T
he gold estuarine anchovy Stolephorusinsularis (Hardenberg 1933) is a small pelagic
fish with a short lifespan that feeds mainly on crustaceans, particularly copepods (Milton et al. 1990). It is widely distributed in tropical coastal waters of the Indo-Pacific to western Pacific Oceans between 27°N and 8°S. This anchovy is generally caught as bait for commercial fisheries. It is also abundant in the coastal waters of Taiwan and around the Penghu Archipelago (the Pescadores) in the Taiwan Strait, where it is a target species of small-fish fisheries in Taiwan
Hatching Period and Early-Stage Growth Rate of the Gold Estuarine
Anchovy Stolephorus insularis in Taiwan as Inferred from Otolith Daily
Growth Increments
Wann-Nian Tzeng1,2,*, Han Chu2, Kang-Ning Shen2, and Yu-Tzu Wang3
1Department of Life Science, College of Life Science, National Taiwan University, Taipei 106, Taiwan 2Institute of Fisheries Science, College of Life Science, National Taiwan University, Taipei 106, Taiwan 3Fisheries Research Institute, Council of Agriculture, Keelung 202, Taiwan
(Accepted February 20, 2008)
Wann-Nian Tzeng, Han Chu, Kang-Ning Shen, and Yu-Tzu Wang (2008) Hatching period and early-stage growth rate of the gold estuarine anchovy Stolephorus insularis in Taiwan as inferred from otolith daily growth increments. Zoological Studies 47(5): 544-554. To understand the reproductive strategies and recruitment dynamics of the gold estuarine anchovy Stolephorus insularis in a subtropical area, their seasonal abundance was investigated, and their hatching period and growth rate were estimated from otolith daily growth increments. Specimens were collected from the Tatu River estuary on the west-central coast of Taiwan during 1997-1998. Juvenile fish dominated the catch composition, suggesting that the estuary is a nursery for newly recruited juveniles of S. insularis. The distribution of hatching dates for anchovy in subtropical Taiwan was similar to that of temperate zone fish, with a major spawning season in spring and a minor spawning season in autumn. This spawning strategy coincided with a new production cycle that was higher in spring than in autumn. Mean standard lengths and ages of the anchovy at recruitment were significantly larger for the autumn than for the spring and summer cohorts (all p < 0.001), while the somatic and otolith growth rates were higher in spring than in summer and autumn (all p < 0.001). Larval anchovy grew faster and reached their maximum growth rate (inflexion point of the growth curve) earlier during summer than during either spring or autumn. The growth rate after the inflexion point was lower in autumn than in either spring or summer. The reproductive and growth rate variability indicated that the spring cohort is the major component of the stock and that the autumn cohort is a minor supplement to overall recruitment. http://zoolstud.sinica.edu.tw/Journals/47.5/544.pdf
Key words: Stolephorus insularis, Otolith, Spawning season, Growth rate, Production cycle.
(Tzeng and Wang 1992, Wang and Tzeng 1997, Tzeng et al. 2002, Chen and Chiu 2003). It also constitutes the dominant component of the larval and juvenile communities in estuaries.
The life cycle of this species is poorly known. It may spawn offshore, with the post-larvae and juveniles migrating to and staying in estuaries until the young stage. The seasonal recruitment of larvae and juveniles in estuaries depends on its spawning regime, which has evolved to adapt to seasonal changes in productivity. Tropical fish are generally characterized by a protracted spawning
* To whom correspondence and reprint requests should be addressed. Tel: 886-2-33662887. Fax: 886-2-23639570. E-mail:wnt@ccms.ntu.edu.tw
season, an adaptation to the continually low productivity in tropical waters, whereas temperate-zone fish usually spawn in 2 seasonal peaks that typically follow the cycle of seasonal production, with a peak of new production in spring and a minor peak of recycled production in autumn (Cushing 1975). The Tropic of Cancer (23°30,N), a boundary between temperate and tropical zones, passes through central Taiwan, and it is not clear whether S. insularis in the estuaries of Taiwan follows a temperate or tropical spawning pattern. Larvae and juveniles of S. insularis are abundantly recruited to the estuaries of Taiwan with a major peak in abundance in spring and a minor peak in autumn (Tzeng et al. 2002). This implies that S. insularis in Taiwan is a temperate-, rather than a tropical-type, spawner. However, this has not been validated by further scientific study. The link between the spawning season and production cycle can accelerate larval growth and reduce the risk of predation thereby increasing the survival rate (match and mismatch hypothesis) (Cushing 1975 1982, Sinclair and Tremblay 1984). The match or mismatch between the timing of production and spawning is a key factor determining the growth rate of the fish in early life and subsequently the year-class strength of the fish stock (Hjort 1914, Cushing 1975, Smith 1985). Understanding the spawning period and growth patterns of the early stages of fish development is very important for analyzing fish recruitment and population dynamics.
