112.1(5) 10.5.7(6) 106.0(5) 122.3(5) 131.7(6) 119.5(6) 1.495(9) 1.508(10) 1.362(9) 1.495(10) 1.405(9) 1.505(9) 1.48900) 1.484(10) 1.38!&13) 1.3820 1) 1.398(13) 1.326(18) 1.314(20) 1.419(14) 1.515(9) 1.37100) 1.362(9) 1.37900) 1.388(12) 1.334(11) 1.385(10) 106.3(5) 126.2(6) 127.5(6) 107.1(6)
K-B. Shiu et al. / Steric effects of diazofylmethane ligands 173 TABLE 1 (continued) NW-MO-C(~) 96.36(22) N(2)-ti7)-c(20) N(4)-MO-C(~) N(4)-MO-C(~) N(4)-MO-C(~) N(4)-MO-C(~) C(l)-MO-C(~) C(l)-MO-C(~) C(l)-MO-C(~) C(2)-MO-C(~) C(2)-Mo-CY4) C(3)-Mo-C(4) MO-N(l)-N(2) MO-NW-C(~) N(2)-NWc(5) N(l)-N(2)-c(7) N(l)-N(2)-C(8) C(7)-N(2)-C(8) N(4)-N(3)-C(8) N(4)-N(3)-C(9) c(8)-N(3)-C(9) MO-N(4)-N(3) MO-N(4)-C(11) N(3)-N(4)-C(11) MO-C(l)-O(l) MO-C(2)-O(2) MO-C(3)-O(3) MO-C(4)-0(4) N(l)-CX5)-C(6) N(l)-c(5)-C(12) CX6)-C(5)-CU2) 94.56(22) 93.53(22) 98.34(22) 176.48(21) 87.1(3) 84.5(3) 88.4(3) 165.9(3) 84.7(3) 83.8(3) 121.3(3) 134xX4) 104.1(5) 111.4(5) 118.2(5) 130.0(5) 118.9(5) 111.1(5) 129.3(5) 120.6(3) 134.5(4) 104.9(5) 176.8(5) 173.2(5) 168.9(6) 178.7(6) 111.1(6) 120.8(6) 128.1(6) c6-c~7j-aioj N(2)-C@)-N(3) N(3)-c(9)-c(lO) N(3)-c(9)-c(21) c(10)-c(9)-c(21) c(9)-ctlo)-c(11) cx9)-cx1o)-cx22) c(ll)-c(1o)-cx22) N(4)-C(ll)-C(10) N(4)-c(ll)-a29) c(1o)-c(ll)-cx29) C(6)-C(13)-C(14) cx13)-c(14)-c(15) ~13)-cu4)-c(19) cx15)-cx14)-cx19) C(14)-CX15)-C(16) Ct15)-Ct16)-c(17) C(16)-CX17)-C08) C(17)-C(18)-CX19) C(14)-C(19)-C(18) CtlOI-c(22XX23) CX22)-c(23)-C(24) CX22)-Ct23)-C(28) C(24)-C(23)-C(28) C(23)-C(24)-C(25) CX24)-C(25)-C(26) (X5)-(X26)X(27) C(26)-C(27)-C(B) C(23)-C(28)-C(27) 121.4(6) 131.5(6) 111.8(5) 107.3(6) 121.9(6) 130.8(6) 105.9(5) 129.0(6) 124.6(6) 110.7(5) 120.8(6) 128.5(5) 116.0(6) 119.2(7) 122.8(8) 118.0(8) 122.2(9) 115.4(10) 127.3(9) 117.5(9) 119.5(9) 112.3(5) 119.9(6) 122.0(6) 118.1(6) 120.7(7) 120.1(7) 119.2(7) 120.6(7) 121.3(6) 2.3.2. Method II
H&(3(5)-PhPz), (0.33 g, 1.10 mmol) and [Mo(CO),l (0.26 g, 1.00 mmol) were added to 20 ml of DME. The mixture was heated under reflux to give a yellow brown solution after 20 min and became brown after 2 h. A similar workup as in method I gave the same product in a slightly higher yield (cu. 20%).
2.4. Preparation of [Mo{H,C(3,5-Me,;4-BzPz),} (CO),] A mixture of H&(3,5-Me,;4-BzPz), (0.38 g, 1.00 mm00 and [Mo(CO),] (0.26 g, 1.00 rnmol) in 20 ml of DME was refluxed for 1 h. The solvent was then removed under vacuum. Recrystallization from CH,Cl,/MeOH gave the yellow product (0.41 g, 69%). Anal. Found: C, 58.33; H, 4.73; N, 9.40. Calcd. for C,,H,,MoN,O,: C, 58.79; H, 4.76; N, 9.46%. ‘H NMR (23°C acetone-d,, 200 MHz): methyl groups on the ring-3 and -5 positions, 6 2.39 (s, 6H), 2.52 (s, 6H); benzyl group, 3.80 (s, 2H), 7.21 (m, 10H); CH,, 6.43 (br, lH), 6.56 (br, 1H). IR (CH,Cl,): Y(CO), 2OOOm, 1870sh, 186Os, 1822s cm-‘.
