行政院國家科學委員會專題研究計畫 成果報告
以 NF-kB 抑制劑及抗凝血蛇毒蛋白抑制眼球之新生血管形
成(3/3)
計畫類別: 個別型計畫 計畫編號: NSC92-2314-B-002-161- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學醫學院眼科 計畫主持人: 楊中美 報告類型: 完整報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 10 月 14 日
Effects of Pyrrolidine Dithiocarbamate, a NF-
κ
B Inhibitor, on Cytokines Expression
and Ocular inflammation in Experimental Autoimmune Anterior Uveitis
I-Mo Fang 1,2, Chang-Hao Yang1, Chang-Pin Lin1, Chung-May Yang1, Muh-Shy Chen1
1
Department of Ophthalmology, National Taiwan University Hospital; 2Department of Ophthalmology,
Taipei Municipal Zhongxiao Hospital, Taipei, Taiwan
Reprint requests to:
Chang-Hao Yang, MD, PhD Department of Ophthalmology National Taiwan University Hospital Chung-Shan S. Rd. No.7, Taipei, Taiwan TEL: 886-2-23123456 ext. 2131
Fax: 886-2-23412875
e-mail: chyang@ha.mc.ntu.edu.tw
ABSTRACT
This study was conducted to investigate effects of pyrrolidine dithiocarbamate (PDTC), a nuclear
factor (NF)-κB inhibitor, on cytokine expressions and suppression of anterior chamber inflammation in
experimental autoimmune anterior uveitis. Uveitis was induced in the Lewis rats with the injection of melanin-associated antigen into peritoneum and footpad. At defined time points, cytokine mRNA expressions in the iris/ciliary body were measured by using a semiquantitative polymerase chain reaction
method. We found that interferon-γ (IFN-γ) and tumor necrosis factor (TNF)-α mRNA expression reached
peak during the active phase of uveitis, whereas interleukin (IL)-10 mRNA increased during the disease resolution. In a separate experiment, PDTC (40mg/kg/day) was administrated intraperitoneally after immunization. We found that PDTC effectively suppressed ocular inflammation, as indicated by reduced clinical scores and inflammatory cells infiltration in aqueous humor and iris/ciliary body. The inhibitory
effects of PDTC are mainly resulted from inhibiting the expression of proinflammatory cytokines, TNF-α
and IFN-γ but augmenting anti-inflammatory cytokines, IL-10 expression. These findings suggest that
application of NF-κB inhibitors may be a potential therapeutic method for the treatment of acute anterior
INTRODUCTION
Uveitis is a major cause of visual defect. The most common form of human uveitis is acute anterior uveitis of unknown etiology (1). The recurrent nature of anterior uveitis may lead to many secondary
complications such as cataract, cystoid macular edema and glaucoma. Because the exact mechanism of acute anterior uveitis remains unknown, nonspecific corticosteroids are still the mainstay for the uveitis treatment. However, complications of corticosteroid therapy limit its clinical applications.
Experimental autoimmune anterior uveitis (EAAU) is a well-established animal model for acute anterior uveitis (2). EAAU is induced by immunizing animals with bovine melanin-associated antigen (MAA) (3, 4). The clinical course in EAAU is very similar to that of human AAU (5, 6). Studies on the molecular mechanisms and treatment of EAAU would help us to gain insights into the pathogenesis of human AAU and to develop effective therapeutic drugs that block the activation of inflammatory signaling pathways.
Cytokines were important inflammatory mediators that had been proven to be involved in a variety of inflammatory diseases (7, 8). Cytokines are mainly secreted by helper T (Th) cells. Based on functional
characteristics, Th cells are classified into Th1 and Th2 (9). Th1 cells produce IL-2 and interferon (IFN)-γ;
Th2 cells produce IL-4, IL-6, IL-10. Th1 cytokines are known as pro-inflammatory cytokines, because they exacerbate inflammation. On the other hand, Th2 cytokines play an anti-inflammatory role since they inhibit inflammation initiated by Th1 cytokines (10, 11). In many human diseases, Th1 cells are associated with the initiation of diseases, whereas Th2 cells are related to suppression of the diseases (12-14).
