INTRODUCTION
The safety of static magnetic fields (SMFs) has been discussed for decades (Drinker and Thomson, 1921). Various studies have examined the effects of SMFs, but their results were often inconclusive (Valentina et al., 2009). With the application of SMFs in clinical practice, the incidence of exposure has greatly increased. A general concern is that the knowledge regarding the health hazards of SMFs lags behind the development of medical technologies, such as magnetic resonance imaging (MRI). Stronger SMFs are required for higher resolution imaging. Weak magnetic fields are used in the treatment of parkinsonism and the motor complications of chronic levodopa therapy (Sandyk et al., 1992). The hazardous effects associated with SMFs on human health have become conspicuous. New studies are needed to fill in the gaps in our knowledge and provide the assurance that novel medical technologies will not cause unwanted health hazards (Leszczynski, 2005).
There have been few studies on the effects of SMFs at the cellular level (Miyakoshi, 2005). SMFs alone do not have a lethal effect on the basic properties of cell growth and survival under normal culture conditions (Nakahara et al., 2002; Sakurai et al., 2009). Most studies have also suggested that SMFs do not affect cell proliferation (Hiraoka et al., 1992) or influence the cell cycle. Morphological analyses indicated that SMFs could induce modifications in cell shape, the cell surface and the cytoskeleton (Yamaguchi et al., 1993; Buemi et al., 2001; Teodori et al., 2002; Pacini et al., 2003; Pate et al., 2003; Pagliara et al., 2005; Tarantino et al., 2005; Gamboa et al., 2007). SMFs may also modulate apoptosis by influencing cytoplasmic calcium ion concentrations (Tarantino et al., 2005). SMFs were able to induce double-stranded DNA breaks in rat brain cells (Lai and Singh, 2004). SMFs are also associated with an increased risk of cancer (McCann, 1998). It is likely that SMFs also affect longevity.
In multicellular organisms, SMFs may cause minor effects at the cellular level that then accumulate and eventually cause distinct symptoms. Early studies did not show any effect of exposure to SMFs on the organogenesis, fetal development or cortical development of mice (Sikov et al., 1979; Konermann and Monig, 1986; Murakami et al., 1992; Okazaki et al., 2001). However, the International Agency for Research on Cancer (IARC) proposed that SMFs might affect embryonic development in amphibians (International Agency for Research on Cancer, 2002). SMFs have been reported to be toxic to rat embryos (Mevissen et al., 1994), and to cause significant time- and dose-dependent increases in the frequency of micronuclei in mice (Suzuki et al., 2001) and in the rates of germination and early growth in Cicer arietinum L. (Ananta and Shantha, 2008). Various studies have focused on the effects of SMFs on the nervous system, behavior, genotoxicity and cancer incidence. Only a few studies have examined the effect of SMFs on reproduction and development, and the results are inconclusive (Valentina et al., 2009).
Caenorhabditis elegans, Maupas, 1900, are a valuable resource for genetic and molecular investigations in a multicellular organism. With a short lifespan, the nematode serves as a powerful model system for studying the molecular mechanisms underlying aging and development. The median lifespan of C. elegans ranges from 11.8days (Van Voorhies, 1992) to 20days (Kenyon et al., 1993) when grown on agar plates at 20°C with Escherichia coli.
Caenorhabditis elegans populations exist primarily as
hermaphrodites. The short lifespan of C. elegans makes it attractive for whole-organism compound screening (Hertweck et al., 2003).
Furthermore, C. elegans may help us to fill the gaps in our knowledge regarding the possible health hazards of SMFs. In C. elegans, a short-term treatment with SMFs induced fluctuations in
The Journal of Experimental Biology 213, 2079-2085 © 2010. Published by The Company of Biologists Ltd doi:10.1242/jeb.039768
Effects of static magnetic fields on the development and aging of Caenorhabditis
elegans
Yao-Ching Hung
1, Jia-Huey Lee
2, Huang-Meng Chen
2and Guewha Steven Huang
2,*
1Section of Gynecologic Oncology, Department of Obstetrics and Gynecology, China Medical University, 91 Hsueh Shih Road, Taichung 404, Taiwan and 2Institute of Nanotechnology, National Chiao Tung University, 1001 University Road, EE137,
Hsinchu 300, Taiwan, ROC
*Author for correspondence (gstevehuang@mail.nctu.edu.tw) Accepted 8 March 2010
SUMMARY
The current study investigated the possible effects of static magnetic fields (SMFs) on the developmental and aging processes of
Caenorhabditis elegans. Nematodes were grown in the presence of SMFs of strengths varying from 0 to 200mT. The rate of development and the lifespan were recorded. Treatment with a 200mT SMF reduced the development time from the L2 to the L3 stage by 20%, from L3 to L4 by 23%, and from L4 to young adult by 31%. After SMF treatment, the average lifespan was reduced from 31days to 24days for wild-type nematodes. The up-regulation of clk-1, lim-7, daf-2, unc-3and age-1after SMF treatment was verified by quantitative real-time RT-PCR. Apparently, induction of gene expression is selective and dose dependent. The total developmental time was significantly reduced for the lin-4, lin-14, lin-41and lim-7mutants, but not for the let-7, clk-1, unc-3and
age-1mutants. Lifespan analyses revealed that the let-7, unc-3and age-1mutants were not affected by SMF treatment. Here we show that SMFs accelerate nematode development and shorten nematode lifespan through pathways associated with let-7,clk-1,
unc-3and age-1.
heat shock protein gene expression (Miyakawa et al., 2001; Kimura et al., 2008). The current study is based on the hypothesis that, given sufficient intensity, SMFs can shorten the life cycle and cause premature aging in nematodes.