2.5. X-Ray diffraction study of [Mo{PhHC(3,5Me- Pz),)(CO),l (11, [Mo{H,C(3-PhPz)(5-PhPz))(CO),l
(2) and [Mo{H,C(3,5-Me,;4-BzPz),}(CO),l (3)
programs were given previously [6b,lll. Absorption correction was performed on both structures using I) scans. The structure of 1 is shown in Fig. 2. Although two independent molecules (2A and 2BI are observed in the asymmetric unit of the crystal of [Mo{H,C(3- PhPzX5-PhPzjKCO),], both have similar bond parame- ters and ORTEP plots; the two molecules differ in the relative orientation of the boat metallacycle (Fig. 1) with (Y = 95.5(5)“, p = 68.6(3Y, y = 127.1(6)“, w =
Cl6 CIS Crystals of l-3 were grown from CH,Cl,/hexane at
room temperature. General procedures and listings of
Fig. 3. ORTEP drawing of [Mo{H,C(3-PhPzXS-PhPz)KCO),l (2). Thermal ellipsoids are drawn at the 50% probability level.
01
Fig. 2. ORTEP drawing of [Mo{PhHC(3,5-Me2P&KCO)J (1). Ther- mal ellipsoids are drawn at the 50% probability level.
R-B. Shiu et al. / Steric effects of diazolylmethane ligands
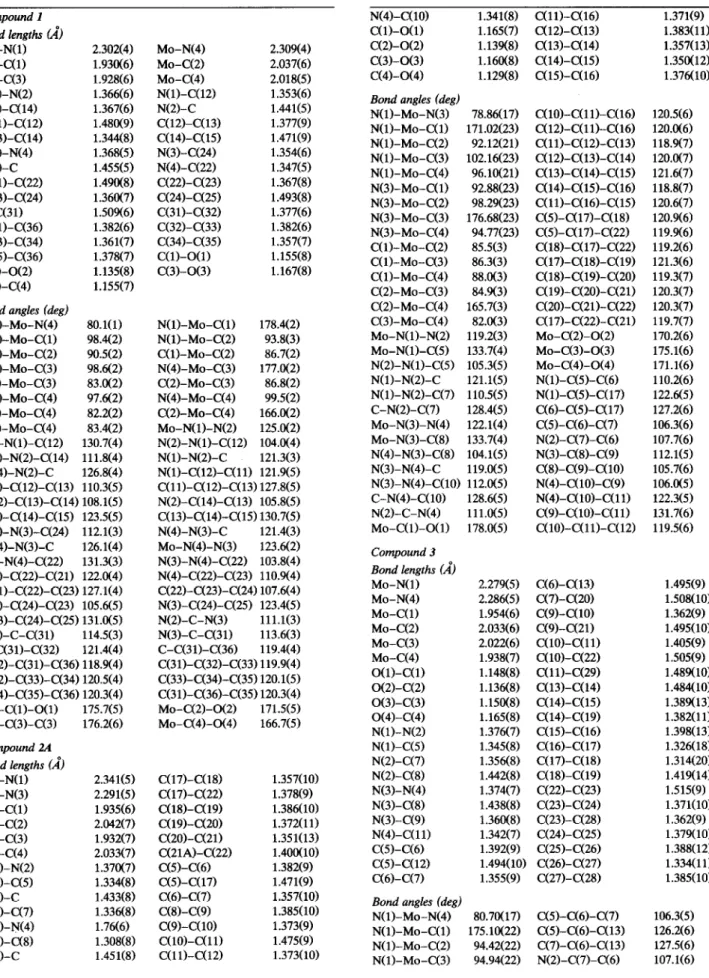
Fig. 4. ORTEP drawing of [Mo(H,C(3,5-Me,;4-BzPz),)(CO),] (3). Thermal ellipsoids are drawn at the 50% probability level. 148.37(25)“ in 2A and (Y = 93.7(6Y, /3 = 748(3Y, y =
127.4(6)“, w = 146.3(3)0 in 2B. Hence, only 2A (Fig. 3) and 3 (Fig. 4) with the relevant numbering schemes are
drawn and the corresponding bond parameters are listed in Table 1. Related crystal data (Table 2) and all the final coordinates of the non-hydrogen atoms (Table
TABLE 2. Crystal data for [MO@-NXCO),l
1 2 3
empirical formula colour
crystal size (mm) space group unit cell dimensions a, b, c, .& a, B, Y, deg volume, A3 Z kc, g/cm3 orientation rflns, range data collected abs car abs coeff, mm- ’ abs. correction applied transm range diffractometer used radiation; A, A temperature (K) scan type 20 range, deg scan speed, deg/min std rfhts
decay; % no. of unique rflns no. of rflns used (NJ no. of parameters (N,) max A/a ratio
R, Ro, S a
weighting factor, bg resid peak/hole, e/K solution
Cz~%&fo%O, G,H,,MoN,Q
yellow green yellow
0.30 x 0.30 x 0.40 0.40 x 0.50 x 0.50
monoclinic, P2,/n (No. 14) monoclinic, P2,/n (No. 14) 9.718(2), 14.198(4), 16.076(4) 90,96.62(2), 90 2203.2(10) 4 1.472 24,15-30 +h, +k, *I ty scan 0.61 applied 18.093(12), 13.138(4), 18.745(6) 90, 98.79(6), 90 4404(4) 8 1.534 24, 18.5” I 28 I 24.3 +h, +k, +I ty scan 0.62 applied 0.931-0.983 Nicolet R3m/V MO Ka, 0.71073 297 e/29 2-50 3.66-14.65 every 50 rfhts <l 3198 2538 with I > 3u(Z) 272 0.119 0.034, 0.037, 1.66 0.0030 0.32/ - 0.37 Heavy atom methods
0.9400-0.9994 Nonius CAD4 MO Ka, 0.7093 298 o/29 2-50 2-8 every 7200 set 52 7744 4465 with Z > 20(I) 578 0.255 0.040, 0.031, 1.98 0 0.41/ - 0.58 Heavy atom methods
GJ-baMoN404
yellow
0.25 x 0.30 x 0.50
orthorhombic, Pbca (No. 61) 12.2387(18), 22.525(4), 20.028(5) 90,90,90 5521.3(19) 8 1.426 24, 18.8” 5 20 5 24.4” +h, +k, +I ty scan 0.50 applied 0.9917-0.9977 Nonius CAD4 MO Ka, 0.7093 298 e/2.9 2-45 2-8 every 7200 set <2 3591 2095 with I > 2&I) 344 0.115 0.035,0.032, 1.45 0 0.25 ‘/ - 0.25 Heavy atom methods