The nuclear factor kappa B (NF-κB) is a transcriptional factor that can regulate the expression of
pro-inflammatory genes, including cytokines, adhesion molecules and chemokines (15, 16). NF-κB has been
proven to play a central role in a variety of inflammatory diseases (17). The identification of NF-κB as a key
player in the pathogenesis of inflammatory reactions suggests that NF-κB-targeted therapeutics might be
effective in the treatment of inflammatory diseases.
This study was designed to investigate the expressions of cytokines in iris/ciliary body using a rat
model of acute anterior uveitis. The effect of pyrrolidine dithiocarbamate (PDTC), a potent NF-κB inhibitor
(18-20), on the expression of cytokines and ocular inflammation in EAAU was evaluated to gain a better insight into the pathogenesis of acute anterior uveitis.
MATERIALS AND METHODS
Antigen and Induction of EAAU
MAA was prepared as previously described by Borekhuyse and Kuhlmann with a modification (2). The iris and ciliary body were carefully obtained from fresh pigmented bovine eyes. The tissue was gently homogenized and filtered through a wire mesh to remove cellular debris and connective tissue. The
homogenate was centrifuged at 1.2 × 105g at 4°C for 15 minutes and washed once with phosphate-buffered saline (PBS) at pH 7.4. The resulting pellet was suspended in 2% sodium dodecyl sulfate (SDS) (Bio-Rad,
Richmond, CA) and incubated at 70°C for 10 minutes. After centrifugation, the pellet was washed three times
with water. The insoluble antigen was dried and stored at –20°C.
Lewis rats, 6–8 weeks old and weighing 125-160 g, were used for the experiment. All animals were treated in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. To induce EAAU, the rats were given two separate injections at the same time: 1). 0.05 ml MAA was suspended in PBS, emulsified (1:1) in complete Freund’s adjuvant (CFA) and injected into left hind footpad
2). 0.05 ml MAA, emulsified with 1µg purified Bordetella pertussis toxin (PTX) was injected
intraperitoneally.
PDTC Treatment
The treatment protocol was conducted in a randomized and double-masked fashion. The rats were divided into two groups as follows:
(1). Group A received intrapertoneal injection of PDTC (40 mg/kg) daily since day 1. (2). Group B received intraperitoneal injecton of PBS daily as a control since day 1.
Clinical Activity Scoring
The rats were clinically observed on a daily basis with slitlamp biomicroscopy for clinical signs of ocular inflammation. Disease severity was assessed with a scale ranging from 0 to 4: 0 = normal; 1 = slight iris-vessel dilatation and some anterior chamber cells; 2 = iris hyperemia, with some limitation in pupil dilation, anterior chamber cells, and a slight flare; 3 = a miotic, irregular, hyperaemic, and (sometimes) slightly damaged iris, with a considerable flare and cells (especially with accumulation near the iris); and 4 = a
seriously damaged and hyperaemic iris, a miotic pupil often filled with protein, and cloudy gel-like aqueous humor (AqH).
Tissue Preparation
Rats were sacrificed on day 3, 9, 11, 14, 18, 25. The eyes were harvested. The eyes were quickly dissected, and the iris and ciliary body were isolated from the remaining ocular tissue by using an operating microscope.
Preparation of RNA and cDNA
Total RNA was extracted from the iris/ciliary body with Trizol reagent (Life, Gaithersburg, MD). One
microgram of total RNA from each sample was annealed for 5 min at 65°C with 300-ng oligo(dT)(Promega,
Madison, WI) and reverse transcribed to cDNA by using 80 U Moloney murine leukemia virus reverse
transcriptase (MMLV-RT) (Gibco, Grand Island, NY) per 50 µg reaction for 1 h at 37°C. The reaction was
Polymerase Chain Reaction
The amplification was performed with a thermocycler. (MJ Research, Waltham, MA) The 50-µl
reaction mixture consisted of 5 µl cDNA, 1 µl of sense and antisense primer, 200 µM of each
deoxynucleotide, 5 µl 10× Taq polymerase buffer, and 1.25 U Taq polymerase (Promega, Madison, WI).
Conditions for amplifying each cytokines were as follows: denaturation, 1 min at 94°C, and elongation, 3 min
at 72°C. For the annealing temperature for cytokines, 62°C to 42°C was designed for IL-4, IL-10 and IFN-γ.
