1998 91: 2658-2663
Shu-Ching Hsu, Chia-Cheng Wu, Tien-Yau Luh, Chen-Kung Chou, Shau-Hwa Han and Ming-Zong Lai
Apoptotic Signal of Fas Is Not Mediated by Ceramide
http://bloodjournal.hematologylibrary.org/cgi/content/full/91/8/2658
Updated information and services can be found at:
http://bloodjournal.hematologylibrary.org/misc/rights.dtl#repub_requests
Information about reproducing this article in parts or in its entirety may be found online at:
http://bloodjournal.hematologylibrary.org/misc/rights.dtl#reprints
Information about ordering reprints may be found online at:
http://bloodjournal.hematologylibrary.org/subscriptions/index.dtl
Information about subscriptions and ASH membership may be found online at:
.
Hematology; all rights reserved
Copyright 2007 by The American Society of
DC 20036.
by the American Society of Hematology, 1900 M St, NW, Suite 200, Washington
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published semimonthly
Apoptotic Signal of Fas Is Not Mediated by Ceramide
By Shu-Ching Hsu, Chia-Cheng Wu, Tien-Yau Luh, Chen-Kung Chou, Shau-Hwa Han, and Ming-Zong Lai
Ceramide has been suggested as the secondary messengermediating the apoptotic signal for Fas engagement. By using different inhibitors, we demonstrated here that ceramide is unlikely a mediator of Fas-initiated apoptosis. First, cAMP prevented cell death induced by ceramide but not by Fas. Second, ceramide-triggered, but not Fas-triggered, apopto-sis was antagonized by the free radical scavenger C60. Third, the metal chelator pyrrolidinedithiocarbamate suppressed ceramide-initiated DNA fragmentation but had no effect on
the Fas-induced cell death. Fourth, the SAPK/ERK kinase dominant negative mutant, which attenuated ceramide-induced cell death, did not prevent Fas-ceramide-induced apoptosis. Finally, activation of NF-kB inhibited ceramide-induced but not Fas-initiated apoptosis. The fact that many antagonists of ceramide-induced apoptosis could not suppress Fas-mediated cell death clearly indicates that ceramide is not the mediator for Fas-initiated apoptotic signal.
r
1998 by The American Society of Hematology.F
as (APO-1) IS A 45-kD membrane protein when engaged
by anti-Fas antibody or Fas ligand triggers programmed
cell death (for review, see Nagata
1). The death pathway initiated
from Fas activation involves a series of death-induced
mol-ecules.
1FADD (Fas-associating protein with death domain) or
MORT1 is recruited to Fas upon its engagement.
2,3FADD then
binds FLICE (FADD-like ICE) or MACH (MORT-1–associated
CED-3 homologue).
4,5The association with the Fas
death-inducing signaling complex activates FLICE,
6followed by
eventual activation of ICE and CPP32.
7The activation of acidic sphingomyelinase that leads to the
hydrolysis of sphingomyelin and generation of ceramide has
also been suggested as the apoptotic pathway downstream of
Fas ligation.
8-12Ceramide is reported as a common
intermedia-tor for stimulation by tumor necrosis facintermedia-tor (TNF),
interleu-kin-1, nerve growth factor, lipopolysaccharide, ionizing
radia-tion, serum withdrawal, and daunorubicin.
12-19The role of
ceramide in the apoptosis induced by some of these stimuli is
illustrated by the ability of membrane-permeable ceramide to
trigger cell death.
13,14,19In addition, defects in the
sphingomy-elinase/ceramide pathway confers the resistance to radiation-,
TNF-
a–, and UV-induced apoptosis,
20-22supporting the role of
ceramide in these types of death induction. Ceramide-induced
cell death involves the activation of c-Jun N-terminal kinase
(JNK).
23In this study, we compared the sensitivity of Fas and
ceramide to various regulatory reagents. We showed that
ceramide-induced apoptosis was antagonized by cAMP, C
60,
and pyrrolidinedithiocarbamate (PDTC). However,
Fas-trig-gered cell death proceeded in the presence of these inhibitors. In
addition, the negative mutant of SAPK/ERK kinase (SEK)
suppressed ceramide-triggered death but did not prevent
Fas-induced apoptosis. Furthermore, activation of NF-
kB
antago-nized ceramide but not Fas-initiated apoptosis. These
observa-tions are not consistent with the model that ceramide acts
downstream of Fas signaling. Together with recent
observa-tions,
24,25our results argue against a role of ceramide in
Fas-mediated apoptosis.