Since Pannella (1971) discovered the daily growth increment in otoliths, it has been widely used to determine larval ages and to back-calculate birthdates and spawning regimes of fish (e.g., Townsend and Graham 1981, Methot 1983, Tzeng 1990, Secor et al. 1992, Wang and Tzeng 1999, Wang and Tzeng 2000). Larval growth rate variability among different spawning seasons can also be elucidated by comparing the otolith daily growth rates and lengths-at-age among seasonal cohorts (Crecco and Savoy 1985, Al-Hossaini et al. 1989, Thorrold and Williams 1989, Rutherford and Houde 1995, Wang and Tzeng 2000).
In this study, we attempted to elucidate the hatching period and growth strategies of S.
insularis in subtropical Taiwan. The hatching
date and early-stage growth rate of the fish were determined from the daily growth increment pattern of otoliths of fish collected in an estuary on the west-central coast of Taiwan during different seasons. The link between seasonal spawning timing and production cycles was also addressed.
MATERIALS AND METHODS
Specimens of S. insularis were collected using an anchored bag-net from the Tatu River estuary on the west-central coast of Taiwan (Fig. 1). The net was set against the tidal current during the nocturnal flood of the spring tide during the new moon from Nov. 1997 to Dec. 1998. The sampling location, procedures, and fishing gear were similar to those described in a study by Tzeng et al. (2002). Surface water temperature and salinity were measured with a microprocessor conductivity meter during sampling. Species were identified following Leis and Rennis (1983), Ozawa (1986), Wang (1987), Okiyama (1988), and Leis and Trnski (1989). In total, 1518 larval and juvenile S.
insularis were collected. The development of the
fish was classified into 4 stages following Leis and Rennis (1983) and Wang (1987): flexion larva (Fl), postflexion larva (Po), juvenile (Ju), and young (Yo). Standard length (SL) was measured to the nearest 0.1 mm.
Sagittal otoliths, the largest of 3 pairs of otoliths, were removed from a fish's head with a sharpened needle, rinsed with distilled water, air-dried, and stored in plastic bottles. Right otoliths were embedded in epofix resin, ground, and polished along the sagittal plane until the primordia were exposed. The polished otoliths were then etched with 10% ethylenediaminetetraacetic acid (EDTA) to enhance the daily growth increments (DGIs). DGIs were counted with the aid of an image processing system, and the radii and DGI width of the otoliths were measured from primordium to rostrum with the aid of SigmaScan Pro 5 software (SPSS science, Chicago, USA) to calculate the otolith growth rate of the fish. Daily ages of fish were directly estimated from DGI counts without adjusting for yolk sac duration, because DGIs were assumed to be deposited daily after hatching (Gjoesaeter et al. 1984, Thorrold and Williams 1989, Hoedt 2002). One study indicated a 1 d delay in the appearance of the 1st increment after hatching in other clupeoids (Hayashi et al. 1989). The hatching (birth) date of the fish was back-calculated from DGIs and the date of capture. Specimens were classified into spring (Mar.-May), summer (June-Aug.), and autumn (Sept.-Nov.) cohorts based on the back-calculated hatching dates.
The relationship between standard length and otolith diameter was fitted by an allometric growth equation, y = axb, and the somatic growth equation of the fish was fitted with length-at-age data by the
exponential equation, y = aeb∙age. The significance of differences in growth equations among cohorts was tested by analysis of covariance (ANCOVA) after logarithmic transformation to fit the normal distribution hypothesis. The difference in increment widths of otoliths among cohorts was tested with repeated-measures analysis of variance (ANOVA). Differences in the transition rate of development between stages among cohorts were tested by a non-parametric ANOVA.
RESULTS Water temperature and salinity
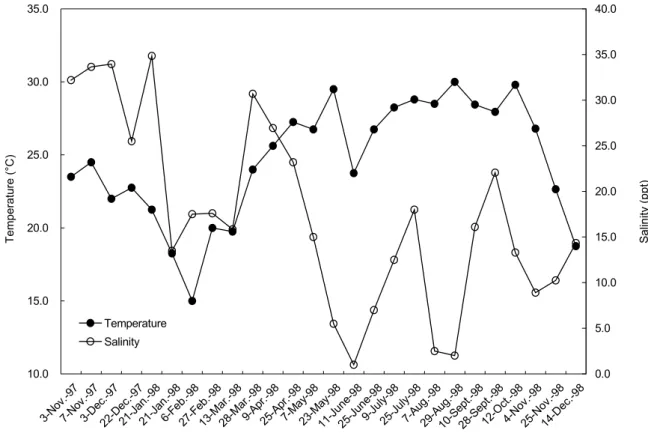
The water surface temperature in the Tatu River estuary on the west-central coast of Taiwan changes seasonally, with a minimum of approximately 15°C in Feb. when the north-eastern monsoon prevails, and a maximum of approximately 30°C in Aug. when the southwestern monsoon prevails. It increased from 15°C in
Feb. to 29.5°C in May, and remained at a high level until early Oct. when it decreased (Fig. 2). The difference in water temperature between the coldest and warmest months was approximately 15°C. The seasonal temperature regime indicated that the study area has a more-temperate than tropical climate.
Seasonal changes in surface salinity in the estuary were irregular, ranging from 1.0 ppt in June to 34.9 ppt in Jan. (Fig. 2).