The temperature then declined at 1°C increments and this was followed by 20 cycles at 55°C. The annealing
temperature for TNF-α, IL-6, TGF-β1 and TGF-β2 was from 67°C to 50°C, declining at 1°C increment and
followed by 21 cycles at 60°C. At the end of amplification, the reaction mixture was heated for 10 min at
72°C and then cooled to 4°C. A 10-µl sample of each polymerase chain reaction (PCR) product was
separated by gel electrophoresis on 2% agarose containing ethidium bromide (Sigma, St. Louis, MO) and then analyzed under ultraviolet light against the DNA molecular length markers. The intensity of the products was analyzed by using an image analyzer (Digital 1D Science; Eastman Kodak, Rochester, NY), and the amount of PCR-amplifiable material in each reverse-transcribed sample was standardized against the amount
of a housekeeping gene rat β-actin. The primers sequences were listed in Table 1.
Quantification of Leukocytes in Aqueous Humor
Aqueous humor (AqH) was collected from the eyes using a 30-gauge needle immediately after the animal was sacrificed on day 18. The AqH was pooled in silicon-treated microcentrifuge tubes (Fisher
Scientific, Pittsburgh, PA). Two microlitre (2 µL) AqH from one rat was stained with 0.4% trypan-blue
solutionand the number of leukocytes was counted under phase-contrast microscopy
Histopathologic Evaluation
On day 18, rats were sacrificed. The eyes were enucleated and embedded in paraffin. Then, 5-µm
sagittal sections were cured and stained with hematoxylin and eosin (H-E)
Statistical Analyses
Differences among the amounts of the cytokines mRNA at different time points were evaluated using Mann-Whitney U-test. Values of p<0.05 were considered as significant.
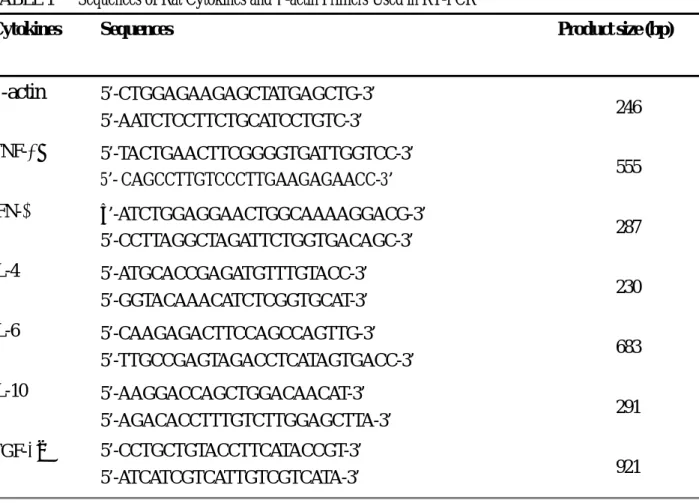
TABLE 1 Sequences of Rat Cytokines and β-actin Primers Used in RT-PCR
Cytokines Sequences Product size (bp)
β
-actin
5’-CTGGAGAAGAGCTATGAGCTG-3’ 5’-AATCTCCTTCTGCATCCTGTC-3’ 246 TNF-α 5’-TACTGAACTTCGGGGTGATTGGTCC-3’ 5’- CAGCCTTGTCCCTTGAAGAGAACC-3’ 555 IFN-γ 5’-ATCTGGAGGAACTGGCAAAAGGACG-3’ 5’-CCTTAGGCTAGATTCTGGTGACAGC-3’ 287 IL-4 5’-ATGCACCGAGATGTTTGTACC-3’ 5’-GGTACAAACATCTCGGTGCAT-3’ 230 IL-6 5’-CAAGAGACTTCCAGCCAGTTG-3’ 5’-TTGCCGAGTAGACCTCATAGTGACC-3’ 683 IL-10 5’-AAGGACCAGCTGGACAACAT-3’ 5’-AGACACCTTTGTCTTGGAGCTTA-3’ 291 TGF-β2 5’-CCTGCTGTACCTTCATACCGT-3’ 5’-ATCATCGTCATTGTCGTCATA-3’ 921 RESULTSClinical Scores of EAAU and Cytokine mRNA Expressions in Iris/CB During EAAU