MATERIALS AND METHODS
Reagents and cell lines. 12-O-tetradecanoylphorbol 13-acetate (TPA), A23187, N6, 28-O-dibutyryladenosine 38,58-cyclic
monophos-phate (Bt2cAMP), forskolin, and PDTC were purchased from Sigma
Chemical Co (St Louis, MO). Anti-Fas antibody CH-1126 was
pur-chased from Upstate Biotech Inc (Lake Placid, NY). C2-ceramide and
C6-ceramide were obtained from Biomol (Plymouth Meeting, PA). ICE
inhibitor z-VAD-FK was purchased from Kamiya (Thousand Oaks, CA). The two regioisomers with C3 or D3 symmetry of water-soluble carboxylic acid C60derivatives (carboxylfullerenes) were synthesized
as previously described.27Both C
60 (C3) and C60(D3) are effective
scavengers of oxgen radicals, with complete elimination of hydroxyl radicals and superoxide radicals at concentrations of 5 to 50 µmol/L, and are potent inhibitors of neuronal apoptosis, which is associated with increased intracellular free radical production.27H-89 and H-85 were
purchased from Seikagaku (Tokyo, Japan). Human T lymphoblastomas CEM (ATCC CCL 119) and human T-cell leukemia Jurkat (ATCC TIB 152) were obtained from American Type Culture Collection (Rockville, MD). Recombinant human TNF-a was purchased from R&D (Minne-apolis, MN).
Plasmids. CMV-RelA(p65) andkB-TATA-CAT28were kind gifts
of Dr Warren C. Greene (University of California, San Francisco, CA). HA-JNK129was obtained from Michael Karin (University of
Califor-nia, San Diego, CA). SEK-negative mutants SEK1(K=R) and SEK1(A=L)30 were obtained from Dr Leonard I. Zon (Harvard
Medical School, Boston, MA). Green fluorescence protein expression vector pEGFP-N1 was obtained from Clontech (Palo Alto, CA).
Transfection. T cells (1.63 107) were washed once with STBS (25
mmol/L Tris-HCl, pH 7.4, 137 mmol/L NaCl, 5 mmol/L KCl, 0.6 mmol/L Na2HPO4, 0.7 mmol/L CaCl2, 0.5 mmol/L MgCl2) and
incubated with a total of 10 µg DNA in 1.2 mL STBS containing 0.5 mg/mL diethylaminoethyl (DEAE)-dextran for 20 minutes at room temperature. T cells were then treated with 15% dimethylsulfoxide for 3 minutes and washed once with STBS.31,32 After 24 to 48 hours,
From the Graduate Institute of Microbiology, National Taiwan University School of Medicine, Taiwan, China; the Institute of Molecu-lar Biology, Academia Sinica, Taiwan, China; the Graduate Institute of Microbiology and Immunology, National Yang-Ming University, Tai-wan, China; the Department of Chemistry, National Taiwan University, Taiwan, China; and the Department of Medical Research, Veteran General Hospital, Taipei, Taiwan, China.
Submitted October 14, 1997; accepted December 31, 1997. Supported by Grant No. DOH86-HR-508 from the Department of Health, by Grant No. NSC 85-2331-B001-050 M30 from National Science Council, and by a grant from Academia Sinica, Taiwan, Republic of China.
Address reprint requests to Ming-Zong Lai, PhD, Institute of Molecular Biology, Academia Sinica, Nankang, Taipei 11529, Taiwan, Republic of China.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked ‘‘adver-tisement’’ in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
r
1998 by The American Society of Hematology. 0006-4971/98/9108-0053$3.00/0transfected cells were then stimulated with anti-Fas or ceramide, and the cell death was quantitated.
