Basic Science
Inducible Nitric Oxide Synthase Promoter
Polymorphism, Cigarette Smoking, and
Urothelial Carcinoma Risk
Cheng-Huang Shen, Yuan-Hung Wang, Wen-Chuang Wang, Yeong-Chin Jou,
Hueih-Shing Hsu, Hsiao-Yen Hsieh, and Hung-Yi Chiou
OBJECTIVES Bladder carcinoma has a high inducible nitric oxide synthase (iNOS) content, and a highly polymorphic (CCTTT)n repeat in the iNOS promoter region has been identified. We explored whether this iNOS promoter polymorphism and cigarette smoking are associated with urothelial carcinoma (UC) risk.
METHODS A total of 250 patients with pathologically confirmed UC and 250 unrelated noncancer controls were serially recruited at the Chia Yi Christian Hospital from August 2002 to May 2005. Multivariate logistic regression analysis was used to calculate the odds ratio and 95% confidence interval (CI).
RESULTS A significantly increased UC risk was found in those who had smoked more than 30 years (odds ratio 2.4, 95% CI 1.5 to 4.2). The study subjects carrying the 12-repeat allele had a significantly increased UC risk (odds ratio 1.7, 95% CI 1.1 to 2.5). We also found the investigated polymorphism was related to clinical stage (P⫽ 0.043). Of those who had ever smoked, those with the short/long (S/L) and long/long (L/L) genotypes (S, 9 to 11 repeats; L, 12 to 18 repeats) and the 12-repeat allele had a significantly increased UC risk of 3.5 (95% CI 1.7 to 7.3) and 4.5 (95% CI 2.2 to 8.9), respectively. Of the study subjects who had smoked longer than 30 years, those with S/L and L/L genotypes and the 12-repeat allele had significantly increased UC risks of 2.4 (95% CI 1.3 to 4.7) and 3.8 (95% CI 1.8 to 8.0), respectively.
CONCLUSIONS These findings suggest that the polymorphic (CCTTT)n repeat in the iNOS promoter region might be involved in the development of UC, especially in those who have ever smoked. UROLOGY69: 1001–1006, 2007. © 2007 Elsevier Inc.
U
rothelial carcinoma (UC) is the second most common cancer and second leading cause of death among malignancies of the genitourinary tract system.1 In general, several types of urothelialcar-cinoma (bladder, renal pelvis, and ureter) have histologic features similar to that of transitional cell carcinoma and are considered to have an analogous etiology.2According
to the annual report of the Taiwan Cancer Registry in 2001, the age-standardized incidence per 100,000 person-years of bladder cancer was 10.15 in males and 4.02 in females.3,4The etiology of UC is heterogeneous,
involv-ing ethnic, environmental, genetic, and dietary factors.5
Cigarette smoking, occupational exposures, inflammatory reactions to parasites or chronic infections, and exposure
to arsenic in the drinking water are known to be risk factors for bladder cancer.6In particular, cigarette smoke
contains polycyclic aromatic hydrocarbons, which might contribute to the etiology of bladder cancer and result in a two to fourfold risk among those who have ever smoked.7
Nitric oxide (NO) is a multifunctional gaseous mole-cule and a reactive free radical that has been shown to play an important role in several diseases, such as vascu-lar disease, immunologic reactions, and cancer.8,9 It is
mainly generated by a family of nitric oxide synthases (NOSs) and is produced by many cell types.10NO is a
product of the conversion ofL-arginine toL-citrulline by
NOS.11 The three NOS isoforms are neuronal NOS
(nNOS or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3).12The activity
of nNOS and eNOS is dependent on the concentration of cytosolic calcium. However, iNOS is independent of the intracellular calcium levels and produces more NO than nNOS and eNOS.13 Overexpression of iNOS has
been reported in several human cancers, including blad-der cancer, and continuous NO production might be
This study was supported by a research grant from the Chia Yi Christian Hospital (R92-14).