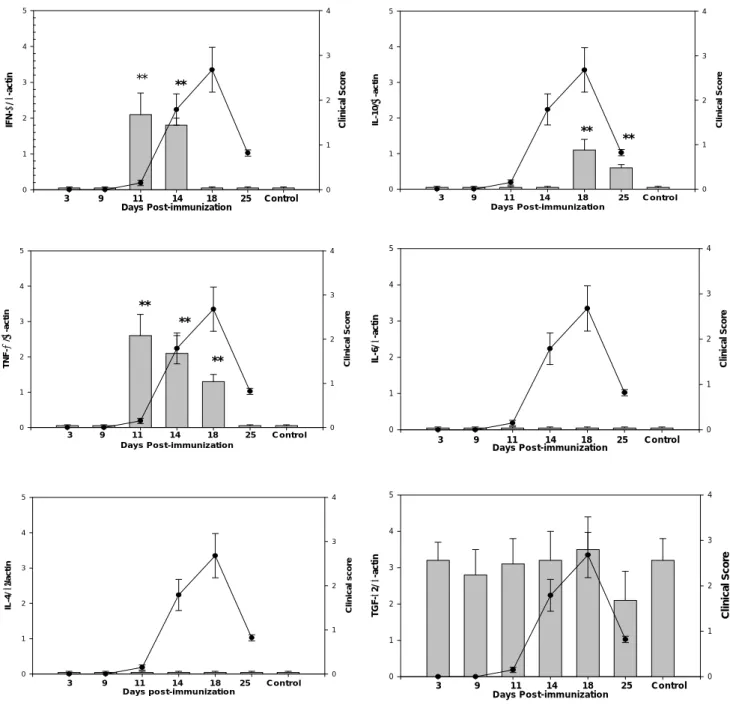
Clinical signs of EAAU showed on day 11 post-immunization. The clinical symptoms reached peak on day 18 and entirely relieved by day 30.
TNF-α mRNA was detected in parallel with the disease progression. As the disease resolved, TNF-α
declined to normal control level. IFN-γ started increasing on day 11, concurrent with the disease onset. At
maximal clinical activity, however, IFN-γ mRNA dropped abruptly to the control level. IL-10 mRNA
increased on day 18 and 25, correlating with remission of the disease. The expression of TGF-β2 mRNA was
remained at a high level. However, when compared with normal control, there was no statistically significant
change in TGF-β2 mRNA during the course of the disease. The mRNA of IL-4 and IL-6 was undetectable
Days Post-im m unization IFN -γ / β -a c ti n 0 1 2 3 4 5 Clinic al S c or e 0 1 2 3 4 3 9 11 14 18 25 Control ** **
D a ys P o st-im m u niz a tio n
IL -1 0/ β -a c ti n 0 1 2 3 4 5 Clin ical S c o re 0 1 2 3 4 3 9 1 1 1 4 1 8 25 C on tro l ** **
D ays P o st-im m u n iz atio n
TN F -α / β -act in 0 1 2 3 4 5 Clin ical S c o re 0 1 2 3 4 3 9 11 14 18 25 C o n tro l ** ** **
D ays P o st-im m u n izatio n
IL-6 / β -a c ti n 0 1 2 3 4 5 C lini c a l S c o re 0 1 2 3 4 3 9 11 14 18 25 C o n tro l
D ays p o st-im m u n izatio n
IL -4 / β− act in 0 1 2 3 4 5 Clin ical sco re 0 1 2 3 4 3 9 11 1 4 1 8 25 C o n tro l
D ays P o st-im m u n izatio n
TG F-β 2/ β -a c ti n 0 1 2 3 4 5 C li n ical Scor e 0 1 2 3 4 3 9 11 14 18 25 C o n tro l
FIGURE 1 Expression of mRNA of cytokines including: TNF-α, ΙFN-γ, TGF-β2, IL-4 and IL-10 in the
iris/ciliary body from Lewis rats at different time points during the course of EAAU. The control represents the normal rats not being immunized. Bar charts represent the relative intensity of mRNA of cytokines
compared to β-actin. Line charts correspond to the clinical scores of the disease. Data are presented as the
mean ± SD in five rats. *p < 0.05, ** p<0.01 when compared to normal control
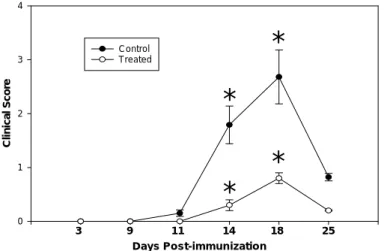
Effects of PDTC on Clinical Activity Scores
Rats treated with PDTC (40 mg/kg) demonstrated a significant reduction in clinical severity than those treated with PBS. The maximal clinical scores in rats treated with PDTC (40 mg/kg) was significantly lower than in those with PBS (Figure 2).