K-B. Shiu et al. / Steric effects of akzolylmethane ligands 175 TABLE 3. Fractional atomic coordinates and anisotropic thermal
parameters in l-3 x Y 2 Ues a or B,, b Compound I, with Cl,, MO 0.0242(l) N(1) - 0.1035(4) N(2) - 0.2444(4) cw 0.0809(7) cx12) - 0.0652(6) Ci13) - 0.1794(8) c(14) - 0.2921(6) Cm) - 0.4394(7) N(3) - 0.3078(4) N(4) - 0.1836(4) c(21) - 0.1025(6) cx22) - 0.2117(6) Ct23) - 0.3486(6) Ct24) - 0.4096(6) CW - 0.5553(6) ;311 - 0.3256(5) - 0.3087(4) CX32) - 0.3298(5) Ct33) - 0.32346) Ct34) - 0.2983(6) Ct35) - 0.2801(6) c(36) - 0.2857(5) c(l) 0.1266(6) o(1) 0.1929(5) c(2) 0.0948(6) o(2) 0.1490(5) c(3) 0.1986(7) o(3) 0.3074(5) c(4) 0.0008(S) o(4) 0.0132(4) Compound 2, with Bes MdA) 0.15252(3) MdB) 0.64730(3) N(lA) 0.0466(3) N(2A) - 0.0126(3) N(3A) 0.11321(25) N(4A) 0.0388(3) C(A) - 0.0170(3) CilA) 0.2404(3) @IA) 0.29464W c(2A) 0.2225(3) O(2AI 0.2673(3) C(3A) 0.1838(3) o(3A) 0.2046(3) C(4A) 0.0994(3) o(4A) 0.0760(3) Ct5A) 0.0337(3) Ct6~) - 0.0322(4) c(7A) - 0.0596(3) Ct8~) 0.1479(3) C(9A) 0.0987(4) CXlOA) 0.0286(3) CtllA) - 0.0464(3) c(12A) - 0.0702(4) c(13A) -0.1414(5) C(14A) -0.1864(4) C(15A) -0.1629(4) C(16A) - 0.0920(4) C07A) 0.0838(3) C(18A) 0.1043(4) 0.6305(l) 0.7660(l) 0.529X2) 0.8384(2) 0.5313(2) 0.8368(2) 0.4199(4) 0.8979(4) 0.4513(3) 0.8838(2) 0.4084(3) 0.9104(3) 0.4580(3) O&x05(3) 0.4434(4) 0.8899(4) 0.6101(2) 0.7068(2) 0.6340(2) O&305(2) 0.6610(4) 0.5416(3) 0.639X3) 0.5966(3) 0.6215(3) 0.5721(3) 0.6019(3) 0.6422(3) 0.5757(5) 0.6529(4) 0.6062(3) 0.7953(3) 0.7004(3) 0.8388(2) 0.7835(3) 0.7950(3) 0.8683(3) 0.8374(3) 0.8705(3) 0.9224(3) 0.7889(4) 0.9664(3) 0.7036(3) 0.9252(3) 0.7168(4) 0.7037(3) 0.7698(3) 0.6710(3) 0.5263(4) 0.6949(4) 0.4743(4) 0.6560(3) 0.6206(4) 0.8360(4) 0.6178(3) 0.8751(3) 0.7469(4) 0.8351(3) 0.8172(3) 0.8713(3) 0.051(l) 0.0490) 0.048(l) 0.105(3) 0.064(2) 0.075(2) 0.062(2) 0.107(3) 0.044(1) 0.045w 0.084(2) 0.053(2) 0.061(2) 0.058(2) 0.091(3) 0.046(2) 0.041(l) 0.055(2) 0.068(2) 0.071(2) 0.071(2) 0.056(2) 0.072(2) 0.109(2) 0.081(2) 0.133(2) 0.083(3) 0.125(3) 0.065(2) 0.1042) 0.71075(4) 0.19302(3) 3.26(3) 0.51823(5) 0.19201(3) 3.29(3) 0.6957(4) 0.2501(3) 2.93) 0.6383(4) 0.2182(3) 3.0(3) 0.5497(4) 0.1593(3) 3.0(3) 0.5262(4) 0.1393(3) 2.9(3) 0.6037(5) 0.1452(3) 3.0(3) 0.7001(5) 0.1462(3) 3.3(3) 0.6941(4) 0.12000@4) 4.6(3) 0.67435) 0.2856(3) 3.6(4) 0.6657(4) 0.33498(25) 5.4(3) 0.8491(5) 0.9323(4) 0.7782(5) 0.8259(4) 0.7127(5) 0.6663(6) 0.6195(6) 0.4634(5) 0.384X5) 0.4273(5) 0.3831(5) 0.3048(6) 0.2662(7) 0.3054(7) 0.3807(6) 0.4192(6) 0.7746(5) 0.8697(5) 0.2158(3) 0.2249(3) 0.1018(3) 0.0535(3) 0.3172(3) 0.3282(4) 0.2651(4) 0.1532(4) 0.1307(4) 0.1229(3) 0.1006(3) 0.1400(4) 0.1197(5) 0.0616(5) 0.0214(4) 0.0408(3) 0.3692(3) 0.3518(3) 3.7(4) 5.3(3) 3.6(3) 6.1(3) 3.2(3) 4.5(4) 4.0(4) 3.9(4) 4.1(4) 3.2(3) 3.1(3) 5.1(4) 7.3(5) 6.8(5) 5.6(4) 3.9(4) 3.4(3) 3.9(3) TABLE 3 (continued) x Y z UcqaorBeqb c(19A) 0.1516(4) 0.9285(6) 0.4004(4) C.fZOA) 