JNK activity assay. The inhibition of JNK by the dominant negative form of SEK was performed with cotransfection of HA-JNK1. T cells were activated as indicated, washed twice with phosphate-buffered saline (PBS), and lysed in ice-cold lysis buffer (20 mmol/L Tris-HCl, pH 8.0, 1% Triton X-100, 10% glycerol, 137 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 50 mmol/L NaF, 1 mmol/L Na3VO4, and 1
mmol/L phenylmethylsulfonyl fluoride). Detergent-insoluble material was removed by centrifugation at 14,000g for 10 minutes at 4°C. For each immuno-precipitation, 200 µg of cell lysate was mixed with anti-HA 12CA5 (Boehring Mannheim, Mannheim, Germany) and incubated at 4°C for 2 hours. Twenty microliters of protein A-sepharose (Pharmacia, Uppsala, Sweden) was then added and incubated for an additional 2 hours at 4°C. Immune complexes were washed three times with lysis buffer and once with kinase buffer (30 mmol/L Tris-HCl, pH 8.0, 20 mmol/L MgCl2, 2 mmol/L MnCl2). Immune complexes were
then incubated in kinase buffer (30 µL) containing 2 mmol/L ATP, GST-c-Jun(1-79), and 5 µCi of (g-32P)ATP for 30 minutes at 30°C.
Incubations were terminated by adding 15 µL of 33 sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiling for 3 minutes. The reaction products were resolved on 15% SDS-PAGE followed by autoradiography and quantitated by Phospho-rimager (Molecular Dynamics, Sunnyvale, CA).33
Cell death measurement. All cultures (except those treated with ceramide) were performed in RPMI with 10% fetal calf serum (both from GIBCO, Grand Island, NY), 10 mmol/L glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 23 1025mol/L 2-ME.34For
apoptosis induced with ceramide, serum free-medium were used throughout the experiment. The extent of apoptosis was determined by propidium iodide (PI) staining or annexin V staining. Cells were treated with different inducers and/or inhibitors, washed with PBS, and fixed with ethanol. DNA content was determined by staining with 20 µg/mL PI and analyzed by FACScan (Becton Dickinson, Mountain View, CA). The fraction of cells with sub-G1DNA content was assessed with the
CELLFIT program (Becton Dickinson). For annexin V staining, the treated cells were washed, resuspended in annexin V-fluorescein isothiocyanate (FITC) (1 µg/mL; Clontech), incubated at room tempera-ture for 15 minutes in the dark, and analyzed on FACScan. For the ceramide/Fas sensitivity of cell transiently transfected with CMV-p65 or pEBG-SEK(A=L), survival was monitored with green fluorescence protein expression vector pEGFP-N1. Treated cells were examined using a Nikon Diaphot 2000 fluorescence microscope (Tokyo, Japan) 48 hours after transfection.
RESULTS AND DISCUSSION
cAMP prevented ceramide-induced cell death but had no
effect on Fas-initiated apoptosis.
Ceramide is a potent inducer
of cell death in T-lymphoma cells Jurkat and CEM (not shown
for CEM). Cell death was assessed by DNA fragmentation as
represented by sub-G
1fraction (Fig 1B). DNA fragmentation
induced by ceramide never exceeded 45%, despite extensive
cell death. A similar observation was also earlier reported.
35We
first identified a few reagents that could block
ceramide-initiated cell death. cAMP was an effective antagonist of
ceramide-induced cell death (Fig 1C). Forskolin (10 µmol/L)
suppressed DNA fragmentation initiated by ceramide by at least
50%. A similar extent of inhibition was observed with dibutyryl
cAMP (Bt
2cAMP) at 1 mmol/L (Fig 2A). There was a
dose-dependent inhibition of cAMP on ceramide-triggered cell death.
The inhibition of ceramide-induced cell death could be detected
with Bt
2cAMP as low as 100 µmol/L. In contrast, apoptosis
triggered by anti-Fas antibody CH-11 was resistant to cAMP.
Treatment with forskolin up to 50 µmol/L or Bt
2cAMP up to 2.5
mmol/L did not reduce the apoptosis triggered by Fas (Fig 2B).
cAMP agonists by itself did not induce any T-cell death at the
highest concentrations used here.
The observation that Fas-initiated cell death cannot be
prevented by cAMP is consistent with an earlier report,
36yet is
in direct contrast to the prominent inhibitory effect of cAMP
recently documented.