MATERIALS AND METHODS Strains and chemicals
The strains of C. elegans used in this research, N2 (wild-type), lim-7, lin-14, lin-41, clk-1, age-1 and unc-3, were obtained from the Caenorhabditis Genetics Center (CGC), University of Minnesota, St Paul (MN, USA). Caenorhabditis elegans populations exist primarily as hermaphrodites. Nematodes were propagated at 20°C on nematode growth medium (NGM) plates (Brenner, 1974) with the E. coli strain OP50 as a food source. All culture media and related chemicals, including Bacto agar, Bacto tryptone and yeast extract, were purchased from Gibco Co. (Gaithersburg, MD, USA). Other chemicals (of analytical grade or higher) were purchased from Sigma (St Louis, MO, USA) or Merck (Hsinchu, Taiwan, ROC). Nd-Fe-B magnets were purchased from the Taiwan Magnetic Corp. Ltd (Taipei, Taiwan, ROC). Magnetic field strength was measured at the National Measurement Laboratory, Taiwan.
Isolation of nematode developmental stages
The developmental stages of nematodes were defined according to the timing of the molting period and the body length, as described previously (Hope, 1999; Cassada and Russell, 1975).
L1: the first larval stage, about 11.5h after fertilization. General structure is similar to the adult with a body length of 250m.
L2: 12h after hatching. The body length of the nematode is 360–380m.
L3: 20h after hatching. The body length of the nematode is 490–510m.
L4: 28h after hatching. The body length of the nematode is 620–650m.
Young adult: 38h after hatching. The body length of the nematode is 900–940m.
Adult: 46h after hatching. The body length of the nematode is 1110–1150m, and the nematode is capable of egg laying.
Quantitative real-time RT-PCR
Total RNA was extracted from 100 worms using TRI-reagent (Talron Biotech, Taipei, Taiwan, ROC) according to the manufacturer’s specifications. Worms were picked and washed three times with M9 medium and collected in a 2ml Eppendorf tube. The pellet was dissolved in 1ml Tri-reagent and disrupted with a homogenizer on ice; the RNA was isolated using chloroform extraction and isopropanol precipitation. The crude RNA extract was immediately purified with an RNeasy Mini Kit (Qiagen, Taipei, Taiwan, ROC) to remove impurities and unwanted organic substances. Purified RNA was resuspended in DEPC water and quantified by absorbance at OD260. The OD260to OD280absorbance
ratio usually exceeded 2.0 at this stage. For cDNA synthesis, 1g total RNA was annealed with 1g oligo-dT, followed by reverse transcription using SuperScript® III Reverse Transcriptase
(Invitrogen, Carlsbad, CA, USA) in a total volume of 50l. Between 0.2 and 0.5l of the reverse transcription reactions was used for quantitative real-time PCR using SYBR Green I on an iCycler iQ5 (Bio-Rad Laboratories, Hercules, CA, USA). Cycling conditions were as follows: 1⫻ (5min at 95°C) and 50⫻ (20s at 95°C, 20s at 60°C, and 40s at 72°C); fluorescence was measured after each 72°C step. Expression levels were obtained as threshold cycles (Ct), which were determined by the iCycler iQ Detection System software.
Because there is no let-7 gene sequence provided by Wormbase and lin-41 gave no results for quantitative real-time PCR, the let-7 and lin-41 data were not available. Relative transcript quantities were calculated using the Ct method. The ribosomal proteins L18 and L21 were used as reference genes and were amplified from the same cDNA samples. The difference in threshold cycles of the sample mRNA relative to ribosomal protein (L18 or L21) mRNA was defined as Ct. The difference between the Ct of the untreated control and the Ct of the SMF-treated sample was defined as Ct. The fold change in mRNA expression was expressed as 2Ct. The results are expressed as the mean ± s.d. of six experiments.
Lifespan assay
Synchronized wild-type nematodes were placed on NGM plates with the E. coli strain OP50 as a food source. The next day, 60 L1 nematode larvae were placed onto three new NGM plates, with 20 nematodes on each plate. We defined this day as the first day. Two groups were developed under the same conditions. One group was treated with a SMF, and the other was given no SMF treatment. The nematodes were moved every day to a new NGM plate with the E. coli strain OP50 as a food source, and the number of live nematodes was recorded to calculate the percentage of living nematodes. The results are expressed as the mean ± s.d. All experiments were performed at 20°C.