0.1791(4) C(21A) 0.1583(4) cx22A) 0.1105(4) N(lB) 0.5373(3) N(2B) 0.4800(3) N(3B) 0.61299Q5) N(4B) 0.5404(3) C(B) 0.4828(3) C(lB) 0.7400(3) 0(1B) 0.79652(23) C(2B) 0.7126(3) Ot2B) 0.7567(3) C(3B) 0.6751(3) 0(3B) 0.69348(24) Ct4B) 0.6016(4) 0(4B) 0.5826(3) CX5B) 0.5209(3) C(~B) 0.4540(3) Ct7B) 0.4308(3) Ct8B) 0.6500(3) CX9B) 0.6047(4) C(lOB) 0.5347(3) CtllB) 0.4620(3) C(12B) 0.4245(4) Ct13B) 0.3573(5) Cf14B) 0.3302(5) Cf15B) 0.3633(5) C(l6~) 0.4314(5) Cf17B) 0.5680(3) C(l8~) 0.5957(4) C(19B) 0.6401(4) c(2OB) 0.6551(4) Ct21B) 0.6268(4) C(22B) 0.5832(4) 0.8891(7) 0.7954(7) 0.7362(6) 0.5312(4) 0.5914(4) 0.6824(4) 0.7106(4) 0.6342(5) 0.5375(5) 0.5529(4) 0.5431(5) 0.5466(4) 0.3763(5) 0.2922(4) 0.4621(5) 0.4193(4) 0.5139(5) 0.5635(5) 0.6107(5) 0.7690(5) 0.8508(5) 0.8124(5) O&510(5) 0.9122(7) 0.9567(7) 0.9515(7) 0.8953(9) 0.8497(8) 0.4535(5) 0.3593(5) 0.3047(5) 0.3455(6) 0.4370(6) 0.4911(6) O&69(4) O&58(4) 0.4366(4) 0.2425(3) 0.2094(3) 0.1682(3) 0.1429(3) 0.1395(3) 0.1542(3) 0.1342(3) 0.288X3) 0.33960(25) 0.2074(3) 0.2124(3) 0.0939(3) 0.0421(3) 0.3089(3) 0.3173(3) 0.2540(4) 0.1728(4) 0.1507(4) 0.1328(3) 0.1050(4) 0.1504(5) 0X50(6) 0.0551(6) 0.0080(5) 0.0351(4) 0.3637(3) 0.3485(3) 0.4027(4) 0.4711(4) 0.4868(4) 0.4331(3) 5.2(4) 6.1(5) 6.0(5) 4.5(4) 2.X3) 3.1(3) 2.8(3) 3.02(25) 3.0(3) 3.2(3) 4.8(3) 3.5(3) 5.7(3) 3.6(3) 5.1(3) 3.7(4) 6xX3) 3.0(3) 3.7(4) 3.%4) 3.9(3) 4.6x4) 3.33) 3.7(4) 6.6(5) 9.6(7) 9.2(7) 10.6(7) 8.6(6) 3.1(3) 3.5(3) 4.5(4) 5.0(4) 5.0(4) 4.1(4) Compound 3, with Bcq MO 0.85751(4) O(1) 0.7207(4) O(2) 0.6751(4) O(3) 0.9643(4) o(4) 0.7039(4) N(l) 0.9703(4) N(2) 1.0796(4) N(3) 1.0795(4) N(4) 0.9710(4) c(l) 0.7701(6) c(2) 0.7454(5) c(3) 0.9363(5) c(4) 0.7624(6) c(5) 0.9566(5) c(6) 1.0554(5) c(7) 1.1316(5) c(8) 1.1234(5) c(9) 1.1327(5) c(10) 1.0574(6) c(11) 0.9582(5) c(12) 0.8464(6) c(13) 1.0722(6) Ct14) 1.0421(6) c(15) 1.063X7) c(l6) 1.0370(9) cx17) 0.9906(9) - - 0.099178&I) 0.18175(3) 2.951(24) 0.00211(20) 0.25291(23) 5.4(3) 0.09792(25) 0.06986(23) 6.1(3) 0.10521(23) 0.1923009) 0.16503(21) 0.15306(21) 0.04718(22) 0.03382(21) 0.0375(3) 0.0983(3) 0.1030(3) 0.1579(3) 0.2187(3) 0.2410(3) 0.1989(3) 0.0997(3) 0.0020(3) 0.0409(3) .0.0202(3) 0.2471(3) 0.2997(3) 0.3528(3) 0.3534(3) 0.4018(5) 0.4472(4) 0.3251703) 0.24664(U) 0.12962(24) 0.12077(25) 0.11952(24) 0.12877(25) 0.2248(3) 0.1069(3) 0.2704(3) 0.2220(3) 0.1014(3) 0.0777(3) 0.0904(3) 0.1502(3) 0.0884(3) 0.0750(3) 0.1015(3) 0.0988(4) 0.0445(4) 0.0843(4) 0.1524(5) 0.1929(5) 0.1618(7) 6.5(3) 4.91(24) 3.03(23) 3.2(3) 3xX25) 3.2(3) 3.6(3) 3.9(3) 4.0(3) 3.5(3) 3.6(3) 3.6(3) 3.5(3) 3.3(3) 3.5(3) 3.3(3) 3.3(3) 4.9(4) 5.1(4) 5.7(4) 7.6t5) 11.8(7) 13.5(8)