37Because DNA fragmentation may not
well represent actual apoptosis,
38,39we also quantitated cell
death by annexin V staining. Figure 3 shows that Fas-induced
apoptosis was associated with an extensive phosphatidylserine
translocation, which was clearly not inhibited by cAMP. cAMP
is an antagonist for ceramide-induced cell death but not for
Fas-initiated apoptosis.
C
60and PDTC prevented ceramide-induced cell death but
had no effect on Fas-initiated apoptosis.
We have futher
observed that two malonic acid derivatives of C
60(carboxy-fullerens)
27were antagonist of ceramide-induced cell death
(Figs 1D and 2A). The pure carbon sphere of C
60(buckminster-fullerene) is known for its avid reactivity with free radicals, yet
the usage is limited by its water insolublility. The two
regioiso-forms with C3 or D3 symmetry of water-soluble C
60derivatives
remain as potent free radical scavengers and are effective
antagonists of apoptotic neronal death induced by serum
deprivation, glutamate receptor agonists, and amyloid peptide.
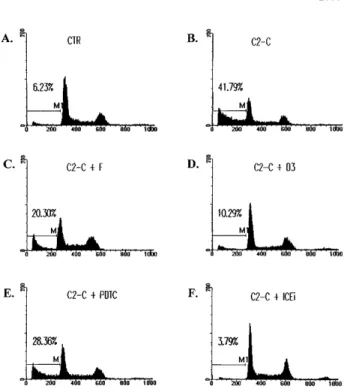
27Fig 1. The inhibition of ceramide-induced cell DNA fragmentation. Jurkat T cells were treated with 5mmol/L of C2-ceramide (C2-C) in the absence or presence of various antagonists for 4 hours, washed with PBS, and fixed with ethanol. DNA content was determined by staining with 20mg/mL PI and analyzed by FACScan (Becton Dickin-son). Fraction of cells with sub-G1DNA content (M1 fraction in the diagram) were assessed with CELLFIT program (Becton Dickinson). CTR, untreated cell control. The inhibitors used were as follows: forskolin (F), 10mmol/L; C60D3 (D3), 100mmol/L; PDTC, 200 mmol/L; Z-VAD-FK (ICEi), 300mmol/L. The sub-G1fractions were less than 6% for cells treated with inhibitors only. The exception was PDTC, in which a slightly elevated background death (8%) was observed.
Ceramide-triggered apoptosis was suppressed by 50% in the
presence of 10 µmol/L C
60(D3) and was inhibited by 90% with
100 µmol/L C
60(D3). We have repeatedly observed that C
60(D3) was more effective than C
60(C3) in the prevention of
ceramide-induced cell death (Fig 2A). In contrast, C
60(D3) and
C
60(C3) (at 100 µmol/L each) had no preventive effect on
Fas-triggered cell death as analyzed by both sub-G
1fraction and
annexin V binding (Figs 2B and 3D). The same contrast was
found with metal chelator PDTC. High concentration of PDTC
antagonized ceramide-induced apoptosis (Figs 1E and 2A), but
did not inhibit Fas-initiated cell death (Fig 2B). Of all the
inhibitors analyzed in this study, PDTC was the only reagent
that by itself increased spontaneous cell death at the
concentra-tion used (1% to 3% over control, data not shown). Because
such increase was minute, the differential effect of PDTC on
ceramide- and Fas-initiated apoptosis was still prominent. As a
control, the potent ICE proteases inhibitor z-VAD-FK
com-pletely abolished cell death induced by either ceramide or Fas
(Figs 1, 2, and 3), consistent with the notion that both
ceramide-and Fas-induced cell death are known to involve caspases such
as ICE.
7,40Therefore, we have identified three types of reagents that
antagonized ceramide-induced cell death but did not prevent
Fas-induced cell death. Between 50% and 90% of
ceramide-triggered cell death was suppressed by these inhibitors. If
ceramide is the major apoptotic messenger downstream of Fas,
a lesser but still prominent inhibition on Fas-induced cell death
by these inhibitors should be detectable. The distinct effect on
Fas- and ceramide-induced apoptosis suggest that ceramide is
dissociated from Fas-induced death cascade.
SEK dominant negative mutant did not interfere Fas-induced
cell death.