From the Departments of Urology, Pathology, and Medical Research, Chia Yi Christian Hospital, Chia Yi City, Taiwan; Department of Urology, Lotung Pohai Hospital, I-Lan County, Taiwan; and Taipei Medical University School of Public Health, Taipei City, Taiwan
Reprint requests: Hung-Yi Chiou, Ph.D., Taipei Medical University School of Public Health, No. 250 Wu-Hsing Street, Taipei 110, Taiwan. E-mail: hychiou@tmu.edu.tw
Submitted: September 11, 2006, accepted (with revisions): February 16, 2007
involved in the inflammatory process associated with many malignancies.14,15 Some studies have found that
iNOS levels are greater in malignant tissue, and the localization of iNOS to the intratumoral macrophages and endothelial cells of the tumor vasculature suggests that the intratumoral microenvironment is conducive to the induction of iNOS.16,17Benzo[a]pyrene is a
carcino-gen found in cigarettes. It results in oxidant stress, which leads to the stimulation of iNOS.18The overexpression of
iNOS is mainly regulated by several polymorphisms, which have been identified in the iNOS promoter. A polymorphic pentanucleotide (CCTTT)n repeat approx-imately 2.5 kilobases upstream the transcription initia-tion site has been elucidated to affect iNOS expression.19
Although the overexpression of NOS occurs in various tumor cell lines and solid tumors, the role of NO in tumor biology is still unclear. In this study, we explored the association among the polymorphic (CCTTT)n repeat in the iNOS promoter, cigarette smoking, and UC risk.
MATERIAL AND METHODS
Study Subject SelectionA case-control study of UC (bladder, renal pelvis, and ureter) was conducted at the Chia Yi Christian Hospital. A total of 250 patients with UC (mean age 64.6⫾ 12.7 years) were serially recruited from the Department of Urology. All cases of UC had been histologically confirmed from August 2002 to May 2005. Patients with recurrent UC or those who received intravesical bacille Calmette-Guérin (BCG) instillations, radiotherapy, or chemotherapy preoperatively were excluded. All the pathologic types were transitional cell carcinoma. One experienced pathol-ogist reviewed all pathology information. The staging and grad-ing of tumors was done accordgrad-ing to the criteria of the TNM staging system and the World Health Organization Interna-tional Society of Urological Pathology.20 The control group
consisted of 250 unrelated subjects (mean age 62.1 ⫾ 12.4 years), who were patients admitted to the same hospital, with an absence of any cancer history or precancerous lesions. All subjects were interviewed by a trained interviewer using a standard questionnaire. The collected information included: (a) demographic characteristics, (b) dietary habits, (c) a history of cigarette smoking and alcohol drinking, and (d) a history of other diseases. Ten milliliters of venous blood was collected into vials from all subjects. Genomic DNA was extracted from the peripheral blood lymphocytes using proteinase K and phe-nol chloroform. The institutional review board at the Chia Yi Christian Hospital approved this study, and all study subjects provided informed consent.
Genotyping of iNOS Promoter Polymorphism The genotyping was determined using polymerase chain reac-tion with the following primers: 5=-ACC CCT GGA AGC CTA CAA CTG CAT-3= (sense); 5=-GCC ACT GCA CCC TAG CCT GTC TCA-3= (antisense). The sense primer was labeled with HEX dye. The thermal cycling was performed with an initial step at 94°C for 5 minutes, 30 cycles of denaturation at 94°C for 20 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 20s, and a final extension at 72°C for 10
min. Genotyping was performed in a mixture of amplified products using an internal size standard by ABI PRISM 310 genetic analyzer (Perkin-Elmer). The alleles were numbered, and representative samples were sequenced with ABI PRISM 310 in both orientations to confirm the GeneScan results.
Statistical Analysis
The frequency distribution of the sociodemographic character-istics, including age, sex, cigarette smoking, and alcohol drink-ing, was examined for all subjects. Cigarette smoking status was categorized into never smoked and ever smoked. Those consid-ered to have ever smoked were those who had smoked more than 100 cigarettes in their lifetime. The association between the iNOS (CCTTT)n promoter polymorphism and the patho-logic and clinical characteristics (grade and stage) was assessed using the chi-square test. The effect of the iNOS (CCTTT)n promoter polymorphism on UC risk was calculated with the odds ratio (OR) and 95% confidence interval (CI), which was derived from unconditional multivariate logistic regression analysis. The Statistical Analysis Systems, version 6.12, soft-ware (SAS Institute, Cary, NC) was used for all statistical analyses. P⬍0.05 was considered statistically significant.