RESULTS
A SMF of 200mT shortens the lifespan of C. elegans
The SMF device was composed of two Nd-Fe-B permanent magnets that sandwich a Petri dish. The field intensity at the center of the SMF device was measured. Adjusting the distance between the magnets varied the intensity from 0 to 200mT (Fig.1). To investigate the possible effects of SMFs on C. elegans lifespan, synchronized nematodes were grown under a 200mT SMF at 20°C. A lifespan assay was performed, and the results were compared with those of the untreated group (Fig.2). The median lifespan of C. elegans was 16days for the untreated group and was reduced to 13days upon treatment with a 200mT SMF. The normal lifespan for C. elegans is 31days at 20°C. SMF treatment shortened the lifespan to 24days. Based on these results, there was a 23% reduction in the normal C. elegans lifespan upon treatment with a 200mT SMF.
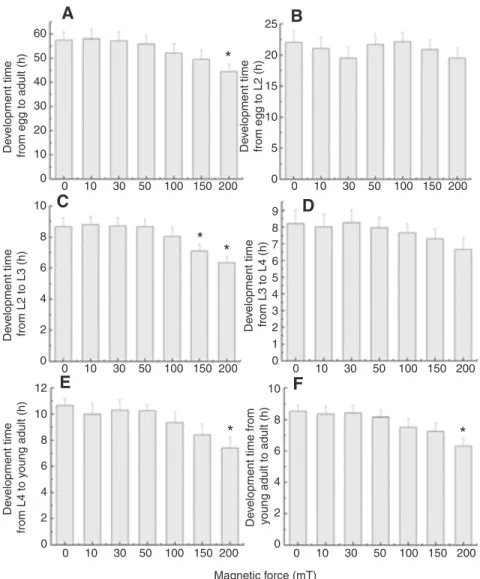
SMFs stronger than 150mT accelerate nematode development time
It is possible that SMFs lead to a shorter nematode lifespan because they interfere with the time required for the early stages of nematode development. The nematode life cycle was measured from the egg stage to the adult stage. Synchronized wild-type nematodes (N2) were treated with 0–200mT SMFs for 3days. The total development time from egg to adult was measured (Fig.3A). At 20°C, the complete life cycle of wild-type nematodes takes 57.5h. The life cycle time was reduced by SMFs in a dose-dependent manner. Treatment with a 200mT SMF led to a 22% reduction in life cycle time compared with the untreated group. Because there is a distinct difference in appearance at each stage of nematode development, it was possible to measure the duration of each stage from egg to adult. No significant differences were observed for the stages from egg to L2 (Fig.3B). A significant difference in development time occurred from L2 to L3; there was an 18% reduction after 150mT treatment and a 23% reduction after 200mT treatment (Fig.3C). Based on these results, SMF treatment seems to accelerate nematode development. For later stages, the development time tended to decrease upon SMF treatment. Although SMFs did not induce a
statistically significant difference in development time from the L3 stage to the L4 stage (Fig.3D), there was a 19% reduction in the development time from the L4 stage to the young adult stage and a 23% reduction from the young adult stage to the adult stage after 200mT SMF treatment (Fig.3E,F). SMFs accelerated nematode development and shortened the development time from the L2 stage to the adult stage. These results are also reflected in the shortened lifespan after SMF treatment.
Genes associated with development and aging are differentially expressed after treatment with SMFs The shortened nematode life cycle indicated that pathways associated with development and aging may have been altered by
SMF treatment. To verify the differential expression of genes in these pathways due to SMF treatment, quantitative real-time RT-PCR was performed. For the preliminary screening, seven genes were selected, and their primer pairs were designed based on the sequences provided in GenBank (Table1). These genes are associated with cell development (lin-4, lin-14, lin-41), growth rate (clk-1), growth of somatic gonadal sheath cells from the L4 stage to adulthood (lim-7), regulation of development and differentiation of B lymphocytes, adipocytes and nerve cells (unc-3), and aging (age-1). Quantitative real-time RT-PCR consistently showed up-regulation of clk-1, lim-7, unc-3 and age-1 when the worms were cultured in the presence of a 200mT SMF (Fig.4A). There was a 13.9-fold, 2450-fold, 5.7-fold and 324-fold up-regulation of clk-1, lim-7, unc-3 and age-1, respectively. These results imply that pathways associated with development and aging play a role in the SMF-induced reduction of lifespan and development time.
Our studies indicated that the life cycle time was reduced by SMFs in a dose-dependent manner. It is not clear whether the aging-related genes exhibit dose-dependent expression. We chose clk-1, daf-2, age-1 and lim-7 to investigate dose-dependent gene expression by quantitative real-time RT-PCR. These four genes exhibited similar trends in expression levels when various intensities of SMFs were applied (Fig.4B). A SMF of 30mT stimulated a minor increase in gene expression. The expression levels increased rapidly, reached a plateau when a 50mT SMF was applied, and remained constant under 100, 150 and 200mT SMFs. The up-regulation of clk-1, daf-2, age-1 and lim-7 was triggered by SMFs in a dose-dependent manner. Under SMF treatments ranging from 50 to 200mT, the average fold change in expression level was 1470, 161, 377 and 2350 for clk-1, daf-2, age-1 and lim-7, respectively.