116 K-B. Shiu et al. / Steric effects of diazolylmethane Iigands TABLE 3 (continued) x Y 2 U,, a or B,, b C(18) 0.9686(7) 0.4518(4) 0.0977(7) 12.3(9) C(19) cc201 c(21) C(22) c(23) c(24) C(25) c(26) C(27) C(28) C(29) 0.9935(6) 1.2527(6) 1.2530(6) 1.0744(6) 1.0665(S) 1.1338(6) 1.1251(8) 1.0469(7) 0.9816(6) 0.9906(6) 0.8490(6) 0.4024(4) 0.197Ot3) 0.0040(3) -0.1019(3) - 0.1501(3) - 0.1484(3) - 0.1906(3) - 0.2350(3) - 0.2366(3) - 0.1942(3) - 0.0493(3) 0.0565(5) 0.0768(3) 0.0754(4) 0.0463(3) 0.0990(3) 0.1537(4) 0.2033(4) 0.1985(4) 0.1453(4) 0.0955(3) 0.1002(3) 8.0(6) 4.9(4) 4.9(4) 4.4(3) 3.4(3) 5 S(4) 7.0(5) 6.2(5) 5.8(4) 4.7(4) 4.5(4) a U,, (A’) is defined as one-third of the trace of the orthogonalized vi tensor. B,, (k) is the mean of the principal axes of the thermal ellipsoid.
3) are also reported. The remaining bond parameters for 2B, the anisotropic displacement coefficients of all the non-hydrogen atoms, the H-atom coordinates and structural factors of l-3 are available from the authors.
3. Results and discussion
3.1. Synthesis of the N-N ligands and related complexes Excess pyrazole with a methyl group on either ring-3 or -5 position is known to react with CH,Cl, under phase transfer conditions to give, as would be ex- pected, three different products, [H&(3-MePz),], [H&(3-MePzXS-MePz)] and [H&(5-MePz),], in a ra- tio of 27/50/23 [7], but it reacts with KBH, in a melt reaction to give only one product, KHB(3-MePz), [12]. Comparison of ‘H and 13C NMR data of [H&(3(5)- MePz),] [7] and of the white product, that we obtained from the reaction of CH,Cl, with [H(3(5)-‘BuPz)], supported by comparison of the results of DEPT and selective proton-proton decoupling experiments [13] of this product, make us confident that we have obtained a single product, [H&(3-‘BuPz),], in a yield of 40%. However we cannot exclude the formation of either [H,C(3-tBuPzX5-tBuPz)] or [H&(5-‘BuPz),] during our procedure. Apparently a bulky group such as a tert-butyl moiety at the ring-3 position favours facile solidification in cold hexane. Similarly, although we believe that the product of our reaction of CH,Cl, with [H(3(5)-‘BuPz)] is a mixture of [H,C(3-PhPz),l and [Mo{H,C(3-PhPzXS-PhPz))(CO)~] in a ratio of 67/33, we admit the possibility that [H&(5-PhPz),l also forms during our reaction procedure.
The piperidine (pip) molecules in [Mo(pip),(CO),l are already known to be readily replaced by a biden- tate ligand under very mild conditions [9,141. Thus, the ligands of H&(3-‘BuPz), and H&(3(5)-PhPz), we
prepared are allowed to react with [Mo(pip),(CO),] in order to obtain [Mo(N-NXCO),]. In either case, how- ever, the yellow suspension, formed by adding [Mo(pip),(CO),] to CH,Cl,, became brown or dark brown soon after the suspension was added with the ligands at room temperature and then heated at 40°C. Although one product, [Mo{H,C(3-PhPzXS-PhPz))- (CO),] (2), is isolated from the reaction mixture when heated in CH,Cl, or DME, only decomposed prod- ucts, shown to have no carbonyl stretching bands in the range 2100-1650 cm-i in the solution IR spectra, are obtained from a similar reaction of [Mo(CO),] or [Mo(MeCN),(CO),] with H&(3-‘BuPz), heated in DME or MeCN. It appears obvious that the antici- pated product, [Mo{H,C(3-‘BuPz),)(CO),L] (L = CO or MeCN), if it is formed at all, decomposed immedi- ately in solution.