D ays Post-im m unization C linic al Scor e 0 1 2 3 4 C ontrol Treated 3 9 11 14 18 25
*
*
*
*
FIGURE 2 Effects of PDTC on clinical scores of Lewis rats with EAAU. Rats treated with PDTC (40 mg/kg) showed a significant reduction in clinical severity than those treated with PBS. * : Mann-Whitney U-test, p < 0.05.
Effects of PDTC on Cellular Infiltration in Aqueous Humor
Rats were sacrificed on day 18 and the cells in the anterior chamber were quantified. PDTC treated eyes showed a lower number of cellular infiltrations; the number of infiltrating cells was 960 ± 160 cells/ ml in the control rats and treatment with PDTC resulted in a significant reduction of the cell number to 240 ± 30 cells/ ml (p = 0.001)
Effects of PDTC on Histologic Changes
Leukocytes, mainly lymphocytes, were found to infiltrate in iris/ciliary body of rats on day 18. (Fig. 3, left panel) The number of leukocytes in iris/ciliary body was markedly reduced after PDTC treatment. (Fig. 3, right panel)
Figure 3 Histological section of eyes on the day 18 after immunization. Rats treated with PBS (left panel); Rats treated with PDTC (40 mg/ml) (right panel). H-E staining; original magnification X 400.
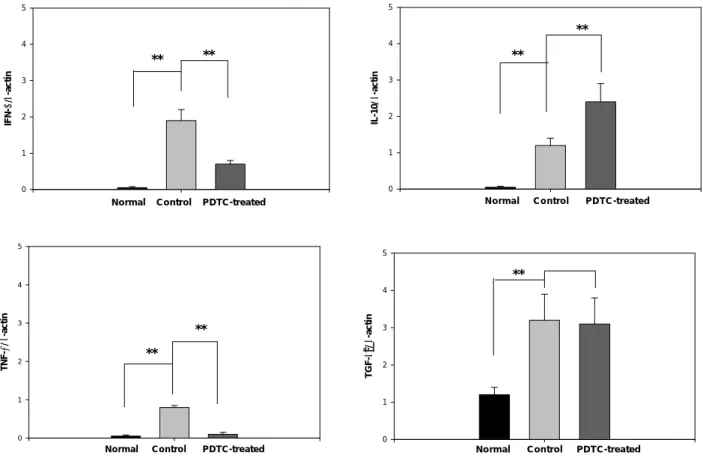
Effects of PDTC of Cytokines mRNA Expression in Iris/Ciliary Body
On day 14, treatment with PDTC demonstrated a significant reduction in IFN-γ mRNA expression in
the iris/ciliary body (p=0.001). On day 18, treatment with PDTC resulted in significantly reduction
inTNF-α mRNA expression (p=0.001). In contrast, treatment with PDTC augmented IL-10 mRNA
expression when compared with control (p=0.001). There was no significant difference in TGF-β2 mRNA
expression between PDTC treated and untreated groups (Fig 4).
Norm al Control PDTC-treated
IF N-γ / β -a ct in 0 1 2 3 4 5 ** ** N o rm a l C o n tro l P D T C -treated IL -10/ β -a c ti n 0 1 2 3 4 5 ** **
Norm al Control PDTC-treated
TN F-α / β -a c ti n 0 1 2 3 4 5 ** **
No rm al Con trol PDT C-treated
TG F -β2 / β -a ct in 0 1 2 3 4 5 **
FIGURE 4 Effects of PDTC on cytokine mRNA expression in iris/ciliary body of Lewis rats with EAAU. Data are presented as the mean ± SD in five rats. ** : Mann-Whitney U-test , p<0.01.