Nematodes mutant for developmental and aging genes are resistant to SMF-induced life cycle reduction
Although the expression levels of genes associated with development and aging were altered upon treatment with SMFs, it has not yet been demonstrated that the inactivation of these genes causes nematodes to be resistant to SMF treatment. Given the expression levels of genes associated with development and aging were altered upon treatment with SMFs, we wanted to find out whether inactivation of these genes causes nematodes to be resistant to SMF treatment. We took advantage of mutant nematodes that carry mutations in genes involved in developmental and aging pathways. Strains mutant for let-7, lin-14, lin-41, clk-1, lim-7, unc-3 and age-1 were scored for total development time with or without 200mT SMF treatment (Fig.5). The gene let-7 is directly involved in the transition from the late larval to adult cell fates. For wild-type nematodes, SMF treatment significantly reduced development time by 22%, from 58h to 45h. The life cycles of lim-7, lin-14, lin-41 and lin-4 mutants were significantly reduced by 20%, 23%, 21% and 22%, respectively, upon SMF treatment. However, the life cycles of let-7, unc-3, clk-1 and age-1 mutants were not affected by SMF treatment. Apparently, pathways associated with development and aging (let-7, unc-3, clk-1 and age-1) are involved in mediating the SMF-induced life cycle reduction in nematodes.
Nematodes mutant for developmental and aging genes are resistant to SMF-induced lifespan reduction
To investigate the possible effects of SMFs on the C. elegans lifespan, synchronized wild-type (N2) and mutant nematodes were grown under a 200mT SMF at 20°C. Lifespan assays were performed on N2, let-7, unc-3 and age-1 nematodes, with or without SMF treatment. The median lifespan of wild-type nematodes was 2000 1500 1000 500 0 10 20 30 40 50 60 M a gnetic f orce (G)
Distance from the surface center (mm) Fig.1. Magnetic field strength at the center of the static magnetic fields (SMFs) used in the current study. The magnetic force (in gauss, G) is plotted against the distance (in mm) between two permanent magnets sandwiching a Petri dish.
100 80 60 40 20 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 N2 N2+SMFs % Aliv e Time (days)
Fig.2. The effects of SMFs on the lifespan of wild-type nematodes. Sixty wild-type nematodes were transferred onto nematode growth medium (NGM) plates with the Escherichia coli strain OP50 as a food source. Lifespan was measured at 20°C. The median lifespan of wild-type nematodes was 16days for the untreated group and was reduced to 13days after treatment with a 200mT SMF. The normal lifespan for wild-type nematodes was 31days at 20°C. SMF treatment shortened the lifespan to 24days. N2 is the wild-type nematode strain.
16days for the untreated group and was reduced to 13days after treatment with a 200mT SMF. The normal lifespan for a wild-type nematode is 31days at 20°C. SMF treatment shortened this lifespan to 24days (Fig.6A). The lifespan of the let-7 mutants showed only a minimal reduction, from 30 to 29days. The lifespan of the unc-3 mutants also showed only a minimal reduction, from unc-34 to unc-3unc-3days
(Fig.6B). Finally, the lifespan of the age-1 mutants also showed only a minimal reduction, from 33 to 32days (Fig.6C). In summary, the lifespans of let-7, unc-3 and age-1 mutants were not affected by SMF treatment. These results show that the developmental and aging pathways associated with these genes are correlated with a SMF-induced reduction in lifespan.
A
B
C
D
E
F
0 10 30 50 100 150 200 0 10 30 50 100 150 200 0 10 30 50 100 150 200 0 10 30 50 100 150 200 0 10 30 50 100 150 200 0 10 30 50 100 150 200 De v elopment time from egg to a d u lt (h) 60 50 40 30 20 10 0*
*
*
*
*
De v elopment time from L2 to L 3 (h) 10 8 6 4 2 0 12 10 8 6 4 2 0 10 8 6 4 2 0 9 8 7 6 5 4 3 2 1 0 25 20 15 10 5 0 Magnetic force (mT) De v elopment time from L4 to y o u ng a d u lt (h) De velopment time from
yo u ng a d u lt to a d u lt (h) De v elopment time from L 3 to L4 (h) De v elopment time from egg to L2 (h)
Fig.3. The effects of SMFs on the life cycle of wild-type nematodes. Synchronized wild-wild-type nematodes were transferred onto NGM plates with the E. coli strain OP50 as a food source. Larval worms were grown under SMFs from 0 to 200mT, and the development time at each stage was measured at 20°C. (A)The total development time of wild-type nematodes was significantly reduced by 23% with a 200mT SMF. (B)No significant reduction was observed in the development time from egg to the L2 stage under SMF treatment. (C)The development time from L2 to L3 was significantly reduced by 18% and 23% with 150mT and 200mT SMFs,
respectively. (D)There was no significant reduction in the development time from L3 to L4 after SMF treatment of 0–200mT. (E)The development time from L4 to young adult was significantly reduced by 19% with 200mT SMF treatment. (F)The
development time from young adult to adult was significantly reduced by 23% with 200mT SMF treatment. Each group contains at least 60 worms. Data are presented as means and s.d. The comparisons between groups were performed through a one-way analysis of variance. The differences between groups were considered statistically significant at P<0.05 (*).