SEK/JNK activation is shown to be essential for
ceramide-initiated apoptosis.
23Despite the fact that JNK is
activated after Fas engagement, its role in apoptosis is less
certain. Lenczowski et al
41demonstrated that the activation of
SEK/JNK is downstream of ICE protease and is not required for
Fig 2. Inhibitors that blocked ceramide-induced DNA fragmenta-tion did not prevent Fas-induced DNA fragmentafragmenta-tion. (A) Jurkat cells were treated with ceramide and various inhibitors for 4 hours. The sub-G1DNA content was quantitated as described in Fig 1. Additional inhibitors used were as follows: dbcAMP, 1 mmol/L; C60(C3/100), 100 mmol/L; C60(D3/100), 100mmol/L; C60(D3/10), 10mmol/L. (B) Jurkat cells were stimulated with anti-Fas antibody CH11 (125 ng/mL) in the absence or presence of the inhibitors for 14 hours, and the sub-G1 DNA content was determined. The inhibitors used were as follows: forskolin, 50mmol/L; dbcAMP, 2.5 mmol/L; C60(C3), 100mmol/L; C60 (D3), 100mmol/L; PDTC, 200 mmol/L; Z-VAD-FK (ICEi), 300 mmol/L. None of the inhibitors alone induced DNA fragmentation at the concentrations used, except PDTC, in which an 1% to 2% increase over control was observed. The result is the average of duplicates, with standard deviation shown as an error bar. Those not shown are too small in scale. Experiments were repeated three times with the same results.
Fig 3. Inability of cAMP and C60(D3) to prevent Fas-induced apoptosis as assessed by annexin V binding. Jurkat cells were treated with CH-11 and inhibitors for 14 hours as described in Fig 2. Cells were washed, resuspended in annexin V-FITC (1mg/mL; Clontech), and analyzed on FACScan. M1 is designated as the fraction of apoptotic cells. In data not shown, cells treated with ceramide were similarly assayed. cAMP, (C60)D3, and ICEi were used at the same concentration as in Fig 2B.
Fas-induced apoptosis. On the contrary, the prevention of JNK
activation by SEK dominant negative mutant is shown to block
Fas-induced cell death in L929 and 293 cells.
42We have thus
studied the effect of the dominant negative mutant of SEK
30on
the same cell when treated with ceramide and anti-Fas. A
transient transfection analysis was used to assess the effect on
cell death in which SEK mutant was cotransfected with green
fluorescence protein. Expression of the dominant negative
mutants of SEK blocked the activation of the cotransfected
HA-JNK1.
32Ceramide triggered cell death was suppressed by
coexpression of SEK (A=L) in Jurkat cells (Fig 4), but the
inhibition was less with SEK (K=R) mutant (data not shown).
This is in contrast to the inability of SEK (A=L) to interfere
with Fas-induced apoptosis (Fig 4). In both Jurkat and CEM
cells (not shown for CEM), the inhibition of SEK activation
prevented ceramide- but not Fas-induced apoptosis. JNK
activa-tion was not essential for Fas-mediated apoptosis at least in
these two T-lymphoma cells.
Activation of NF-
kB did not interfere Fas-induced cell death.
In accordance with the recent finding that activation of NF-
kB
prevents the apoptotic signal of TNF-
a, we also found that
NF-
kB activation antagonized ceramide-induced DNA
fragmen-tation (Fig 5). Transient expression of NF-
kB p65, which led to
activation of
kB-CAT (not shown), prevented ceramide-induced
apoptosis. On the contrary, Fas-induced cell death was not
affected by the activation of NF-
kB in Jurkat cells. These results
further showed that ceramide is not a mediator of Fas-induced
apoptosis, for otherwise Fas would be equally sensitive to the
inhibition by NF-
kB.
In summary, we have presented evidence that ceramide is not
essential for Fas-induced cell death. Ceramide-triggered cell
death was sensitive to the inhibition of cAMP, yet its blockage
did not prevent Fas-initiated apoptosis. Two other antagonists,
C
60and PDTC, also demonstrate such selectivity in suppressing
ceramide-induced apoptosis. We have further shown that
block-ing SEK activation diminished ceramide-induced cell death in T
lymphomas Jurkat and CEM, yet had no effect on Fas-triggered
apoptosis on the same cells (Fig 4). A similar distinction was
also found with NF-
kB activation (Fig 5). Therefore, a large
fraction of Fas-triggered death pathways must be
ceramide-independent. Even though ceramide has been suggested as the
mediator for Fas-initiated apoptosis, the linkage between
ce-ramide and Fas-induced cell death is less than confirmative.