RESULTS
No significant differences were found in the distribution of age or sex between those with UC and the controls. After adjustment for age, sex, and alcohol drinking, a significantly increased UC risk of 2.3 and 2.4 was found for those who had ever smoked and those who had smoked more than 30 years, respectively. Regarding al-cohol drinking, a significantly increased UC risk was found for those who had ever consumed alcohol, after adjustment for age, sex, and cigarette smoking (Table 1). The distribution of the polymorphic (CCTTT)n repeats is shown in Figure 1; the repeats range from 6 to 21. Among them, four repeats (10, 11, 12, and 13) with a greater frequency (more than 10%) and five repeats (6, 8 19, 20, and 21) with a rare frequency (less than 1%) were observed. Additionally, three longer repeat lengths were found in a greater proportion of the controls, including the 15-repeat allele (5.2% in patients with UC and 6.0% in controls; chi-square test, P⫽ 0.5882), 16-repeat allele (4.6% in patients with UC and 6.4% in controls; chi-square test, P ⫽ 0.2119), and 17-repeat allele (3.0% in patients with UC and 3.6% in controls; chi-square test,
P ⫽ 0.5954). However, we found nonsignificant
differ-ences between those with UC and controls among these three repeats. We then excluded the five rare repeats and defined alleles of less than 12 repeats as the short form (S) and alleles of 12 or more repeats as the long form (L). The study subjects were classified into three genotypes: S/S, S/L, and L/L. After we had combined the S/L and L/L genotypes, a nonsignificantly increased UC risk was shown (Table 1). However, the study subjects carrying the 12-repeat allele had a significantly increased UC risk. The patients with UC were then categorized into two groups (superficial and invasive) and a significant associ-ation was found with the iNOS (CCTTT)n promoter
polymorphism (S/S, S/L, and L/L; P⫽ 0.043). However, we found no significant association between the patho-logic grade and the investigated polymorphism (Table 2). The UC risk related to the (CCTTT)n promoter poly-morphism was further examined in those with diverse
cigarette smoking status (Table 3). After adjustment for age, sex, and alcohol drinking, those who had ever smoked had a significantly increased UC risk of 3.5 and 4.5 for those with the S/L and L/L genotypes and the 12-repeat allele, respectively. In addition, those who had smoked more than 30 years had a significantly increased UC risk of 2.4 and 3.8 for those with the S/L and L/L genotypes and the 12-repeat allele, respectively.
COMMENT
In this study, we observed that the polymorphic (CCTTT)n repeat in the iNOS promoter and cigarette smoking were significantly associated with UC risk. Con-sistent with the findings of previous studies,21,22cigarette
smoking was the major risk factor for bladder cancer. We expected such a finding because cigarettes contain many carcinogens that put smokers at an increased risk of UC. We also found a significantly increased UC risk in alcohol drinkers. A meta-analysis suggested a slightly increased cig-arette smoking-adjusted UC risk for men (relative risk 1.3).22Minor differences could have resulted from the
dif-ferent drinking patterns of various populations.