Developmental stage composition and seasonal abundances
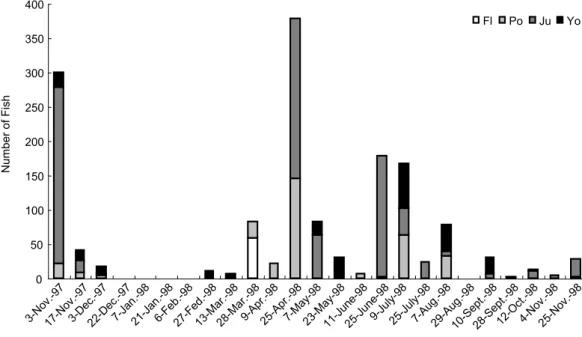
Juvenile S. insularis dominated the compo-sition of specimens collected in the Tatu River estuary and accounted for approximately 60% of the total catch, followed by postflexion larvae and young stages, which accounted for approximately 20% each. Flexion larvae accounted for approximately 2% (Fig. 3).
The seasonal abundance by stage indicated that S. insularis was recruited to the estuary mainly
Fig. 1. Location of the sampling site in the Tatu River estuary (white arrow).
N 120° 121° 122°E 25° 24° 23° 22°N 5 km TAIWAN Taiwan Strait CHINA Changhua City Taichung City Tatu River Taiwan Strait 0 30 60 km
10.0 15.0 20.0 25.0 30.0 35.0 3-Nov .-97 7-Nov .-97 3-Dec .-97 22-D ec.-97 21-Ja n.-98 21-Ja n.-98 6-Feb .-98 27-Fe b.-98 13-Mar.-9828-Mar.-989-A
pr.-98 25-A pr.-98 7-May -98 23-M ay-98
11-June-9825-June-989-July-9825-July-987-A ug.-98 29-A ug.-98 10-S ept.-9 8 28-S ept.-9 8
12-Oct.-984-Nov.-9825-Nov.-9814-Dec.-98
0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 Temperature Salinity Sampling date Temperature (°C) Salinity (p pt)
Fig. 2. Seasonal changes in surface water temperature and salinity in the Tatu River estuary.
0% 10% 20% 30% 40% 50% 60% 70% o Y u J o P l F Developmental stage Fr eq ue nc y N = 1471
Fig. 3. Stage composition of larvae and juveniles of Stolephorus insularis collected in the Tatu River estuary. Fl: flexion larva; Po: postflexion larva; Ju: juvenile; Yo: young.
0 50 100 150 200 250 300 350 400 3-Nov .-97 17-N ov.-9 7 3-Dec .-97 22-D ec.-97 7-Jan .-98 21-Ja n.-98 6-Feb .-98
27-Fed.-9813-Mar.-9828-Mar.-989-Apr.-9825-A pr.-98 7-May -98 23-M ay-98 11-Ju ne-98 25-Ju ne-98 9-July -98 25-Ju ly-98 7-Aug.-9829-Aug .-98 10-Sept.-9828-S ept.-98 12-O ct.-98 4-Nov .-98 25-N ov.-9 8 Collecting date N um be r o f F is h Fl Po Ju Yo
Fig. 4. Monthly changes, by stage, of abundance in numbers of Stolephorus insularis. as juveniles, with peak abundances in spring (Apr.)
and early summer (June) and a minor peak in autumn (Nov.). A peak in abundance was found in Nov. 1997 but not in Nov. 1998 (Fig. 4).
Flexion and postflexion larvae occurred mainly in Nov., Mar.-Apr., and June-Aug. These timings indicate that the fish has protracted spawning behavior with a major spawning season from spring to early summer, a minor season in autumn, and no spawning in winter.
Comparison of mean age and length among cohorts
The standard-length frequency distributions of
S. insularis at recruitment to the estuary are shown
by stage and cohort in figure 5. Post hoc tests indicated that the size of the autumn cohort was significantly larger than the spring and summer cohorts, irrespective of the stage (postflexion, larval, juvenile, and young fish, all p < 0.001). Lengths did not significantly differ between spring and summer cohorts (p = 0.98, 0.20) except at the juvenile stage where the spring cohort was longer than the summer cohort (p < 0.001).