DISCUSSION
In the present study, we demonstrated that pro-inflammatory cytokines, IFN-γ and TNF-α, increased
recovery periods of the disease. In addition, we investigated the in vivo effects of PDTC, a NF-κB inhibitor, on the expression of cytokines in rat model of acute anterior uveitis. We found that PDTC effectively suppressed ocular inflammations, as indicated by reduced clinical activity scores, diminished leukocyte infiltration in iris/ciliary body and aqueous humor. These anti-inflammatory effects are mainly by means of
decreasing TNF-α, IFN-γ , as well as augmenting IL-10 expression. These results indicated that timely
expression of certain cytokines is critical in the pathogenesis of EAAU and PDTC exerted it anti-inflammatory effect by regulating the expressions of specific cytokines.
Previous studies reported that expressions of TNF-α and IFN-γ are correlated with disease
progression in several autoimmune diseases (21, 22). TNF-α was reported to play a critical role in the
initiation of inflammatory reactions, including the activation of T cells (23), induction of vascular cell adhesion lmoleucle-1 (24), and activation of leukocytes (25), all of which could contribute to the tissue
destruction in the autoimmune diseases. IFN-γ enhanced the cytotoxic effect of TNF-α and also activated
macrophages (26, 27). These mechanisms presumably mediate the pro-inflammatory roles of TNF-α and
IFN-γ in generating uveitis. In our study, decreased IFN-γ and TNF-α expression by PDTC resulted in
diminished clinical severity of uveitis, further supporting the pivotal role of IFN-γ and TNF-α in the
pathogenesis of uveitis.
In our study, IL-10 mRNA increased in association with the disease resolution, whereas IL-4, IL-6 mRNA did not change during EAAU. Previous report demonstrated that IL-6 was activated to antagonize anterior chamber-associated immune deviation (ACAID) in rat model of endotoxin-induced uveitis (28). The difference between our study and that reported was due to difference in the methods of inducing the
experimental uveitis. IL-10 was reported to be a potent suppressor of macrophages, T-cells, and NK cells and suppressed the growth and transformation of Th1 cells (29). Therefore, at disease resolution, IL-10 helps to reduce inflammation and restore ocular immune privilege.
PDTC, a NF-κB inhibitor, may suppress pro-inflammatory cytokine, TNF-α, expression but enhance
anti-inflammatory cytokine, IL-10, expression, thereby reduce inflammations. Nemeth et al demonstrated
the reduction in TNF-α and increase in IL-10 expression after PDTC treatment in LPS-induced endotoxemia
mouse (30). However, since both IL-10 and TNF-α genome contain NF-κB-like recognition sites (31, 32), it
is possible that PDTC may influence the expression of these two cytokines with different mechanisms. Recent studies demonstrated that PDTC could augment protein-1 (AP-1) activation, which was found to be presented in the IL-10 genome (33, 34). We therefore postulated that the enhanced activation of AP-1 by PDTC was responsible for the increased IL-10 levels. However, further studies are required to support this finding.
TGF-β is thought to contribute to immunosuppressive properties and is important for the maintenance
of the immune-privileged environment of the anterior chamber (35, 36). Several studies demonstrated
reduction of TGF-β activity in aqueous humor of uveitis (37, 38). In the present study, however, we could not
find any significant alterations in mRNA expression of TGF-β2 during EAAU. TGF-β is secreted in a latent
complex that needs to be transformed into mature TGF-β to become biologically active (39). In this study, we
TGF-β may therefore have little relationship to biological function.
PDTC represents a class of antioxidants reported to be a potent inhibitor of NF-κB (19, 20, 40). In
addition, PTDC has been shown to block IκB-α phosphorylation, precluding the dissociation of NF-κB from
IκB-α and subsequent NF-κB translocation from the nucleus in response to inflammatory stimulation. The
potential for modulating cell activation suggests that PDTC and its analogs may offer therapeutic benefit in
inflammatory conditions in which activation of NF-κB plays a major role.
There are several limitations in our study, which was designed to be a pilot experiment to examine the
expressions of various cytokines and then determines the effects of nuclear factor (NF)-κB inhibitor, on the
expressions of these cytokines. Therefore, we screened the expression and regulation of cytokines only at the transcription level. To obtain more information on the roles of cytokines in uveitis, further study with expressions of major cytokine protein in iris/ ciliary body is needed. Secondly, despite PDTC successfully alleviated the clinical signs of EAAU, PDTC-treated rats showed a retarded body weight gain, suggesting the presence of a toxic action of PDTC (41). The ultimate benefit of such targeted therapy will depend on the delicate balance between suppressing inflammatory and interfering with normal cellular functions. By
selectively targeting specific NF-κB subunits that have a degree of tissue specificity, one might attain
therapeutic efficacy and minimize systemic toxicity.