This is the first study to explore an association between
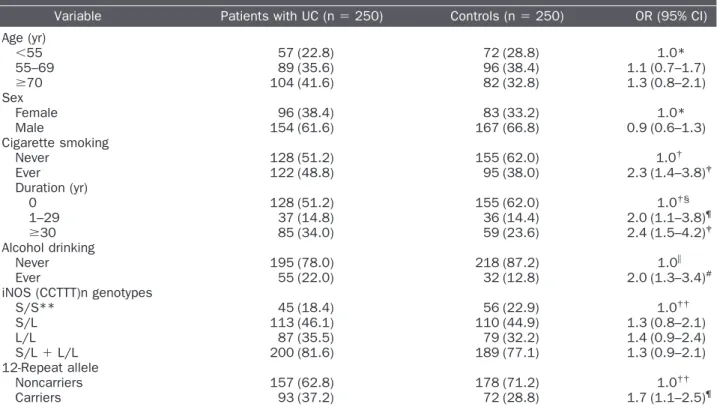
Table 1. Risk of urothelial cancer by sociodemographic characteristics and iNOS (CCTTT)n promoter polymorphism among patients with UC and controls
Variable Patients with UC (n⫽ 250) Controls (n⫽ 250) OR (95% CI) Age (yr) ⬍55 57 (22.8) 72 (28.8) 1.0* 55–69 89 (35.6) 96 (38.4) 1.1 (0.7–1.7) ⱖ70 104 (41.6) 82 (32.8) 1.3 (0.8–2.1) Sex Female 96 (38.4) 83 (33.2) 1.0* Male 154 (61.6) 167 (66.8) 0.9 (0.6–1.3) Cigarette smoking Never 128 (51.2) 155 (62.0) 1.0† Ever 122 (48.8) 95 (38.0) 2.3 (1.4–3.8)‡ Duration (yr) 0 128 (51.2) 155 (62.0) 1.0†§ 1–29 37 (14.8) 36 (14.4) 2.0 (1.1–3.8)¶ ⱖ30 85 (34.0) 59 (23.6) 2.4 (1.5–4.2)‡ Alcohol drinking Never 195 (78.0) 218 (87.2) 1.0储 Ever 55 (22.0) 32 (12.8) 2.0 (1.3–3.4)# iNOS (CCTTT)n genotypes S/S** 45 (18.4) 56 (22.9) 1.0†† S/L 113 (46.1) 110 (44.9) 1.3 (0.8–2.1) L/L 87 (35.5) 79 (32.2) 1.4 (0.9–2.4) S/L⫹ L/L 200 (81.6) 189 (77.1) 1.3 (0.9–2.1) 12-Repeat allele Noncarriers 157 (62.8) 178 (71.2) 1.0†† Carriers 93 (37.2) 72 (28.8) 1.7 (1.1–2.5)¶
iNOS⫽ inducible nitric oxide synthase; UC ⫽ urothelial cancer; OR ⫽ odds ratio; CI ⫽ confidence interval; S ⫽ short; L ⫽ long. * Adjusted for age and sex.
†
Adjusted for age, sex, and alcohol drinking.
‡P⬍0.001. §
P for trend⬍0.001. ¶P⬍0.05.
储Adjusted for age, sex, and cigarette smoking.
** Five “rare” repeat numbers (frequency⬍1%) were excluded; other repeat numbers divided into two groups: S (9–11 repeats) and L (12–18 repeats).
††Adjusted for age, sex, cigarette smoking, and alcohol drinking.
Figure 1. Distribution of (CCTTT)n promoter polymorphism in patients with UC (black bars) and controls (white bars).
the polymorphic (CCTTT)n repeat in the iNOS pro-moter and UC risk. The distribution of the (CCTTT)n repeats was similar to that reported previously for the Japanese and was different from that of other popula-tions.23In our study, the repeats were spread between 6
and 21 in those with UC and 6 and 19 in the controls. Several reports have divided the length into short and long forms, depending on their ability to elevate iNOS expression. We defined alleles of 9 to 11 repeats as the short form (S) and alleles of 12 to 18 repeats as the long form (L). An in vitro study showed that iNOS promoter activity increases with the repeat numbers (9 to 15 re-peats), suggesting that longer forms have greater tran-scription activity.24However, no significantly increased
UC risk was found in our study subjects carrying the S/L and L/L genotypes. It is likely that different repeats have
diverse effects on the transcription ability of the pro-moter. Therefore, the transcription effect was not solely dependent on the promoter length because the longer forms (15 and 17 repeats) were no more effective than the 14 repeat in mediating interleukin-1beta induction of iNOS.24In addition, we found a significantly increased
UC risk in those carrying the 12-repeat allele. A previous study indicated that the frequent repeat was the 12-repeat allele and that 41% of the patients with colorectal cancer and 38% of controls carried this allele.25 Moreover, a
sig-nificantly greater frequency of the 12-repeat allele was observed in Chinese patients with diabetes compared with white patients with diabetes (P ⫽ 0.001).26
Al-though the association between the 12-repeat allele and UC risk found in our study is not evidence of causation, we cannot exclude the possibility that the 12-repeat
Table 2. Associations of iNOS (CCTTT)n promoter polymorphism with pathologic grade and clinical stage
Variable S/S* S/L L/L P Value†
12-Repeat Allele
Noncarriers Carriers P Value†
Grade 0.541 0.673 Low (G1) 16 (21.6) 35 (47.3) 23 (31.1) 45 (60.8) 29 (39.2) High (G2–G3) 29 (17.0) 78 (45.6) 64 (37.4) 112 (63.6) 64 (36.4) Stage 0.043 0.240 Superficial (ⱕT1) 33 (23.7) 61 (43.9) 45 (32.4) 93 (66.0) 48 (34.0) Invasive (T2–T4) 12 (11.3) 52 (49.1) 42 (39.6) 64 (58.7) 45 (41.3) Abbreviations as in Table 1.