It is also probably true that the only isolable car- bonyl-containing product from the reaction of [Mo(CO),] or [Mo(pip),(CO),] with H&(3(5)-PhPz), is not [Mo{H,C(3-PhPz),)(CO),] but [Mo{H,C(3- PhPzXS-PhPz))(CO)J, in 15 or 20% yield, based on the starting metal carbonyl complex. This yield is in a sharp contrast to 85% yield for the formation of [Mo{PhHC(3,5-Me,Pz),)(CO),] (1) or [MoIH,C(3,5- Me,;4-BzPz),)(CO),] (3) from the mixture of [Mo(CO),] and the corresponding N-N ligand, refluxed in DME. The difference in yields for the formation of [Mo{H,C(3-PhPzXS-PhPz))(CO),l is compatible with the mixed composition of more than one isomer of the ligand, H&(3(5)-PhPz),. If the mixed ratio of 67/33 is considered, the conversion percentage to [Mo{H,C(3- PhPzXS-PhPz))(CO),] can be as high as 60%. Appar- ently the presence of a rather bulky substituent such as a phenyl or a tert-butyl group on the ring-3 position of both the pyrazolyl skeletons of N-N inhibits the forma- tion of the stable complexes, [Mo(N-NXCO),] (N-N = H ,C(3-‘ BuPz), or H&(3-PhPz),).
3.2. Comparison of the solid-state structures of [M(N- N) (CO),] (M = MO or W)
From Figs. 2-4 and Table 1, one can observe clearly that the structures of [Mo{PhHC(3,5-Me,Pz),)(CO),l (l), [Mo(H ,C(3-PhPzX5-PhPz))(CO),] (2) and [Mo(H,C(3,5-Me,;4-BzPz),)(CO),] (3) are very simi- lar to those of [W{H,C(3,4,5-Me,Pz),)(COI,l (4),
[W{H,C(3,5-Me,Pz),)(CO),] (5) [6cl and [MO-
{H&(3,5-Me,Pz),)(CO),] (6) [6bl in having a six-mem- bered boat metallacycle, as shown in Fig. 1, and two distorted cis-carbonyls. Thus, presence of a large sub- stituent such as the phenyl group in 1 or 2, or of the benzyl group in 3 does not change the basic boat structure. However, there are some important differ- ences in these structures.
K.-B. Shiu et al. / Steric effects of diazolylmethane ligands 177
Firstly, the twoobond lengths of 2.291(5) A for Mo- N(3) and 2.341(5) Aofor MO-N(~) in 2A (Fig. 3) (2.270(5) A versus 2.337(5) A in 2B) are appreciably different whereas the two correspfnding bonds are all similar in
1 (MO-N(~) = 2.302(4) A and MO-N(~) = 2.309(4) A, CJ? Fig. 2)oor 3 (MO-N(~) = 2.279(5) A and MO-N(~) = 2.286(5) A, cc Fig. 4) or in 4-6 [6b,cl.
Secondly, the angle, formed by two cis-carbonyls and the central metal atom, that was used previously [6] to represent best the conformational modification of the boat, is found rather unexpectedly to be similar in l-3 (166.0(2Y in 1, 165.7(3)0 in ZA, 164.6(3>0 in 2B, and 165.9(3>0 in 3) though smaller than those of 170.80(21)” in 4, 167.7(3>0 in 5, and 167.3(1)0 in 6 [5]. By comparing the MO-C-O angles, one observes that the more distorted carbonyl of the two cis-carbonyls, or the most distorted carbonyl group in each molecule, is C(4)0(4) in 1 (Fig. 21, C(210(2) in 2 (Fig. 31, and C(3)0(3) in 3 (Fig. 4).
Thirdly, the (Y angle of 94.4(5>0 in 3 is close to the average value of those of 95.5(5)0 in 2A and 93.7(6>0 in 2B while the /3 angle of 130.1(3>0 in 3 is much larger than those of 68.6(3)0 in 2A and 74.8(3)0 in 2B. This feature with similar (Y angles and different /3 angles (i.e., cr = 82.9”, p = 120.8” in 4; (Y = 85.0”, p = 68.2” in 5 [6c] and (Y = 86.1”, /3 = 68.1” in 6 [6bl) was reported previously; when the hydrogen atom at the 4-position is replaced by a larger methyl group, the p angle in- creases appreciably from 68” to 121”. The p angle of 130.1(3)” in 3 is the largest found so far in any [M(N- NXCO),] (M = MO or W) structure, including 1-3, apparently reflecting the much higher steric effect of the benzyl group relative to the methyl group.
Fourthly, the angles, y, are 159.7” for 1 (Fig. 21, 148.37(25)0 for 2A, 146.3(3)0 for 2B (Fig. 3) and 150.51(24)0 for 3 (Fig. 4) and the angles, w, are 128.7” for 1, 127.1(6>0 for 2A, 127.4(6)0 for 2B and 123.9(6) for 3, showing that the shallow end of the boat metalla- cycle is still closer to the metal atom in l-3 as in 4-6 despite the presence of the bulky substituents in the N-N ligands used in l-3.