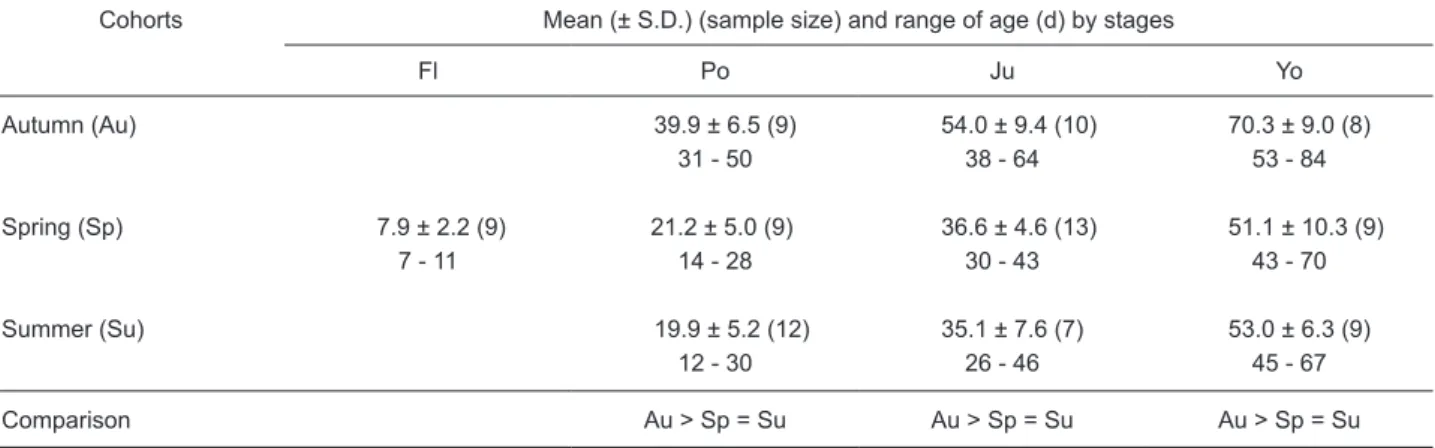
Similarly, the mean ages at recruitment were also significantly older in the autumn than in the spring and summer cohorts (p < 0.001-0.002), but did not significantly differ between the spring and summer cohorts (p = 0.47-0.96), irrespective of the
developmental stage (Table 1). The mean (± S.D.) ages of juveniles at recruitment to the estuary were 36.6 ± 4.6 d in spring and 35.1 ± 7.6 d in summer, but increased to 54.0 ± 9.4 d in autumn. This indicated that the age at recruitment to the estuary was delayed by approximately 18 d for the autumn cohort.
Relationship between otolith radius and standard length
Relationships between maximum otolith radius (R, μm) and standard length (L, mm) of
S. insularis were fitted by the allometric growth
equations by season as follows:
Autumn R = 2.4365L1.7296 (R2 = 0.89), Spring R = 0.8274L2.0648 (R2 = 0.98), and Summer R = 0.5511L2.1895 (R2 = 0.96).
ANCOVA indicated that there were no significant differences in either slope or adjusted mean among the equations (p = 0.7528). Accordingly, the relationship between otolith radius and standard length of S. insularis was calculated from pooled data as follows (Fig. 6):
R = 0.8416L2.0567 (R2 = 0.97).
Comparison of somatic growth among cohorts
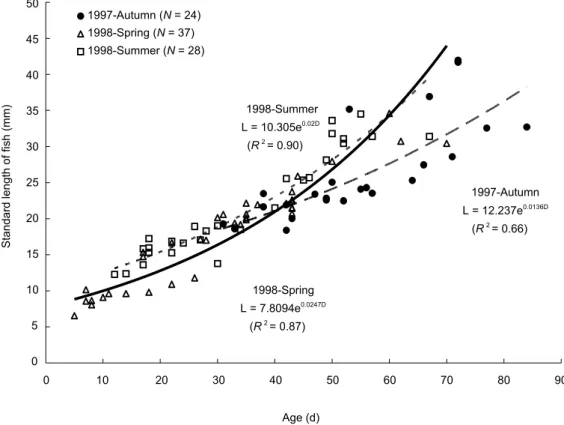
Growth curves of S. insularis were fitted by exponential equations as follows (Fig. 7):
Spring R = 7.8094e0.0247D (R2 = 0.87), Summer R = 10.305e0.020D (R2 = 0.90), and Autumn R = 12.237e0.0136D (R2 = 0.66);
where R and D are otolith radius (μm) and age (d), respectively.
ANCOVA indicated that the slope (growth rate) of the equation significantly differed among cohorts (p < 0.0001). Post hoc tests further
indicated that growth rates in decreasing order were spring > summer > autumn (all p < 0.001).
Differences in otolith daily growth patterns among cohorts
Patterns of daily growth increments in otoliths were compared among 3 selected young S.
5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50 Young Juvenile Postflexion Autumn 35 30 25 20 15 10 5 0 Spring Summer Fr eq ue nc y (% ) Fr eq ue nc y (% ) Fr eq ue nc y (% ) N = 34 17.9 ± 3.9 mm N = 190 13.6 ± 2.2 mm N = 86 13.5 ± 2.7 mm N = 279 23.9 ± 1.8 mm N = 473 21.6 ± 3.4 mm N = 77 19 ± 1.5 mm N = 64 33.1 ± 6.5 mm N = 50 24.8 ± 3.3 mm N = 62 26.9 ± 4.3 mm
Standard length of fish (mm)
35 30 25 20 15 10 5 0 35 30 25 20 15 10 5 0
Fig. 5. Standard-length frequency distributions of larval Stolephorus insularis by stage and cohort. Spring cohort, Mar.-May; summer cohort, June-Aug.; autumn cohort, Sept.- Nov.