Recent biochemical analysis have questioned the role of
ceramide in Fas-induced apoptosis. In one study, the direct
measurement of sphingosine-based ceramide failed to detect
induction of ceramide up to 2 hours after Fas triggering.
25In
another study, ceramide increase was found to be slower than
Fas-induced cell death and can be inhibited by Z-VAD-FMK.
24Our results are fully in support of these observations and
suggest that ceramide is not the mediator of Fas-initiated
apoptosis.
Mixed effect of cAMP and C
60on TNF-
a–induced apoptosis.
We also examined the effect of cAMP, C
60, and PDTC on
TNF-
a–induced apoptosis, which is reported to be mediated by
ceramide.
14,16The study was performed on L929 cells because
TNF-
a alone does not induce cell death in Jurkat cells. The
effect of these inhibitors on TNF-
a–induced cell death was not
as unequivocal as on Fas-mediated apoptosis. PDTC (5 µmol/L)
induced spontaneous cell death (70%) in L929 cells, and its
inhibitory effect on TNF-
a–induced cell death cannot assessed.
TNF-
a–triggered apoptosis was completely prevented by 50
µmol/L of C
60(C3) and C
60(D3), yet was resistant to treatment
with forskolin and Bt
2cAMP (Fig 6). In contrast to the
NF-
kB–independent Fas-mediated apoptosis (Fig 5), TNF-a–
induced cell death is also known to be suppressed by the
activation of NF-
kB.
43-45Some of the discrepancy may be
attributed to the difference in apoptotic signaling between Fas
and TNF-
a previously reported.
36For example, the different
sensitivity to C
60(Figs 2 and 6) is well correlated with the
involvement of reactive oxgen radicals in TNF-
a–induced, but
not in Fas-induced, cell death.
36It may also be noted that the
mixed effect of the inhibitors on TNF-
a–mediated apoptosis is
Fig 4. Dominant negative mutant of SEK prevented ceramide- but not Fas-induced apoptosis. Jurkat cells were transfected with 5mg of either SEK1(A=L) or pEBG vector, together with 5mg of pEGFP-N1 (Clontech). Thirty-six hours later, cells were untreated (Ctr) or treated with either C2-ceramide (5mmol/L) or anti-Fas antibody CH11 (100 ng/mL) (Induction). Ceramide-induced cell death was analyzed 6 hours later, whereas Fas-induced cell death were quantitated 12 hours later. Data are the average of duplicates.
Fig 5. Differential sensitivity of ceramide- and Fas-triggered cell death to activation of NF-kB. Jurkat cells were transfected with 5 mg of either CMV-RelA or CMV vector, together with 5mg of pEGFP-N1 (Clontech). Thirty-six hours later, cells were untreated or treated with either C2-ceramide (5mmol/L) or anti-Fas antibody CH11 (100 ng/mL). Ceramide-induced cell death was analyzed 6 hours later, whereas Fas-induced cell death were quantitated 14 hours later. Data are the average of duplicates.
consistent with the observation that ceramide contributes to a
fraction, but not all, of TNF-
a–induced cell death.
35The distinction between the Fas-induced apoptotic cascade
and ceramide-induced death pathway shows that death
pro-cesses induced by different stimulation can be clearly
indepen-dent of each other. The combination of different stimulations
may hence be more effective in the induction of apoptosis,
especially in tumor cells for therapeutic purposes, notably,
ceramide-mediated cell death initiated by TNF-
a, daunorubicin,
and
g-irradiation, which are reagents widely used in tumor
treatment.
20-22The dissociation of Fas-induced death signals
from ceramide may indicate an additional dimension in
manipu-lation of apoptosis.
ACKNOWLEDGMENT
The authors thank Dr Leonard Zon for SEK(A=L) and SEK(K=R), Dr Warren C. Greene forkB-TATA-CAT, and Dr Michael Karin for HA-JNK1. We also thank Douglas Platt for editorial correction of the manuscript.