Fifthly, the LY angle of 108.4” and the p angle of 127.6” in 1 are much larger than those in 6 (86.1” and 68.1”) [6b], respectively. Apparently, the phenyl sub- stituent at the carbon end of the boat metallacycle can induce quite large nonbonded interaction if it takes the same geometry as 6. In principle, two possible isomers, r.e., a pair of epimers with the phenyl group either frans (i-tram with R’ = Ph,cf. Fig. 11 or cis (Ccis with R6 = Ph, c$ Fig. 11 to C(4)0(4), can exist. A model reveals the unfavourable steric repulsion between the phenyl and the methyl groups in the structure of 6-trans. We believe this also drives the regiospecific formation of [Mo{PhHC(3,5-Me,Pz),KCO),(q3-allylXBr)l, either
from [Mo{PhHC(3,5-Me,Pz),](CO),] or from
[Mo(PhHC(3,5-Me,Pz),)(CO),] and ally1 bromide [15]. Since the geometry of 6 is 6-ci.r (Fig. 2), with the more distorted &carbonyl group C(4)0(4), it is quite possi- ble that this carbonyl would be lost during thermolysis and the phenyl group located at the right orientation is ready to supply one of the three phenyl a-electron pairs to fill the coordination site, forming the T2-arene compound, [Mo{PhHC(3,5-Me,Pz),]~CO),] [5].)
From the first two considerations outlined above, it is quite obvious that the different MO-N bond lengths in 2 are due to the asymmetric presence of the phenyl groups at the neighbourhood <cf Fig. 3); the calculated nonbonded distances between the phenyl atoms and C(3)0(3) reveals that this carbonyl group is almost equidistant, with a range of 3.42-3.81 A, to C(17), C(18) and C(22). If the MO-N(~) distance is shortened to the extent of 2.291(5) A as found for MO-N(~), the observed angle, C(2)-MO-C(~), should be much smaller than 165.7(3)“.
Based on the different angles, (Y, p, y and w, found in [M(N-NXCO),], one can also infer from the flexibil- ity of the boat conformation that the (Y angle remains almost constant around 84” for the methyl substituent and around 94” for the phenyl or benzyl substituent at the pyrazolyl skeleton while the /3 angle increases largely with any non-hydrogen substituent at the 4- position. Interestingly, the shorter nonbonded dis- tances for 1A (Fig. 2) between the phenyl atoms (i.e., C(17)-C(22) and H(18)-H(22) for one phenyl group and C(ll)-C(16) and H(12)-C(16) for the other) and the atoms either in the metal carbonyl fragment (i.e., MO, C(l)-C(4), and 0(1)-O(4)) or in the methylene group (i.e.& C, H(CAl), and H(CA2)) are thvse of 2.700(7) A for C(3) . . . H(18), 2.850(5) A for O(3) . . . H(18), 2.619(6) A for C(11) - * - H(CAl), and 2.610(7) A for Cc161 . . . H(CA1) with similar C-N bond 1Engths (1.433(8), 1.451(8) A in 1A and 1.433(g), l&1(8) A in 1B). The angles between the phenyl and the connected pyrazolyl planes are 59.3(1)0 at the 5-posi- tion and 52.7(3)0 at the 3-position (75.1(3>0 verSuS 46.1(3>0 for lB1, showing that the phenyl plane at the ring-5 position is twisted more than that at the ring-3 position. Local steric congestion around the methylene carbon can apparently be relieved by twisting the phenyl plane along the C(lO)-C(11) bond and the efficient way to remove the local steric congestion around the metal carbonyl fragment is to twist the phenyl plane along the C(5)-C(17) bond and simultaneously to @rigthen the MO-N(~) bond from 2.291(5) to 2.341(5) A (Fig. 2). Thus, it is probably true that both MO-N bonds in the unknown [Mo{H,C(3-PhPz),}(CO),] com- plex should be weakened to be around 2.34 A or even longer due to the larger steric repulsion between the
178 K-B. Shiu et al. / Steric effects of diazolylmethane ligands
two phenyl groups at the 3-position, explaining the observed decomposition products, from the unstable [Mo{H,C(3-PhPz),}(CO),], accompanied by the forma- tion of 1. A simple structural model supports that the steric repulsion between the metal carbonyl fragment and [H&(3-‘BuPz),] is much larger than that found in 1, and also explains that only decomposed products are obtained from the reaction of [Mo(CO),] or [Mo(MeCN),(CO),] with H&(3-‘BuPz),. Quite obvi- ously, the coordination-limiting character is present not only in the tridentate ligands, HB(3-RPz); (R = ‘Bu or Ph), but also in the bidentate ligands, H&(3- RPz),. Although the flexible boat may help to remove some steric repulsion by varying various q /3, y and o angles (Fig. 1) as shown in 1-6, this flexibility is not large enough to remove all the imposed steric con- gestion, allowing the isolation of [Mo{H,C(3- PhPz)&CO),] or [Mo{H,c(3-‘BuPz),)(CO),]. We thus believe that the N-N ligand, H&(3-RPz),, can have potential for use in the preparation of model com- pounds for dioxygen activation or ester hydrolysis and in synthesis of similar ‘zinc and magnesium alkyl com- pounds with unusual reactivity as reported previously [2-41. Research in this field will be reported in due course.
