Plant Growth Regulation 16: 249-252.1995.
@ 1995 Kluwer Academic Publishers. Printed in the Netherlands.
249
Glutathione reduces the inhibition of rice seedling root growth caused by
cadmium
Sheng Lee Chen & Ching Huei Kao*
Department of Agronomy, National Taiwan University, Taipei, Taiwan, Republic of China; * Author for correspondence
Received 18 July 1994; accepted 29 July 1994
Key words: cadmium, glutathion, Oryza sativa L., root growth
Abstract
We investigated the effects of agents known to affect cellular glutathione (reduced form, GSH) levels on the growth of rice seedlings treated with Cd. CdClz was more effective than CdS04 in inhibiting root growth. However, CdCl2 had no effect on shoot growth. GSH, a substrate for phytochelatin synthesis, was effective in counteracting growth inhibition of roots by CdC12. Root growth in the CdCl2 medium was found also to be enhanced by the addition of L-glutamic acid and L-cysteine, both of which are substrates for GSH formation. Buthionine sulfoximine, an
inhibitor of GSH synthesis, rendered the roots susceptible to growth inhibition by Cd. Our results suggest that GSH level may play a role in regulating Cd-inhibited growth of rice roots.
Abbreviations: BSO = buthionine sulfoximine; GSH = reduced form glutathione
1. Introduction 2. Materials and methods
Cd is one of the most toxic among the heavy metals. It is supplied to soil, air and water mainly by efflu- ent from industries, mining, burning and leakage of waste, and by fertilization with phosphates and sewage sludge. In Taiwan, Cd poses a serious problem for rice production. Cd interferes with seedling growth [ 1, 81. Plant cells subjected to Cd rapidly synthesize phy- tochelatins, i.e. Cd-binding peptides, whose function is to sequester and to detoxify excess Cd ions [lo, 141. Failure to synthesize these peptides results in growth inhibition and cell death [lo, 141. Phytochelatins are synthesized from GSH [4,13]. It has been reported that BSO, an inhibitor of GSH biosynthesis [3], caused no reduction of growth of tobacco cells in the absence of Cd, but growth was greatly reduced in cultures exposed to BSO and Cd [l 11. Hence it is of interest to study the effects of agents known to affect cellular GSH levels on the Cd-inhibited root growth of rice seedlings.
Rice (Oryza sativa L. cv. Taichung Native 1) seeds were sterilized with 2.5% sodium hypochlorite for 15 min and washed thoroughly with distilled water. These seeds were then germinated in Petri dish (20 cm) con- taining distilled water at 37 ‘C under dark condition. After l-day incubation, uniformly germinated seeds were selected and transferred to Petri dishes (9.0 cm) containing two sheets of Whatman No. 1 filter paper moistened with 10 ml of distilled water or test solu- tions. Each Petri dish contained 20 germinated seeds. Each treatment was replicated 4 times. The germinated seeds were allowed to grow at 27 ‘C in darkness and 3 ml of distilled water or test solutions was added to each Petri dish on day 3 of growth. Length and fresh weight of roots and shoots were measured after 5 days in darkness. All experiments described here were repeated three times. Similar results of identical trends were obtained each time. The data reported here are from a single experiment.
250 80 60 E E St 40 5 f20 c 20 m ;16 . 12 E .- ,“8 z f 4 al Ii 0
I
OShoot \ .Root 0 0.1 0.2 0.3 CdC12 , mMFig. I. Effects of CdC12 on the growth of rice seedlings. Seedling growth was measured after 5 days of treatment. Vertical bars repre- sent standard errors.
3. Results and discussion
Figure 1 shows the effect of CdCl:! on the growth of rice seedlings. CdC12 significantly reduced root growth, as judged by root length and root fresh weight, of rice seedlings. Increasing concentrations of CdC12 from 0.1 to 0.3 mM progressively decreased root growth. However, no reduction of shoot growth by CdCl2 was observed. The differential effect of Cd on root and shoot growth could be accounted for by the fact that Cd is accumulated mainly in roots and to a minor extent in shoots [5,6, 151. Our results indicate that the primary effect of Cd is to take place in roots of rice seedlings. When the effect of CdS04 on root growth of rice seedlings was compared with that of CdCl2, it was found that CdS04 was less effective in reduc- ing root growth (Fig. 2). Sulphate has been shown
80 ES0 s-4 0 F 520 0 I I I I 0 0.1 0.2 0.3 mM
Fig. 2. Effects of CdCl2 and CdS04 on root growth of rice seedlings. Root growth was measured after 5 days of treatment. Vertical bars represent standard errors.
5 12 c -Cd2+ + Cd2+
0 0 0.5 1 2 3
GSH 9 mM
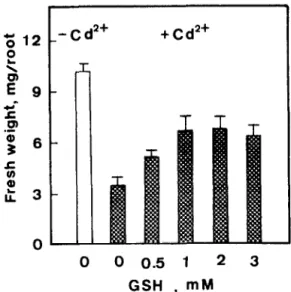
Fig. 3. Effects of GSH on root growth of rice seedlings in the presence of CdC12 (Cd ‘+, 0.3 mM). Root growth was measured after 5 days of treatment. Vertical bars represent standard errors.
251
-Cd2+
r
0 0 0.5 1.0 5.0 10 L-Cysteine , mM
Fig. 4. Effects of L-cysteine on root growth of rice seedlings in
the presence of CdClz (Cd2+, 0.3 mM). Root growth was measured after 5 days of treatment. Vertical bars represent standard errors.