Table 1. Comparison, among cohorts, of mean age by stage for Stolephorus insularis. Fl: flexion larva, Po:
postflexion larva, Ju: juvenile, Yo: young
Cohorts Mean (± S.D.) (sample size) and range of age (d) by stages
Fl Po Ju Yo Autumn (Au) 39.9 ± 6.5 (9) 54.0 ± 9.4 (10) 70.3 ± 9.0 (8) 31 - 50 38 - 64 53 - 84 Spring (Sp) 7.9 ± 2.2 (9) 21.2 ± 5.0 (9) 36.6 ± 4.6 (13) 51.1 ± 10.3 (9) 7 - 11 14 - 28 30 - 43 43 - 70 Summer (Su) 19.9 ± 5.2 (12) 35.1 ± 7.6 (7) 53.0 ± 6.3 (9) 12 - 30 26 - 46 45 - 67 Comparison Au > Sp = Su Au > Sp = Su Au > Sp = Su
Age (d) S ta nd ar d le ng th o f f is h (mm) 1997-Autumn L = 12.237e0.0136D (R2= 0.66) 1998-Summer L = 10.305e0.02D (R2= 0.90) 1998-Spring L = 7.8094e0.0247D (R2= 0.87) 0 5 10 15 20 25 30 35 40 45 50 0 10 20 30 40 50 60 70 80 90 1997-Autumn (N = 24) 1998-Spring (N = 37) 1998-Summer (N = 28)
Fig. 7. Somatic growth equations, by cohort, of Stolephorus insularis.
R = 0.8416L2.0567 R2 = 0.97 0 200 400 600 800 1000 1200 1400 1600 1800 2000 0 5 10 15 20 25 30 35 40 45
Standard length of fish (mm)
M ax im um ra di us o f o to lit h (μ m ) 1997-Autumn (N = 30) 1998-Spring (N = 43) 1998-Summer (N = 30)
insularis which represented each of the cohorts
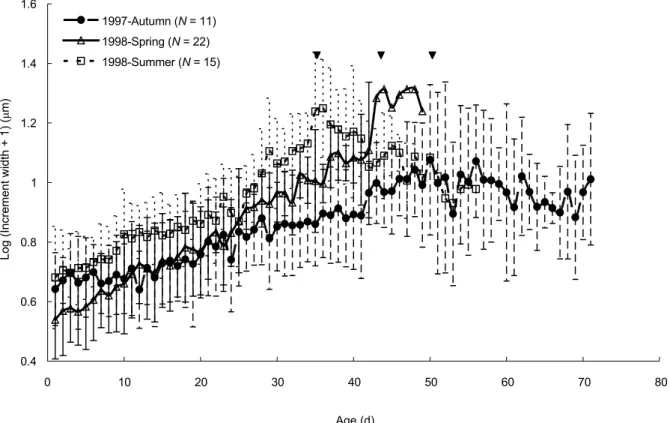
(Fig. 8). Results indicated that the increment width (otolith daily growth rate) and the timing at the transition of the otolith growth rate differed among cohorts. The increment width was narrower in the core than outer layer of the otolith in each of the 3 cohorts, while the timing of the growth transition from slower to faster was earlier in the summer (at approximately 28 d old) than in the spring (35 d old) and autumn cohorts (43 d old).
Repeated-measures ANOVA indicated that the otolith mean daily growth rates significantly differed among cohorts (p < 0.001). Non-parametric ANOVA further indicated that the timing at inflexion from positive to negative growth acceleration occurred fastest for the summer cohort (on average at approximately 35 d old), followed by the spring (44 d old) and autumn (50 d old) cohorts (Fig. 9).
DISCUSSION
The gold estuarine anchovy S. insularis is mainly recruited in the Tatu River estuary at the juvenile stage during the period from spring
0.4 0.6 0.8 1 1.2 1.4 1.6 0 10 20 30 40 50 60 70 80 1997-Autumn (N = 11) 1998-Spring (N = 22) 1998-Summer (N = 15) Age (d) Lo g (In cr em en t w id th + 1 ) ( μm)
Fig. 9. Comparison of temporal changes, among cohorts, in mean (± S.D.) otolith increment widths of Stolephorus insularis. Arrows indicate the timing at the transition of the growth rate.
Fig. 8. Patterns of daily growth increments in otoliths of 3 young Stolephorus insularis. (A) Autumn cohort, 28.93 mm standard length (SL) collected on 3 Nov. 1997; (B) spring cohort, 34.59 mm SL, 23 May 1998; (C) summer cohort, 30.44 mm SL, 7 Aug. 1998.
100 μm (A)
(B)
to early summer (Apr.-July) and autumn (Nov.) (Figs. 3, 4), at a mean age of 1 mo for the spring and summer cohorts and 1.5 mo for the autumn cohort (Table 1). Anchovy ages at recruitment and seasonal occurrences and abundances indicated that spawning occurred mainly from spring to early summer, with a minor spawning peak in autumn and no spawning in winter. On the other hand, the back-calculated hatching date distribution demonstrated that larger juveniles which appeared in early spring had spawned the previous autumn. These overwintering juveniles are the first ones discovered in Taiwan. The abundance of the juvenile stage relative to other stages (flexion and postflexion larvae and young) indicated that these fish were spawned offshore and not in the estuary. After hatching, the larvae passively disperse and are completely recruited to the estuary at the juvenile stage, and then leave the estuary at the young stage. The estuary obviously plays an important role as a nursery for juvenile anchovy, similar to other species (Raynie and Shaw 1994, MacGregor and Houde 1996).