Data in parentheses are percentages.
* Five “rare” repeat numbers (frequency⬍1%) were excluded; other repeat numbers divided into two groups: S (9–11 repeats) and L (12–18 repeats).
†
Chi-square test.
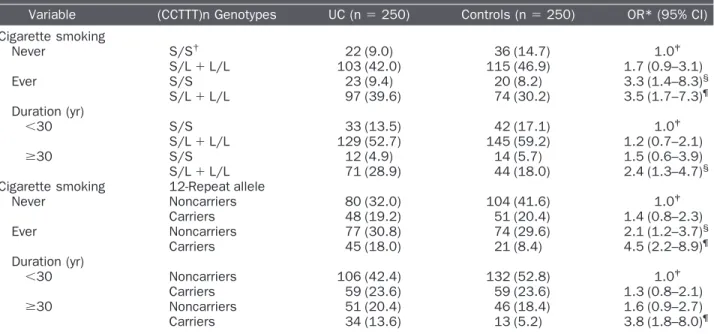
Table 3. Joint effect on risk of urothelial cancer between iNOS (CCTTT)n promoter polymorphism and cigarette smoking among UC cases and controls
Variable (CCTTT)n Genotypes UC (n⫽ 250) Controls (n⫽ 250) OR* (95% CI) Cigarette smoking Never S/S† 22 (9.0) 36 (14.7) 1.0‡ S/L⫹ L/L 103 (42.0) 115 (46.9) 1.7 (0.9–3.1) Ever S/S 23 (9.4) 20 (8.2) 3.3 (1.4–8.3)§ S/L⫹ L/L 97 (39.6) 74 (30.2) 3.5 (1.7–7.3)¶ Duration (yr) ⬍30 S/S 33 (13.5) 42 (17.1) 1.0‡ S/L⫹ L/L 129 (52.7) 145 (59.2) 1.2 (0.7–2.1) ⱖ30 S/S 12 (4.9) 14 (5.7) 1.5 (0.6–3.9) S/L⫹ L/L 71 (28.9) 44 (18.0) 2.4 (1.3–4.7)§
Cigarette smoking 12-Repeat allele
Never Noncarriers 80 (32.0) 104 (41.6) 1.0‡ Carriers 48 (19.2) 51 (20.4) 1.4 (0.8–2.3) Ever Noncarriers 77 (30.8) 74 (29.6) 2.1 (1.2–3.7)§ Carriers 45 (18.0) 21 (8.4) 4.5 (2.2–8.9)¶ Duration (yr) ⬍30 Noncarriers 106 (42.4) 132 (52.8) 1.0‡ Carriers 59 (23.6) 59 (23.6) 1.3 (0.8–2.1) ⱖ30 Noncarriers 51 (20.4) 46 (18.4) 1.6 (0.9–2.7) Carriers 34 (13.6) 13 (5.2) 3.8 (1.8–8.0)¶ Abbreviations as in Table 1.