4. Acknowledgment
We wish to thank the National Science Council of the Republic of China for the financial support of this research.
5. References
1 (a) J.C. Calabrese, S. Trofimenko and J.S. Thompson, J. Chem. Sot., Chem. Commun., (1986) 1122; (b) S. Trofimenko, J.C. Cal- abrese and J.S. Thompson, Inorg. Chem., 26 (1987) 1507; (cl S.
Trofimenko, J.C. Calabrese, P.J. Domaille and J.S. Thompson,
2 6 7 8 9 10 11 12 13 14 15
Inorg. Chem., 28 (1989) 1091; (d) J.C. Calabrese, P.J. Domaille, J.S. Thompson and S. Trofimenko, Znorg. Chem., 29 (1990) 4429; (e) J.C. Calabrese, P.J. Domaille S. Trofimenko and G.J. Long, Irwrg. Chem., 30 (1991) 4429.
(a) N. Kitajima, K. Fujisawa, C. Fujimoto and Y. Moro-oka, Chem. Lett. (1989) 421; (b) J.W. Egan, Jr., B.S. Haggerty, A.L. Rheingold, S.C. Sendlinger and K.H. Theopold, J. Am. Chem. Sot., I12 (1990) 2445; (c) V.S. Joshi, M. Nandi, H. Zhang, B.S. Haggerty and A. Sarkar, Inorg. Chem., 32 (1993) 1301; (d) R. Alsfasser, AK. Powell and H. Vahrenkamp, Angew. Chem., Int.
Ed. En& 29 (1990) 898.
R. Han, A. Looney and G. Parkin, J. Am. Chem. Sot., lZl(1989) 7276.
LB. Gorrell, A. Looney and G. Parkin, J. Chem. Sot., Chem. Commun., (1990) 220.
(a) K.-B. Shiu, C.-C. Chou, S.-L. Wang and S.-C. Wei,
Organometallics, 9 (1990) 286, 2632; (b) K.B. Shiu, Proceedings of the 12th Seminar on Science and Technology: Crystallography,
Tokyo, Japan, 1990, p. 195.
(a) K.-B. Shiu and C.-J. Chang, J. Chin. Chem. Sot. (Taipei), 34 (1987) 297; (b) K.-B. Shiu, C.-J. Chang, Y. Wang and M-C. Cheng, J. Chin. Chem. Sot. (Taipei), 36 (1989) 25; (c) K.-B. Shiu, K.-S. Liou, Y. Wang, M.-C. Cheng and G.-H. Lee, J. Organomet.
Chem., 453 (1993) 201.
S. Julia, P. Sala, J. de1 Maze, M. Sancho, C. Ochoa, J. Elguero, J.-P. Fayet and M.-C. Vertut, J. Heterocyclic Chem., 19 (1982) 1141.
B.S. Furniss, A.J. Hannaford, V. Rogers, P.W.G. Smith and A.R. Tatchell (eds.), VogeZZ Textbook of Practical Organic Chemistry,
4th ed., Longman, New York, 1980, p. 881.
D.J. Darensbourg and R.L. Kumps, Znorg. Chem., 17 (1978) 2680.
D.P. Tate, W.R. Knipple and J.M. Augl, Inorg. Chem., 1 (1962) 433.
K.-B. Shiu, F.-M. Shen, S.-L. Wang and S.-C. Wei, .I. Organomet. Chem., 372 (1989) 251.
T.J. Desmond, F.J. Lalor, G. Fergusson and M. Parvez, J.
Organomet. Chem., 277 (1984) 91.
R.M. Silverstein, G.C. Bassler and T.C. Morrill (eds.), Spectro- metric Identification of Organic Compounds, fifth edition, John Wiley&Sons, New York, 1991, pp. 276 and 198.
(a) K.-B. Shiu, S.-L. Wang and F.-L. Liao, .I. Organomet. Chem., 420 (1991) 207; (b) K.-B. Shiu, S.-M. Peng, M.-J. Cheng, S.-L. Wang and F.-L. Liao, J. Organomet. Chem., 461 (1993) 111. K.-B. Shiu, C.-J. Chang, S.-L. Wang and F.-L. Liao, J. Organomet.
![Fig. 1. Structural plot of [M(N-NMCO),] (M = MO or W; N-N = H,C(3,5-Me,Pz)2 (R3 = R3’= R5 = R5’= Me, R4 = R4’= H, R6 = R7 = H), H,C(3,4,5-Me3Pz)2 (R3 = R3’= R4 = R4’= R5 = R5’= Me, R6 = R7 = H), PhHC(3,5-Me,Pz), (](https://thumb-ap.123doks.com/thumbv2/9libinfo/8664795.195351/2.868.64.330.112.289/fig-structural-plot-nmco-mo-pz-phhc-pz.webp)

![Fig. 4. ORTEP drawing of [Mo(H,C(3,5-Me,;4-BzPz),)(CO),] (3). Thermal ellipsoids are drawn at the 50% probability level](https://thumb-ap.123doks.com/thumbv2/9libinfo/8664795.195351/6.864.65.595.103.368/ortep-drawing-bzpz-thermal-ellipsoids-drawn-probability-level.webp)