Although the Tropic of Cancer passes through central Taiwan, the seasonal temperature variation in the Tatu River estuary in west-central Taiwan is more temperate than tropical (Fig. 2). The spawning behavior of S. insularis in the estuary followed Cushing’s match-mismatch theory (Cushing 1975), and coincides with the spring and autumn phytoplankton blooms in temperate areas (Lalli and Parsons 1993). Although primary productivity data in the study area are unavailable to validate the relationship between seasonal productivity and spawning behavior of the anchovy, seasonal changes in phytoplankton and zooplankton in the nearby Tanshui River estuary of northern Taiwan seem to reflect this phenomenon (Chern and Tzeng 1993). Water temperatures in shallow waters of the continental shelf of the Taiwan Strait dramatically change with the seasonal monsoon. The Tanshui and Tatu Rivers respectively on the north and west-central coasts of Taiwan are both influenced by the prevailing northeast monsoon in winter and southwest winds in summer, which produce seasonal changes in both temperature and coastal currents (Chu 1963, Tzeng et al. 2002). Such physical oceanographic processes may influence biological processes such as the seasonal spawning activity of S.
insularis, plankton production that provides food
for fish larvae, and the transport and retention of larvae in estuaries as well. Navaluna and Pauly (1986) found a relationship between monsoon
winds and the seasonal recruitment of stolephorid anchovies in the Philippines. Dalzell (1987) also found that the spawning of stolephorid anchovies followed seasonal changes in the hydrological environment driven by the seasonal monsoons, which subsequently influenced spring planktonic production.
Growth curves of both length-at-age and otolith daily growth increment width indicated that
S. insularis grew faster in spring/summer than in
autumn, and the mean fish ages indicated that the duration of vulnerability to predation mortality might be longer in autumn than in spring/summer (Table 1, Figs. 7-9). Differences in growth strategies among cohorts during the early life stages were evident. The bigger-is-better hypothesis states that juvenile fish that grow slowly are exposed to predation over a longer period and therefore have lower survival rates than faster-growing fish (Houde 1987, Miller et al. 1988, Rice et al. 1993, Cushing and Horwood 1994, Ottersen and Loeng 2000). Based on the otolith increment width, the summer cohort grew faster and reached the growth rate inflexion point earlier than did the spring and autumn cohorts. In addition, the growth rate after the inflexion point was lower in autumn than in the spring and summer cohorts. Also, somatic growth was slower and the durations of larval and juvenile stages were longer in autumn than in the spring and summer cohorts; thus, autumn-hatched larvae are expected to have lower survival rates. Small annual differences in growth or mortality rates in early stages can lead to large annual fluctuations in recruitment (Leggett et al. 1984, Houde 1987, Frank 1991, Fey 2001).
A peak recruitment occurred in Nov. 1997 but the recruitment failed in autumn 1998 (Fig. 4). This may have been due to interactions of physical and biological processes (Boehlert and Mundy 1988). The spring and summer cohorts of the fish experienced higher temperatures than the autumn cohorts (Fig. 2) and subsequently had higher growth rates (Figs. 7, 9), because higher temperatures can cause high production of prey items leading to higher growth and survival rates through the vulnerable larval and juvenile stages (Ottersen and Loeng 2000). Anderson (1988) also proposed that the survival of a cohort was directly related to the growth rate during the pre-recruitment period. The seasonal growth period of phytoplankton is shorter and the magnitude of productivity is lower in autumn than in spring and early summer (Chern and Tzeng 1993). This suggests that the autumn cohort is more
susceptible to recruitment failure, due to its mismatch with the production cycle, than is the spring or summer cohort. Alternatively, Nov.-Dec. salinities were lower in 1998 than in 1997 which may indicate that environmental conditions for the transport or retention of larvae and juveniles in the estuary were unfavorable in autumn 1998. The drastic decrease in salinity observed from Apr. to June was probably due to high river discharge as a result of typhoon-associated rains. The higher freshwater discharge in autumn 1998 might have prevented the fish from entering the estuary, either due to strong currents, or the inability of the fish to tolerate fresh water. Factors causing the recruitment failure observed in Nov. 1998 (Fig. 4) are presently unclear. That failure was probably due to simple biological processes during migration from the spawning ground to the nursery ground (member-vagrant hypothesis, Sinclair 1988), or due to a negative effect of unstable oceanographic conditions on larval growth and survival during the pre-recruitment period (ocean stability hypothesis, Lasker 1975 1978, Blaber and Blaber 1980, Anderson 1988, Ottersen and Loeng 2000). Dalzell (1987) also found 2 stolephorid anchovy species that have extreme variability in reproductive success and recruitment, with almost no spawning in some years. These uncertainties underline the need for more research on a long-term basis.