* Adjusted for age, sex and alcohol drinking.
†Five “rare” repeat numbers (frequency⬍1%) were excluded; other repeat numbers divided into two groups: S (9–11 repeats) and
L (12–18 repeats).
‡P for trend⬍0.001. §
P⬍0.01. ¶P⬍0.001.
allele is in linkage with the primary associated polymor-phism.
Although patients with UC at an advanced stage (T2– T4) seemed to have a greater percentage of a longer iNOS (CCTTT)n promoter length, we have merely shown an association between stage and polymorphisms. However, previous studies have indicated that iNOS expression in bladder cancer tissue might play an impor-tant role in tumor angiogenesis and recurrence.16,17
Be-cause of the lack of follow-up of the patients with UC, we can only speculate whether the polymorphisms in the
iNOS gene will lead to varying iNOS expression and alter
the individual’s susceptibility to UC. Furthermore, our results have indicated that of those who have ever smoked and those who have smoked more than 30 years, the individuals with S/L and L/L genotypes and the 12-repeat allele have significantly increased UC risks, respectively. One study has suggested that ciga-rette smoking results in oxidative stress, which leads to the stimulation of iNOS, together with the protein tyrosine phosphorylation, promoting the development of UC.27
Bladder instillation with BCG induces a local produc-tion of NO, likely because of the inducproduc-tion of NOS activity in urothelial cells.28,29To avoid the confounding
from BCG instillations, those with recurrent UC and those who had received intravesical BCG instillations, radiotherapy, or chemotherapy preoperatively were ex-cluded. In addition, the results of a recent study have refuted the correlation between urine or serum levels of NO and bladder cancer risk.30 However, it had some
limitations, including a small sample size, absence of the evaluation of healthy controls, and irregular and insuf-ficient follow-up in the patients with cancer. There-fore, additional studies with longer follow-up and a larger number of patients with UC, controlling for treatment variation, are necessary to evaluate the true correlation between polymorphisms in the iNOS gene and UC risk.
CONCLUSIONS
This is the first study, to our knowledge, on the associa-tion among the (CCTTT)n repeat of iNOS promoter, cigarette smoking and UC risk. Our findings have sug-gested that the polymorphic (CCTTT)n repeat in the iNOS promoter might be involved in the development of UC, especially in those who have ever smoked.
Acknowledgment. To Chia Yi Christian Hospital for sup-porting the completion of this research by providing a suitable research environment and by making their research resources available; and to the urology department and all colleagues for their assistance and support.
References
1. Konety BR, and Getzenberg RH: Urine based markers of urological malignancy. J Urol 165: 600 – 611, 2001.
2. Komiya Y, Tsukino H, Nakao H, et al: Human glutathione S-transferase A1 polymorphism and susceptibility to urothelial cancer in the Japanese population. Cancer Lett 221: 55–59, 2005. 3. Department of Health: The Executive Yuan Health Statistics 2004.
Republic of China, Taipei, Department of Health, The Executive Yuan, 2004.
4. Department of Health: The Executive Yuan Cancer Registry An-nual Report 2005. Republic of China, Taipei, Department of Health, The Executive Yuan, 2005.
5. Bid HK, Manchanda PK, and Mittal RD: Association of interleu-kin-1Ra gene polymorphism in patients with bladder cancer: case control study from North India. Urology 67: 1099 –1104, 2006. 6. Negri E, and La VC: Epidemiology and prevention of bladder
cancer. Eur J Cancer Prev 10: 7–14, 2001.
7. Kellen E, Zeegers M, Paulussen A, et al: Fruit consumption reduces the effect of smoking on bladder cancer risk: the Belgian case control study on bladder cancer. Int J Cancer 118: 2572–2578, 2006.
8. Ohshima H, and Bartsch H: Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 305: 253–264, 1994.
9. Fukumura D, Kashiwagi S, and Jain RK: The role of nitric oxide in tumour progression. Nat Rev Cancer 6: 521–534, 2006. 10. Forstermann U, Gath I, Schwarz P, et al: Isoforms of nitric oxide
synthase: properties, cellular distribution and expressional control. Biochem Pharmacol 50: 1321–1332, 1995.