Table 1. Primer sequences for genes involved in development and aging
Symbol Annotation Primer sequence (5⬘j3⬘)
lin-4 Abnormal cell lineage F: gtgccagcctcacggaaagg
R: gggaggagtagctgaaggag
lin-14 Abnormal cell lineage F: aaccagcatcgccgacattac
R: ggagtggtggagctgtttcaac
lin-41 Abnormal cell lineage F: tcccgcaagactcctttcgg
R: gcgtcggagacaggtacatc
lim-7 LIM domain family F: accaccgatggcagtttgtgc
R: caggcaacacacgcaaagcag clk-1 Clock (biological timing) abnormality F: aggtgcaatggcttgtacaattgc
R: tccatcgtgttctactccagtatc
unc-3 Uncoordinated F: gatgtgccgagtgcttctcac
R: gcatcggtgcgcttagttctc
age-1 Aging alteration F: agagctccacggcactttcc
R: ctcagcttggcagccttgac daf-2 Abnormal dauer formation (insulin/IGF-1-like receptor) F: tactgtttgaagacactctgcca
R: aaactgtgctacacgaaaacgat F denotes the sequence of the forward primer.
DISCUSSION
SMFs accelerate the development and aging of nematodes Few studies have examined the effect of SMFs on fertility and the development of embryos and fetuses. Slight changes in spermatogenesis and embryogenesis in mice exposed to 1.5T for 30min have been reported (Narra et al., 1996). The maturation of sperm movement in mice as well as postnatal testicular and epididymis development were largely unaffected by either single, short-term exposure or continuous, long-term exposure at 500–700mT (Tablado et al., 1996; Tablado et al., 1998; Tablado et al., 2000).
Strong magnetic field gradients may affect embryonic development in amphibians (International Agency for Research on Cancer, 2002). The abnormal growth and increased incidence of malformations in embryos exposed to a static field of 1T have been observed (Neurath, 1968; Ueno et al., 1984). Later studies indicated
a lack of developmental effects after exposure to a SMF (Ueno et al., 1994). Exposure to SMFs of up to 17T induced abnormalities in the first three cleavages of the African clawed toad, Xenopus laevis, embryo (Denegre et al., 1998; Ueno et al., 1984; Ueno et al., 1994).
Teratogenic effects did not occur in mammalian embryos when they were exposed to a static field of up to 6.3T (Sikov et al., 1979; Konermann and Monig, 1986; Okazaki et al., 2001; Murakami et al., 1992). However, when rats were exposed to a 30mT static field during the entire gestation period, a significant decrease in the number of live fetuses per litter was reported (Mevissen et al., 1994). Abnormalities in mammalian development as a result of strong MRI exposures of up to 5T have been reported (Tyndall, 1993; Tyndall, 1990; Tyndall and Sulik, 1990; Carnes and Magin, 1996; Magin et al., 2000). These include neuronal and eye abnormalities. However, despite the differences in the experimental protocols, the effects described in the two studies are not likely to be reproducible. Although developmental abnormalities in individuals have been observed, the statistical analyses and molecular studies are missing. The current study provides, for the first time, quantitative measurements of gene expression that correlate with developmental and aging processes. The functional analyses using nematode mutants further provide strong evidence for molecular pathways mediating the SMF-induced acceleration of both the development and aging processes.
Global effects of SMFs
We expect the effects of SMFs to be global. Among the genes selected for measurement of transcriptional activity, most were 65,536 16,384 4096 1024 256 64 16 4 1 16,384 4096 1024 256 64 16 4 1
0.25 lin-4 lin-14 clk-1 lim-7 unc-3 age-1
Control 30 mT 50 mT 100 mT 150 mT 200 mT
A
B
F old ch a ngeclk-1 lim-7 daf-2 age-1
Fig.4. Quantitative real-time RT-PCR for genes associated with development and aging pathways. (A)Treatment with a 200mT SMF elevated the expression levels of clk-1, lim-7, unc-3 and age-1. The expression level of the corresponding gene from an untreated group served as the normalization control. The fold changes were calculated from the difference in cycle numbers of real-time RT-PCR by the Ct method as described in Materials and methods. (B)Upregulation of age-1, daf-2, lim-7 andclk-1 corresponds to increasing doses of SMFs. Nematodes were cultured under SMFs at strengths of 30, 50, 100, 150 and 200mT. The expression levels of age-1, daf-2, lim-7 and clk-1 for the treated worms relative to the untreated controls were obtained by quantitative real-time RT-PCR and are expressed as a fold change. The data are averaged from six independent experiments. Data are presented as means and s.d.