Acknowledgments: We acknowledge financial
support from the National Science Council of Taiwan (NSC92-2313-B-002-004 awarded to Prof. W.N. Tzeng). Thanks to C.W. Chang, S.H. Lin, and M.Y. Chang for specimen collection, Ms. H.Y. Teng for specimen sorting, and Mr. Brian M. Jessop and 2 anonymous reviewers for helpful comments on an early draft of the manuscript.
REFERENCES
Al-Hossaini M, Q Liu, TJ Pitcher. 1989. Otolith microstructure indicating growth and mortality among plaice,
Pleuronectes platessa L., post-larval subcohorts. J. Fish
Biol. 35(Supplement A): 81-90.
Anderson JT. 1988. A review of size dependent survival during pre-recurit stages of fishes in relation to recruitment. J. Northw. Atl. Fish. Sci. 8: 55-66.
Blaber SJM, TG Blaber 1980. Factors affecting the distribution of juvenile estuarine and inshore fish. J. Fish Biol. 17: 143-162.
Boehlert GW, BC Mundy. 1988. Roles of behaviour and physical factors in larval and juvenile recruitment to estuarine nursery areas. Am. Fish. Soc. Symp. 3: 51-67.
Chen CS, TS Chiu. 2003. Early life history traits of Japanese anchovy in the northeastern waters of Taiwan, with reference to larval transport. Zool. Stud. 42: 248-257. Chern YT, WN Tzeng. 1993. Feeding strategy of
Encrausicholina punctifer and Stolephorus insularis larvae
in the estuary of Tanshui River, Taiwan 3/4 I. Ontogenetic dietary shifts and morphological correlation. J. Fish. Soc. Taiwan 20: 313-328.
Chu TY. 1963. The oceanography of the surrounding waters of Taiwan. Taipei, Taiwan: Report of the Institute of Fishery Biology of the Ministry of Economic Affairs and National Taiwan Univ. Vol. 1, pp. 29-44.
Crecco VA, TF Savoy. 1985. Effects of biotic and abiotic factors on growth and relative survival of young American shad, Alosa sapidissima, in the Connecticut River. Can. J. Fish. Aquat. Sci. 42: 1640-1648.
Cushing DH. 1975. Marine ecology and fisheries. Cambridge, UK: Cambridge Univ. Press, 278 pp.
Cushing DH. 1982. Climate and fisheries. London, UK: Academic Press, 373 pp.
Cushing DH, JW Horwood. 1994. The growth and death of fish larvae. J. Plankton Res. 16: 291-300.
Dalzell P. 1987. Some aspects of the reproductive biology of stolephorid anchovies from northern Papua New Guinea. Asian Fish. Sci. 1: 86-91.
Fey DP. 2001. Differences in temperature conditions and somatic growth rate of larval and early juvenile spring-spawned herring from the Vistula Lagoon, Baltic Sea manifested in the otolith to fish size relationship. J. Fish Biol. 58: 1257-1273.
Frank KT. 1991. Predicting recruitment variation from year class specific vertebral counts: an analysis of the potential and the plan for verification. Can. J. Fish. Aquat. Sci. 48: 1350-1357.
Gjoesaeter J, P Dayaratne, OA Bergstad, H Gjoesaeter, MI Souza, IM Beck. 1984. Ageing tropical fish by growth rings in the otoliths. Rome, Italy: Food and Agricultural Organization, Fisheries Circular, No. 776, pp. 1-54. Hardenberg JDF. 1933. New Stolephorus species of the
Indo-Australian seas. Natuurkd. Tijdschr. Neder. Indië 93: 258-263.
Hayashi A, Y Yamashita, K Kawaguchi, T Ishii. 1989. Rearing method and daily otolith ring of Japanese sardine larvae. Nippon Suisan Gakkaishi 55: 997-1000.
Hjort J. 1914. Fluctuation in the great fisheries of northern Europe viewed in the light of biological research. Rapports Procès-Verbaux Réunions Conseil Int. l’ Exploration Mer Vol. 20, pp. 1-228.
Hoedt FE. 2002. Growth in eight species of tropical anchovy determined from primary otolith increments. Mar. Freshwater Res. 53: 859-867.
Houde ED. 1987. Fish early life dynamics and recruitment variability. Am. Fish. Soc. Symp. 2: 17-29.
Lalli CM, TR Parsons. 1993. Biological oceanography: an Introduction. New York: Pergamon Press, 301 pp. Lasker R. 1975. Field criteria for survival of anchovy larvae:
the relation between inshore chlorophyll maximum layers and successful first feeding. Fish. Bull. 73: 453-462. Lasker R. 1978. The relationship between oceanographic
conditions and larval anchovy food in the California Current: identification of factors contributing to recruitment failure. Rapports Procès-Verbaux des Réunions Conseil Int. l’Exploration Mer Vol. 173, pp. 212-230.
and hydrographical regulation of year-class strength in capelin (Mallotus villosus). Can. J. Fish. Aquat. Sci. 41: 1191-1201.
Leis JM, DS Rennis. 1983. The larvae of Indo-Pacific coral reef fishes. Kensington, Australia: New South Wales Univ. Press, 269 pp.
Leis JM, T Trnski. 1989. The Larvae of Indo-Pacific Shore Fishes. Hawaii University Press, Honolulu.