In conclusion, the present study demonstrated the roles of IFN-γ and TNF-α in the disease initiation
and prorogation and IL-10 in resolution of EAAU. PDTC showed important modulatory effects on cytokine expression and effectively suppressed ocular inflammation. These effects are mainly by means of inhibiting
the expression of proinflammatory cytokines, TNF-α but augmenting anti-inflammatory cytokines
expression. These findings suggest that PDTC may be a valuable therapeutic alternative to uveitis.
ACKNOWLEDGMENTS
This study was supported by research grant from the Department of Health, Taipei City Government and grant NSC 92-2314-B-002-161 from the National Science Council, the Executive Yuan, Republic of China. We are grateful to Miss Lee-Run Lin for preparing the manuscript.
REFERENCE
1. Nussenblatt RB, Palestine AG. Uveitis: Fundamentals and Clinical Practice. Chicago: Year Book Medical Publishers; 1989.
2. Broekhuyse RM, Kuhlman ED, Winkens HJ, Van Vugt AHM. Experimental autoimmune anterior uveitis (EAAU), a new form of experimental uveitis, I: induction by a detergent-insoluble, intrinsic
3. Broekhuyse RM, Kuhlman ED, Winkens HJ. Experimenal autoimmune anterior uveitis (EAAU). II: dose-dependent induction and adaptive transfer using a melanin-bound antigen of the retina pigment epithelium. Exp. Eye Res. 55:401-411, 1992.
4. Broekhuyse R.M, Winkens H.J, Kuhlmann ED. Intraperitoneally injected melanin is highly uveitogenic. Exp. Eye Res. 62:199-200, 1996.
5. Bora NS, Kim MC, Kabeer NH. Experimental autoimmune anterior uveitis (EAAU): induction with melanin associated antigen from the iris and cilialry body. Invest. Ophthalmol. Vis. Sci. 36:1056-1066, 1995.
6. Chan CC, Hikjta N, Dastgheib K, Whitcup SM, Gery I, Nussenblatt RB. Experimental melanin-protein induced uveitis in the Lewis rat: Immunopathologic process. Ophthalmology 101:1275-1280, 1994.
7. Feldmann M, Brennan FM, Chantry D, Haworth C, Turner M., Abney E., Buchan G., Barrett K, Barkley D, Chu A.. Cytokine production in the rheumatoid joint: implications fro treatment. Annals
Rheumatic Diseases. 49:480-486, 1990.
8. Harigai M, Hara M, Kitani A, Morioka K., Hirose T., Hirose W., Suzuki K., Kawakami M., Masuda
K., Shinmei M. Interleukin I and tumor necrosis factor-α synergistically increase the production of
interleukin 6 in human synovial fibroblast. J. Clin. Lab. Immunol. 34:107-113, 1991.
9. Mosmann TR, Coffman RJ. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173, 1989.
10. Clerici M, Sheater GM. A Th1 to Th2 switch is a critical step in the etiology of HIV infection.
Immunol. Today 14:107-111, 1993.
11. Murray HW, Spitalny GL, Nathan CF. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon- gamma. J. Immunol. 134: 1619-1622, 1985.
12. Fujioka T, Jimi T, Hilliard BA, Ventura ES, Rostami A. The expression of cytokine mRNA in the cauda equina of Lewis rats with experimental allergic neuritis. J. Neuroimmunol. 84: 223-229, 1998.
13. Nakaura S, Sugita M, Tanaka SI, Ohno S. Ennanced production of in vitro tumor necrosis factor-alpha in Behcet’s disease. Nippon Ganka Gakkai Zasshi. 96: 1282-1285, 1992.
14. Doherty GM, Lange JR, Langstein HN, Alexander HR, Buresh CM, Norton JA. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J. Immunol. 149: 1666-1670, 1992.
15. Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J. Clin. Invest. 107:7-11, 2001.
16. Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappa B, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 45:7-17, 1999.
17. Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu. Rev.