REFERENCES
1. Nagata S: Apoptosis by death factor. Cell 88:355, 1997
2. Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM: FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505, 1995
3. Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D: A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem 270:7795, 1995
4. Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM: Flice, a novel FADD-homologue
ICE/CED-3-like protease, is recruited to the CD95(Fas/APO-1) death-inducing signaling complex. Cell 85:817, 1996
5. Boldin MP, Goncharov TM, Goltsev YV, Wallach D: Involvement of MACH, a novel MORT1/FADD-interacting protease in Fas/APO-1-and TNF receptor-induced cell death. Cell 85:803, 1996
6. Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME: FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J 16:2794, 1997
7. Enari M, Talanian RV, Wong WW, Nagata S: Sequential activation of ICE-like and CPP32-like protease during Fas-mediated apoptosis. Nature 380:723, 1996
8. Cifone MG, Maria RD, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R: Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med 179:1547, 1994
9. Cifone MG, Roncaioli P, De Maria R, Camarda G, Santoni A, Giovna R, Testi R: Multiple pathways originate at the Fas/APO-1 (CD95) receptor: Sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphigomyelinase in the propagation of apoptotic signal. EMBO J 14:5859, 1995
10. Gill BM, Nishikata H, Chan G, Delovitch TL, Ochi A: Fas antigen and sphingomyelin-ceramide turnover-mediated signalling: Role in life and death of T lymphocytes. Immunol Rev 142:113, 1994
11. Gulbins E, Bissonnette R, Mahboubi A, Martin S, Nishioka W, Brunner T, Baier G, Baier-Bitterlich G, Byrd C, Lang F, Kolesnick R, Altman A, Green D: Fas-induced apoptosis is mediated by a ceramide-initiated Ras signalling pathway. Immunity 2:341, 1995
12. Tepper CG, Jayadev S, Lin B, Bielawska A, Wolff R, Yonehara S, Hannun A, Seldin MF: Role for ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci USA 92:8443, 1995
13. Obeid LM, Linardic CM, Karolak LA, Hannun YA: Programmed cell death induced by ceramide. Science 259:1769, 1993
14. Kolesnick R, Golde DW: The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell 77:325, 1994
15. Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, Mcloughlin M, Fuks Z, Kolesnick RN: Ionization radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med 180:525, 1994
16. Dbaibo GS, Obeid LM, Hannun YA: Tumor necrosis factor-a (TNF-a) signal transduction through ceramide. J Biol Chem 268:17762, 1993
17. Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R: Ceramide synthetase mediates daunorubicin-induced apoptosis: An alternative mechanism for generating death signals. Cell 82:405, 1995
18. Jaffrezou JP, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N, Vermeersch S, Rousse A, Laurent G: Daunorubicin-induced apoptosis: Triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J 15:2417, 1996
19. Spiegel S, Foster D, Kolesnick R: Signal transduction through lipid second messengers. Curr Opin Cell Biol 8:159, 1996
20. Wright SC, Zheng H, Zhong J: Tumor cell resistance to apoptosis due to a defect in the activation of sphigomyelinase and the 24 kDa apoptotic protease (AP24). FASEB J 10:325, 1996
21. Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R: Acid sphinomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 86:189, 1996
22. Cai Z, Bettaieb A, Mahdani NE, Legres LG, Stancou R, Masliah J, Chouaib S: Alteration of the sphingomyelin/ceramide pathway is associated with resistance of human breast carcinoma MCF7 cells to tumor necrosis factor-a-mediated cytotoxicity. J Biol Chem 272:6981, 1997
23. Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer
Fig 6. Mixed effect of cAMP and C60 on TNF-a–induced apoptosis. L929 cells were treated with recombinant TNF-a (250 mg/mL) in the absence or presence of the indicated inhibitors for 18 hours. The sub-G1DNA content was quantitated as described in Fig 1. The concentration of the inhibitors used were as follows: forskolin, 10 mmol/L; dbcAMP, 1.0 mmol/L; C60 (C3), 50 mmol/L; C60 (D3), 50 mmol/L; Z-VAD-FK (ICEi), 100 mmol/L.
MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN: Requirement for ceramide-initiated SAPK/JNK signal-ing in stress-induced apoptosis. Nature 380:75, 1996
24. Sillence DJ, Allen D: Evidence against an early signalling role for ceramide in Fas-mediated apoptosis. Biochem J 324:29, 1997
25. Watts JD, Gu M, Polverino AJ, Patterson SD, Aebersold R: Fas-induced apoptosis of T cells occurs independently of ceramide generation. Proc Natl Acad Sci USA 94:7292, 1997
26. Yonehara S, Ishii A, Yonehara M: A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with receptor of tumor necrosis factor. J Exp Med 169:1747, 1989
27. Dugan LL, Tureysky DM, Du C, Lobner D, Wheeler M, Almli CR, Shen CKF, Luh TY, Choi DW, Lin TS: Carboxyfullerenes as neroprotective agents. Proc Natl Acad Sci USA 94:9434, 1997
28. Ballard DW, Dixon EP, Perfer NJ, Bogerd H, Doeree S, Stein B, Greene WC: The 65-kDa subunit of human NF-kB functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci USA 89:1875, 1992
29. Hibl M, Lin A, Smeal T, Minden A, Karin M: Identification of an oncoprotein- and UV-responsive protein kinase that bind and potentiate the c-Jun activation domain. Genes Dev 7:2135, 1993
30. Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis MJ, Zon LI: Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 372:794, 1994
31. Hsueh YP, Liang HE, Ng SY, Lai MZ: CD28 costimulation activates CREB in T lymphocytes. J Immunol 158:85, 1997
32. Ho HY, Lee HH, Lai MZ: Overexpression of mitogen-activated protein kinase kinase kinase reverses cAMP inhibition of NF-kB in T cells. Eur J Immunol 27:222, 1997
33. Hsueh YP, Lai MZ: c-Jun N-terminal kinase but not mitogen-activated protein kinase is sensitive to cAMP in T lymphocytes. J Biol Chem 270:18094, 1995
34. Lee MR, Liou ML, Liou ML, Yang YF, Lai MZ: cAMP analogs
prevent activation-induced apoptosis of T cell hybridoma. J Immunol 151:5208, 1993
35. Higuchi M, Singh S, Jaffrezou JP, Aggarwal BB: Acidic sphingo-myelinase-generated ceramide is needed but not sufficient for TNF-induced apoptosis and nuclear factor-kB activation. J Immunol 156: 297, 1996
36. Schulze-Osthoff K, Krammer PH, Droge W: Divergent signal-ling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J 13:4587, 1994
37. Wilson DJ, Fortner KA, Lynch DH, Mattingly RR, Macara IG, Posada JA, Budd RC: JNK, but not MAPK, activation is associated with Fas-mediated apoptosis in human T cells. Eur J Immunol 26:989, 1996 38. Schulze K, Walczak H, Droge W, Debatin KM, Krammer PH: Cell nucleus and DNA fragmentation are not required for apoptosis. J Cell Biol 127:15, 1994
39. Nakajima H, Golstein P, Henkart PA: The target cell nucleus is not required for cell-mediated granzyme- or Fas-based cytotoxicity. J Exp Med 181:1905, 1995
40. Smyth MJ, Perry DK, Zhang J, Poirier GG, Hannun YA, Obeid LM: prICE: A downstream target for ceramide-induced apoptosis and for inhibitory action of Bcl-2. Biochem J 316:25, 1996
41. Lenczowski JM, Dominguez L, Eder A, King LB, Zacharchuk CM, Aswell JD: Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol Cell Biol 17:170, 1997
42. Yang X, Khosravi-Far R, Chang HY, Baltimore D: Daxx, a novel fas-binding protein that activates JNK and apoptosis. Cell 88:1067, 1997
43. Beg AA, Baltimore D: An essential role for NF-kB in preventing TNF-a-induced cell death. Science 274:782, 1996
44. Wang CY, Mayo MW, Baldwin AS: TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-kB. Science 274:784, 1996
45. Van Antewerp DJ, Martin SJ, Kafri T, Green DR, Verma IM: Suppression of TNF-a-induced apoptosis by NF-kB. Science 274:784, 1996