MacGregor JM, ED Houde. 1996. Onshore-offshore pattern and variability in distribution and abundance of bay anchovy Anchoa mitchilli eggs and larvae in Chesapeake Bay. Mar. Ecol.-Prog. Ser. 138: 15-25.
Methot RD. 1983. Seasonal variation in survival of larval
Engraulis mordax estimated from the age distributions of
juveniles. Fish. Bull. 81: 741-750.
Miller TJ, LB Crowder, JA Rice, EA Marschall. 1988. Larval size and recruitment mechanisms in fishes: towards a conceptual framework. Can. J. Fish. Aquat. Sci. 45: 1657-1670.
Milton DA, SJM Blaber, NJF Rawlinson. 1990. Diet and prey selection of six species of tuna baitfish in three coral reef lagoons in the Solomon Islands. J. Fish Biol. 37: 205-244.
Navaluna NA, D Pauly. 1986. Seasonality in the recruitment of Philippine fishes as related to monsoon wind patterns. Workshop on Recruitment in Tropical Demersal Communities, 21-25 Apr. 1986, Campeche, Mexico. Paris, France: Intergovernmental Oceanographic Commission/Food and Agricultural Organization, pp. 167-180.
Okiyama M. 1988. An Atlas of the Early Stage Fishes in Japan. Tokai University Press, Tokyo.
Ottersen G, H Loeng. 2000. Covariability in early growth and year-class strength of Barents Sea cod, haddock, and herring: the environmental link. J. Mar. Sci. 57: 339-348. Ozawa T. 1986. Studies on the Oceanic Ichthyoplankton in
the Western North Pacific. Kyushu University Press, Fukuoka, Japan.
Pannella G. 1971. Fish otoliths: daily growth layers and periodical patterns. Science 173: 1124-1127.
Raynie RC, RF Shaw. 1994. Ichthyoplankton abundance along a recruitment corridor from offshore spawning to estuarine nursery ground. Estuar. Coast. Shelf S. 39: 421-450. Rice JA, TJ Miller, KA Rose, LB Crowder, EA Marschall, AS
Trebitz, DL DeAngelis. 1993. Growth rate variation and larval survival: interfaces from an individual-based size-dependent predation model. Can. J. Fish. Aquat. Sci. 50: 133-142.
Rutherford ES, ED Houde. 1995. The influence of temperature
on cohort-specific growth, survival, and recruitment of striped bass, Morone saxatilis, larvae in Chesapeake Bay. Fish. Bull. 93: 315-332.
Secor DH, JM Dean, EH Laban. 1992. Otolith removal and preparation for microstructure examination. Can. Spec. Publ. Fish. Aquat. Sci. 117: 19-57.
Sinclair M, MJ Tremblay. 1984. Timing of spawning of Atlantic herring (Clupea harengus harengus) populations and the match-mismatch theory. Can. J. Fish. Aquat. Sci. 41: 1055-1065.
Smith PE. 1985. Year-class strength and survival of 0-group clupeoids. Can. J. Fish. Aquat. Sci. 42: 69-82.
Thorrold SR, DMcB Williams. 1989. Analysis of otolith microstructure to determine growth histories in larval cohorts of a tropical herring (Herklotsichthys castelnaui). Can. J. Fish. Aquat. Sci. 46: 1615-1624.
Townsend DW, JJ Graham. 1981. Growth and age structure of larval Atlantic herring, Clupea harengus harengus, in the Sheepscot River Estuary, Maine, as determined by daily growth increments in otoliths. Fish. Bull. 79: 123-130. Tzeng WN. 1990. Relationship between growth rate and age
at recruitment of Anguilla japonica elvers in a Taiwan estuary as inferred from otolith growth increments. Mar. Biol. 107: 75-81.
Tzeng WN, YT Wang. 1992. Structure, composition and seasonal dynamics of the larval and juvenile fish community in the mangrove estuary of Tanshui River, Taiwan. Mar. Biol. 113: 481-490.
Tzeng WN, YT Wang, CW Chang. 2002. Spatial and temporal variations of estuarine larval fish community on the west coast of Taiwan. Mar. Freshwater Res. 53: 419-430. Wang CH, WN Tzeng. 2000. The timing of metamorphosis and
growth rates of American and European eel leptocephali – a mechanism of larval segregative migration. Fish. Res. 46: 191-205.
Wang YT. 1987. Studies on the eggs, larvae and juveniles of fishes in the estuary of Tansui and Shuang-hsi Rivers, northern Taiwan. MSc Thesis, Institute of Marine Science, Chinese Culture Univ., 306 pp. (in Chinese with English abstract)
Wang YT, WN Tzeng. 1997. Temporal succession and spatial segregation of clupeoid larvae in the coastal waters off the Tanshui River Estuary, northern Taiwan. Mar. Biol. 129: 23-32.
Wang YT, WN Tzeng. 1999. Differences in growth rates among cohorts of Encrasicholina puntifer and Engraulis
japonicus larvae in the coastal waters off Tanshui River
Estuary, Taiwan, as indicated by otolith microstructure analysis. J. Fish Biol. 54: 1002-1016.