Immunol. 12:141-179, 1994.
18. Liu SF, Ye X, Malik AB. Pyrrolidine dithiocarbamate prevent I-kB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol .Pharmacol. 55: 658-667, 1999.
19. Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA.. Dithiocarbamates as potent inhibitors of nuclear factor KB activation in intact cells. J. Exp.Med. 175:1181-1194, 1992.
20. Ohta K, Nakayama K, Kurokawa T, Kikuchi T., Yoshimura N.. Inhibitory effects of Pyrrolidine Dithiocarbamate on endotoxin-induced uveitis in Lewis rats. Invest. Ophthalmol. Vis. Sci. 43:744-775, 2002.
21. Furlan R, Villa P, Senaldi G., Martino G.. TNF alpha in experimental diseases of the CNS. Methods
Mol. Med. 98:171-190, 2004.
22. Woon MD, Kaplan HJ, Bora NS. Kinetics of cytokine production in experimental autoimmune anterior uveitis. Curr. Eye Res. 17: 955-961, 1998.
23. Yokota S, Geppert TD, Lipsky PE. Enhancement of antigen- and mitogen-induced human T lymphocyte proliferation by tumor necrosis factor- alpha. J. Immunol. 140:531-536, 1988.
24. Carlos TM, Schwartz BR, Kovach NL, Yee E, Rosa M., Osborn L, Chi-Rosso G., Newman B, Lobb R., Rosso M.. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to
cytokine-activated cultured human endothelial cells. Blood 76:965-970, 1990.
25. Salyer JL, Bohnsack JF, Knape WA, Shigeoka AO, Ashwood ER, Hill HR. Mechanisms of tumor necrosis factor-alpha alternation of PMN adhesion and migration. Am. J. Pathol. 136:831-841, 1990.
26. Bancroft GJ, Kelly JP, Kaye PM, McDonalk V, Cross CE. Pathways of macrophages activation and innate immunity. Immunol. Lett. 43:67-70, 1994.
27. Young HA, Hardy KJ. Role of interferon-gamma in immune cell regulation. J. Leukocyte Boil. 58:373-381, 1995.
28. Ohta K, Yamagami S, Taylor AW, Streilein W. IL-6 antagonizes TGF-β and abolishes immune
privilege in eyes with endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 41:2591-2599, 2000.
29. Moore KW, O’garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu. Rev.
Immunol. 11:165-190, 1993.
30. Nemeth ZH, Hasko G, Vizi ES. Pyrrolidine Dithiocarbamate Augments IL-10, inhibits TNF-α,
MIP-1α IL-12 and nitric oxide production and protects from the lethal effect of endotoxin. Shock
10:49-53, 1998.
31. Collart MA, Baeuerle PA, Vassalli P. Regulation of tumor necrosis factor alpha transcription in
macrophages: involvement of four κB-like motifs and inducible forms of NF-κB. Mol Cell Biol
10:1498-1506, 1990.
32. Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human gene. J. Immunol. 148: 3618-3623, 1992.
33. Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of
NF-kappa B and AP-1 in intact cells. EMBO J. 12: 2005-2015, 1993.
34. Pinkus R, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of AP-1,
NF-kappa B, and glutathione S-transferase gene expression. J. Biol. Chem. 271:13422-13429, 1996.
35. Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest. Ophthalmol. Vis. Sci. 32:2201-2211, 1991.
36. Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-β in human aqueous
humor. Curr. Eye Res. 9:963-969, 1990.
38. Murray PI, Clay CD, Mappi C, Salmon M. Molecular analysis of resolving immune response in uveitis. Clin. Exp. Immunol. 117:455-461, 1999.
39. Wakefield LM, Smith DM, Masui T, Harris CC, Sporn MB. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J. Cell Biol. 105:965-975, 1987.
40. Liu SF, Ye X, Malik AB. Inhibition of NF-κB activation by pyrrolidine Dithiocarbamate prevents in
vivo expression of proinflammatory genes. Circulation 100:1330-1337, 1999.
41. Tamada S, Nakatani T, Asai T, Tashiro K, Komiya T, Sumi T, Okamura M. Inhibition of NF-κB
activation by pyrrolidine dithiocarbamate prevents chronic FK506 nephropathy. Kidney Int. 63:306-314, 2003.