60 50 40 30 20 10 0
*
*
*
**
*
N2 lin-4 lin-14 lin-41 lim-7 clk-1 let-7 unc-3 age-1
To
ta
l de
v
elopment time (h)
Fig.5. The effects of SMFs on the life cycle of mutant nematodes. Synchronized wild-type nematodes were transferred onto NGM plates with the E. coli strain OP50 as a food source. Larval worms were grown at 20°C under SMFs from 0 to 200mT, and the development time of each stage was measured. Each group contained at least 60 worms. Data are presented as means and s.d. Comparisons between groups were performed through a one-way analysis of variance. The differences between groups were considered statistically significant at P<0.05 (*) or P<0.01 (**). The hatched bars depict data for the 200mT SMF-treated group. The filled bars depict data for the untreated group. For wild-type nematodes, SMF treatment significantly reduced the development time by 22%, from 58h to 45h. The life cycles of let-7, unc-3, clk-1 and age-1 mutants were not affected by SMF treatment. The life cycles of lim-7, lin-14, lin-41 and lin-4 mutants were significantly reduced by 20%, 23%, 21% and 22%, respectively, after SMF treatment.
affected to different extents. However, genes associated with development and aging were particularly up-regulated. The clk-1, lim-7, daf-2 and age-1 genes are associated with development and aging; however, these genes are members of distinct pathways within the development and aging processes. The clk-1 (clock abnormal protein 1) gene encodes an enzyme (demethoxyubiquinone mono-oxygenase) that is necessary for ubiquinone biosynthesis in the worm C. elegans and other eukaryotes (Liu et al., 2005). The mouse version of the gene is called mclk1, and the human, fruit fly and yeast homologs are called COQ7. The gene lim-7 encodes one of seven C. elegans LIM-homeodomain (LIM-HD) proteins and is the sole C. elegans member of the Islet subclass of LIM-HD transcription factors. Loss of lim-7 activity results in L1 larval lethality characterized by locomotion and morphological defects. A later role for lim-7 in gonad development has been suggested. The gene age-1 encodes a homolog of the page-1age-10 subunit of phosphatidylinositol 3-kinase, and 2 encodes an insulin receptor family member. daf-2 and age-1 are involved in regulating the entry into an alternative developmental pathway, the dauer pathway. For these genes, the increase in lifespan is primarily due to an increase in adult lifespan. Although we focused on genes and pathways associated with development and aging, it is likely that SMFs may affect pathways of other biological processes. A genomic survey will reveal the global effect of SMFs on the biological system.
Triggering effects of SMFs on transcription
Given the minor effects of SMF on development time and lifespan, it is intriguing to observe the sensitive and enhanced response of gene expression after application of SMFs. SMFs higher than 50mT seem to trigger the up-regulation of clk-1, lim-7, unc-3 and age-1,
and gene expression instantly reached a plateau. A switch in expression seemed to occur if the applied SMF was higher than a threshold level, which resides somewhere between 30 and 50mT. The strong change in gene expression at 50mT exhibited by the nematode was unexpected because a SMF at this intensity did not induce a significant variation in development time. Further translational studies and functional investigations will reveal the molecular correlation between SMFs and development.
Genetic analyses of mutants reveal possible mechanisms underlying the SMF-induced gene expression
Here we took advantage of nematode genetics to quantify expression levels with quantitative real-time RT-PCR. The results are consistent with the mutant analyses in both development time and lifespan (Figs5 and 6). The only exception occurs with lim-7. SMFs up-regulated lim-7; however, development in the mutant strain occurred at wild-type rates. It is likely that SMFs affect development through alternative pathways, since we have shown the global effect of SMFs. Thus, although the expression levels of lim-7 were not increased in the mutant nematode, SMFs shortened the development time through other pathways.
CONCLUSIONS
Here, we have shown that exposing nematodes to SMFs above 150mT significantly shortens the life cycle of C. elegans. The application of SMFs accelerated nematode development from the L2 to adult stages. The greatest effect, a 23% reduction in development time, was seen in the progression from the L2 stage to the L3 stage. Using real-time quantitative RT-PCR, we identified genes (clk-1, lim-7, unc-3 and age-1) that were differentially
0 2 4 6 8 10 12 1416 18 20 22 24 2 28 30 32 100 80 60 40 20 0 age-1 age-1+SMFs Time (days)
C
100 80 60 40 20 0 % Aliv eA
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 let-7 let-7+SMFs 0 2 4 6 8 10 12 1 16 1 20 22 24 2 28 30 32 3 36 100 80 60 40 20 0 Time (days)B
unc-3 unc-3+SMFs % Aliv eFig.6. The effects of SMFs on the lifespan of mutant nematodes. (A)The lifespan of let-7 mutants in the presence and absence of a 200mT SMF. (B)The lifespan of unc-3 mutants in the presence and absence of a 200mT SMF. (C)The lifespan of age-1 mutants in the presence and absence of a 200mT SMF. Each experiment contained 60 nematodes. The lifespan experiment was performed at 20°C.
expressed following SMF treatment. A dose–response analysis indicated that a 50mT SMF was sufficient to trigger the induction of transcription. Genetic analysis using mutant strains indicated that developmental and aging pathways, including the insulin receptor pathway, are involved in the SMF-induced development time and lifespan reductions. SMFs of sufficient intensity induced a reduction in life cycle time and accelerated the aging process in nematodes. The current study provides insight into the molecular consequences and mechanisms underlying SMF treatment.
ACKNOWLEDGEMENTS
This study was supported in part by the National Science Council in Taiwan (grants NSC96-2320-B-009-001 and NSC 97-2320-B-009-002-MY3), the Bureau of Animal and Plant Health Inspection and Quarantine Council of Agriculture in Taiwan (grant 98AS-9.2.4-BQ-B1), and by China Medical University Hospital (DMR-93-52 and DMR-97-075).