11. Moncada S, and Higgs A: The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993.
12. Forstermann U, and Kleinert H: Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn Schmiede-bergs Arch Pharmacol 352: 351–364, 1995.
13. Aktan F: iNOS-mediated nitric oxide production and its regula-tion. Life Sci 75: 639 – 653, 2004.
14. Wolf H, Haeckel C, and Roessner A: Inducible nitric oxide syn-thase expression in human urinary bladder cancer. Virchows Arch
437: 662– 666, 2000.
15. Shochina M, Fellig Y, Sughayer M, et al: Nitric oxide synthase immunoreactivity in human bladder carcinoma. Mol Pathol 54: 248 –252, 2001.
16. Sandes EO, Faletti AG, Riveros MD, et al: Expression of inducible nitric oxide synthase in tumoral and non-tumoral epithelia from bladder cancer patients. Nitric Oxide 12: 39 – 45, 2005. 17. Klotz T, Bloch W, Jacobs G, et al: Immunolocalization of inducible
and constitutive nitric oxide synthases in human bladder cancer. Urology 54: 416 – 419, 1999.
18. Chen J, Yan Y, Li J, et al: Differential requirement of signal pathways for benzo[a] pyrene (B[a]P)-induced nitric oxide synthase (iNOS) in rat esophageal epithelial cells. Carcinogenesis 26: 1035–1043, 2005.
19. Xu W, Liu L, Emson PC, et al: Evolution of a homopurine-homopyrimidine pentanucleotide repeat sequence upstream of the human inducible nitric oxide synthase gene. Gene 204: 165–170, 1997.
20. Knowles MA: Molecular subtypes of bladder cancer: Jekyll and Hyde or chalk and cheese. Carcinogenesis 27: 361–373, 2006. 21. Cao W, Cai L, Rao JY, et al: Tobacco smoking, GSTP1
polymor-phism, and bladder carcinoma. Cancer 104: 2400 –2408, 2005. 22. Zeegers MP, Kellen E, Buntinx F, et al: The association between
smoking, beverage consumption, diet and bladder cancer: a system-atic literature review. World J Urol 21: 392– 401, 2004. 23. Tatemichi M, Sawa T, Gilibert I, et al: Increased risk of intestinal
type of gastric adenocarcinoma in Japanese women associated with long forms of CCTTT pentanucleotide repeat in the inducible nitric oxide synthase promoter. Cancer Lett 217: 197–202, 2005. 24. Warpeha KM, Xu W, Liu L, et al: Genotyping and functional analysis of a polymorphic (CCTTT)(n) repeat of NOS2A in dia-betic retinopathy. FASEB J 13: 1825–1832, 1999.
25. Fransen K, Elander N, and Soderkvist P: Nitric oxide synthase 2 (NOS2) promoter polymorphisms in colorectal cancer. Cancer Lett
225: 99 –103, 2005.
26. Liao L, Lim MC, Chan SW, et al: Nitric oxide synthase gene polymorphisms and nephropathy in Asians with type 2 diabetes. J Diabetes Complications 20: 371–375, 2006.
27. Chang WC, Lee YC, Liu CL, et al: Increased expression of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MEK1/ERK2 proteins in terminal bronchiole lesions in the lungs of rats exposed to cigarette smoke. Arch Toxicol 75: 28 –35, 2001.
28. Alvarez V, Lodillinsky C, Umerez S, et al: Inhibition of bacillus Calmette-Guérin-induced nitric oxide in bladder tumor cells may improve BCG treatment. Int J Mol Med 16: 565–571, 2005.
29. Hosseini A, Koskela LR, Ehren I, et al: Enhanced formation of nitric oxide in bladder carcinoma in situ and in BCG treated bladder cancer. Nitric Oxide 15: 337–343, 2006.
30. Kilic S, Bayraktar N, Beytur A, et al: Can the levels of nitric oxide in the urine, serum and tumor tissue be putative markers for bladder cancer? Int J Urol 13: 1079 –1085, 2006.