+Cd2+
0 0 1.0 2.0 3.0 5.0 L-Glutamic acid, mM
Fig. 5. Effects of L-glutamic acid on root growth of rice seedlings in the presence of CdC12 (Cd’+, 0.3 rnhf). Root growth was measured after 5 days of treatment. Vertical bars represent standard errors.
to induce accumulation of GSH [2]. If high levels of GSH play an important role in regulating Cd-induced growth inhibition of rice roots, then the growth of roots in CdC12 is expected to be enhanced by adding GSH. As indicated in Fig. 3, root growth was indeed significantly improved by GSH in the CdC12 treat-
$j 8.0 t if 6.0 m E -p 4.0 s L & 2.0 0 0 0 0.1 0.5 1 .o BSO , mM
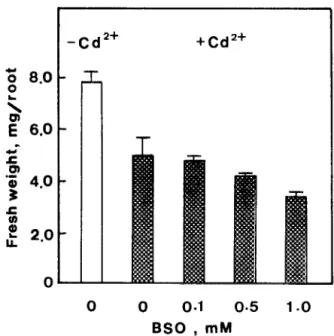
Fig. 6. Effect of BSO on root growth of rice seedling in the presence of CdC12 (Cd’+, 0.1 mM). Root growth was measured after 5 days of treatment. Vertical bars represent standard errors.
ment. Additional experiments were conducted to see if glycine, L-glutamic acid and L-cysteine, all of which are substrates for GSH synthesis [ 121, can also reduce growth inhibition by CdCla. Root growth in the CdCla medium was indeed enhanced by the addition of L- glutamic acid or L-cysteine (Figs. 4 and 5). L-Cysteine is even more effective than L-glutamic acid in counter- acting growth inhibition by CdClz. However, glycine had no effect on Cd-inhibited root growth (data not shown), suggesting that the glycine level is sufficient for GSH synthesis in Cd-treated roots.
Since D-cysteine is ineffective in counteracting growth inhibition of roots by CdC12 (data not shown), the observed positive effect of L-cysteine on the recovery of root growth in the presence CdCla is unlikely to be due to binding of Cd with the cysteine thiol, leading to a reduced level of Cd in the medium.
To further confirm the beneficial effect of GSH on root growth of rice seedlings in CdC12 medium, BSO, an inhibitor of GSH synthesis, was used to test its effect on root growth of rice seedlings in the presence of low concentration (0.1 mM) of CdC12. As indicated inFig. 6, BSO rendered the roots susceptible to growth inhibition by Cd.
Taking all data into account, we conclude that GSH levels in roots may play an important role in regulating Cd-inhibitedroot growth of rice seedling. The effect of
252
GSH on Cd-inhibited growth of rice roots is unlikely to be due to its characteristic of being an antioxidant, since ascorbic acid was found to be ineffective in coun- teracting Cd-inhibited root growth (data not shown). It has been shown that GSH stimulated the accumulation of phytochelatins in Cd treated cells [7]. It seems most likely that the positive effect of GSH on root growth is mediated through the synthesis of phytochelatins, which leads to a reduced level of free Cd ions in root cells. Clearly a deeper knowledge of GSH-induced phytochelatin accumulation in rice roots is necessary for a better understanding of Cd-inhibited growth of rice roots.
References
Bishnoi NR, Sheoran IS and Singh R (1993) Effect of cadmium and nickel on mobilisation of food reserves and activities of hydrolytic enzymes in germinating pigeon pea seeds. Biol Plant 35: 583-589
de Kok LJ, de Kan PJL, Tanczos OG and Kuiper PJC ( 198 1) Sulphate induced accumulation of glutathione and frost- tolerance of spinach leaf tissue. Physiol Plant 53: 435-438 Griffith OW and Meister A (1979) Potent and specific inhi- bition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254: 7558-7560
Grill E, Loffler S, Winnaker E-L and Zonk MH (1989) Phy- tochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific y-glutamycysteine
5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15.
dipeptidyl tmnspeptidase (phytochelatin synthase). Proc Nat1 Acad Sci USA 86: 6838-6842
Jarvis SC and Jones LHP (1978) Uptake and transport of cad- mium by perennial ryegrass from flowing solution culture with a constant concentration of cadmium. Plant Soil 49: 333-342 Jarvis SC, Jones LHP and Hopper MJ (1976) Cadmium uptake from solution by plants and its transport from roots to shoots. Plant Soil 44: 179-191
Mendum ML, Gupta SC and Goldsbrough PB (1990) Effect of glutathione on phytochelatin synthesis in tomato cells. Plant Physio193: 484-488.
Mrozek El (1980) Effect of mercury and cadmium on germi- nation of Sprtina alternif7ora Loisel seeds at various salinities. Environ Exp Bot 20: 367-377
Orzech KA and Burke JJ (1988) Heat shock and the protection against metal toxicity in wheat leaves. Plant Cell Environ 11: 711-714
Rauser WE (1990) Phytochelatins. Annu Rev Biochem 59: 61-86
Reese RN and Wagner GJ (1987) Effects of buthionine sul- foximine on Cd-binding peptide levels in suspension-cultured tobacco cells treated with Cd, Zn, or Cu. Plant physiol 84: 574577
Rennenberg H (1982) Glutathione metabolism and possible biological roles in higher plants. Phytochemistry 2 1: 2771- 2781
Scheller HV, Huang B, Hatch E and Glodsbrough PB (1987) Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physio185: 1031-1035
Steffens JC ( 1990) The heavy metal-binding peptides of plants. Annu Rev Plant Physiol Plant Mol Biol41: 553-575
Weigel JH and Jarger HJ (1980) Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol 65: 480-482