REFERENCES
Ananta, V. and Shantha, N. (2008). Exposure of seeds to static magnetic field
enhances germination and early growth characteristics in chickpea (Cicer arietinum L.).Bioelectromagnetics 29, 571-578.
Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94.
Buemi, M., Marino, D., Di Pasquale, G., Floccari, F., Senatore, M., Aloisi, C., Grasso, F., Mondio, G., Perillo, P., Frisina, N. et al. (2001). Cell proliferation/cell
death balance in renal cell cultures after exposure to a static magnetic field. Nephron
87, 269-273.
Carnes, K. I. and Magin, R. L. (1996). Effects of in utero exposure to 4.7 T MR
imaging conditions on fetal growth and testicular development in the mouse. Magn. Reson. Imaging 14, 263-274.
Cassada, R. C. and Russell, R. L. (1975). The dauerlarva, a post-embryonic
developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326-342.
Denegre, J. M., Valles, J. M., Lin, K., Jordan, W. B. and Mowry, K. L. (1998).
Cleavage planes in frog eggs are altered by strong magnetic fields. Proc. Natl. Acad. Sci. USA 95, 14729-14732.
Drinker, C. K. and Thomson, R. M. (1921). Does the magnetic field constitute an
industrial hazard? J. Ind. Hyg. 3, 117-129.
Gamboa, O. L., Gutierrez, P. M., Alcalde, I., De la Fuente, I. and Gayoso, M. J.
(2007). Absence of relevant effects of 5 mT static magnetic field on morphology, orientation and growth of a rat Schwann cell line in culture. Histol. Histopathol. 22, 777-780.
Hertweck, M., Hoppe, T. and Baumeister, R. (2003). C. elegans, a model for aging with high-throughput capacity. Exp. Gerontol. 38, 345-346.
Hiraoka, M., Miyakoshi, J., Li, Y. P., Shung, B., Takebe, H. and Abe, M. (1992).
Induction of c-fos gene expression by exposure to a static magnetic field in HeLaS3 cells. Cancer Res. 52, 6522-6524.
Hope, I. A. (1999). C. elegans. Oxford: Oxford University Press.
International Agency for Research on Cancer (IARC) (2002). IARC Monographs on
the Evaluation of Carcinogenic Risks to Humans. Non-Ionising Radiation. Part 1, Static and Extremely Low Frequency (ELF) Electric and Magnetic Fields. IARC 80.
Kenyon, C., Chang, J., Gensch, E., Rudner, A. and Tabtiang, R. (1993). A C. elegans mutant that lives twice as long as wild type. Nature 366, 461-464.
Kimura, T., Takahashi, K., Suzuki, Y., Konishi, Y., Ota, Y., Mori, C., Ikenaga, T., Takanami, T., Saito, R., Ichiishi, E. et al. (2008). The effect of high strength static
magnetic fields and ionizing radiation on gene expression and DNA damage in Caenorhabditis elegans. Bioelectromagnetics 29, 605-614.
Konermann, G. and Monig, H. (1986). Studies on the influence of static magnetic
fields on prenatal development of mice. Radiologie 26, 490-497.
Lai, H. and Singh, N. P. (2004). Magnetic-field-induced DNA strand breaks in brain
cells of the rat. Environ. Health Perspect. 112, 687-694.
Leszczynski, D. (2005). Rapporteur report: cellular, animal and epidemiological
studies of the effects of static magnetic fields relevant to human health. Prog. Biophys. Mol. Biol. 87, 247-253.
Liu, X., Jiang, N., Hughes, B., Bigras, E., Shoubridge, E. and Hekimi, S. (2005).
Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 19, 2424-2434.
Magin, R. L., Lee, J. K., Klintsova, A., Carnes, K. I. and Dunn, F. (2000). Biological
effects of long-duration, high-field (4 T) MRI on growth and development in the mouse. J. Magn. Reson. Imaging 12, 140-149.
Maups, E. (1900). Modes et formes de reproduction des nematodes. Arch. Zool. Exp. Gén. 7, 563-628.
McCann, J. (1998). Cancer risk assessment of extremely low frequency electric and
magnetic fields: a critical review of methodology. Environ. Health Perspect. 106, 701-717.
Mevissen, M., Butenkotter, S. and Loscher, W. (1994). Effects of static and
time-varying (50-Hz) magnetic fields on reproduction and fetal development in rats. Teratology 50, 229-237.
Miyakawa, T., Yamada, S., Harada, S., Ishimori, T., Yamamoto, H. and Hosono, R.
(2001). Exposure of Caenorhabditis elegans to extremely low frequency high magnetic fields induces stress responses. Bioelectromagnetics 22, 333-339.
Miyakoshi, J. (2005). Effects of static magnetic fields at the cellular level. Prog. Biophys.Mol.Biol. 87, 213-223.
Murakami, J., Torii, Y. and Masuda, K. (1992). Fetal development of mice following
intrauterine exposure to a static magnetic field of 6.3 T. Magn. Reson. Imaging 10, 433-437.
Nakahara, T., Yaguchi, H., Yoshida, M. and Miyakoshi, J. (2002). Effects of
exposure of CHO-K1 cells to a 10-T static magnetic field. Radiology 224, 817-822.
Narra, V., Howell, R. W., Goddu, S. M. and Dandamudi, V. (1996). Effects of a
1.5-Tesla static magnetic field on spermatogenesis and embryogenesis in mice. Invest. Radiol. 31, 586-590.
Neurath, P. W. (1968). High gradient magnetic field inhibits embryonic development of
frogs. Nature 219, 1358-1359.
Okazaki, R., Ootsuyama, A., Uchida, S. and Norimura, T. (2001). Effects of a 4.7T
static magnetic field on fetal development in ICR Mice. J. Radiat. Res. 42, 273-283.
Pacini, S., Gulisano, M., Peruzzi, B., Sgambati, E., Gheri, G., Gheri, B. S., Vannucchi, S., Polli, G. and Ruggiero, M. (2003). Effects of 0.2 T static magnetic
field on human skin fibroblasts. Cancer Detect. Prev. 27, 327-332.
Pagliara, P., Lanubile, R., Dwikat, M., Abbro, L. and Dini, L. (2005). Differentiation
of monocytic U937 cells under static magnetic field exposure. Eur. J. Histochem. 49, 75-86.
Pate, K., Benghuzzi, H., Tucci, M., Puckett, A. and Cason, Z. (2003). Morphological
evaluation of MRC-5 fibroblasts after stimulation with static magnetic field and pulsating electromagnetic field. Biomed. Sci. Instrum. 39, 460-465.
Sakurai, T., Terashima, S. and Miyakoshi, J. (2009). Effects of strong static
magnetic fields used in magnetic resonance imaging on insulin-secreting cells. Bioelectromagnetics 30, 1-8.
Sandyk, R., Anninos, P. A., Tsagas, N. and Derpapas, K. (1992). Magnetic fields in
the treatment of Parkinson’s disease. Int. J. Neurosci. 63, 141-150.
Sikov, M. R., Mahlum, D. D., Montgomery, L. D. and Decker, J. R. (1979).
Development of mice after intrauterine exposure to direct-current magnetic fields. Phillips 462-473.
Suzuki, Y., Ikehata, M., Nakamura, K., Nishioka, M., Asanuma, K., Koana, T. and Shimizu, H. (2001). Induction of micronuclei in mice exposed to static magnetic
fields. Mutagenesis 16, 499-501.
Tablado, L., Pérez-Sánchez, F. and Soler, C. (1996). Is sperm motility maturation
affected by static magnetic fields? Environ. Health Perspect. 104, 1212-1216.
Tablado, L., Pérez-Sánchez, F., Núñez, J., Núñez, M. and Soler, C. (1998). Effects
of exposure to static magnetic fields on the morphology and morphometry of mouse epididymal sperm.Bioelectromagnetics 19, 377-383.
Tablado, L., Soler, C., Núñez, M., Núñez, J. and Pérez-Sánchez, F. (2000).
Development of mouse testis and epididymis following intrauterine exposure to a static magnetic field. Bioelectromagnetics 21, 19-24.
Tarantino, P., Lanubile, R., Lacalandra, G., Abbro, L. and Dini, L. (2005).
Post-continuous whole body exposure of rabbits to 650 MHz electromagnetic fields: effects on liver, spleen, and brain. Radiat. Environ. Biophys. 44, 51-59.
Teodori, L., Grabarek, J., Smolewski, P., Ghibelli, L., Bergamaschi, A., De Nicola, M. and Darzynkiewicz, Z. (2002). Exposure of cells to static magnetic field
accelerates loss of integrity of plasma membrane during apoptosis. Cytometry 49, 113-118.
Tyndall, D. A. (1990). MRI effects on the teratogenicity of x-irradiation in the C57BL/6J
mouse. Magn. Reson. Imaging. 8, 423-433.
Tyndall, D. A. (1993). MRI effects on craniofacial size and crown-rump length in
C57BL/6J mice in 1.5T fields.Oral Surg. Oral Med. Oral Pathol. 76, 655-660.
Tyndall, D. A. and Sulik, K. K. (1990). Effects of magnetic resonance imaging on eye
development in the C57BL/6J mouse. Teratology 43, 263-275.
Ueno, S., Umar, H., Bambauer, H. J. and Ueck, M.(1984). Ultracytochemical localization of Ca++-ATPase activity in the paraphyseal epithelial cells of the frog, Rana esculenta Cell Tissue Res. 235, 3-11.
Ueno, S. and lwasaka, M. (1994). Early embryonic development of frogs under
intense magnetic fields up to 8 T. J. Appl. Phys. 75, 7165-7167.
Valentina, H., Giulio, G., Nicola, V., Massimo, L., Luigi, L. and Silvana, S. (2009).
Biological effects and safety in magnetic resonance imaging: a review.Int. J. Environ. Res. Public Health 6, 1778-1798.
Van Voorhies, W. A. (1992). Production of sperm reduces nematode lifespan. Nature
360, 456-458.
Yamaguchi, H., Hosokawa, K., Soda, A., Miyamoto, H. and Kinouchi, Y. (1993).
Effects of seven months’ exposure to a static 0.2 T magnetic field on growth and glycolytic activity of human gingival fibroblasts. Biochim. Biophys. Acta. 1156, 302-306.