Contents lists available atScienceDirect
Journal of Hazardous Materials
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j h a z m a tDegradation of carbofuran in aqueous solution by ultrasound and Fenton
processes: Effect of system parameters and kinetic study
Ma Ying-Shih
a,∗, Sung Chi-Fanga
a, Lin Jih-Gaw
baDepartment of Environmental Engineering and Health, Yuanpei University, Hsinchu, Taiwan bInstitute of Environmental Engineering, National Chiao Tung University, Hsinchu, Taiwan
a r t i c l e i n f o
Article history:Received 28 September 2009 Received in revised form 24 December 2009 Accepted 14 January 2010 Available online 1 February 2010 Keywords: Carbofuran Degradation First-order kinetics Ultrasound/Fenton process
a b s t r a c t
To establish an efficient oxidation process for carbofuran degradation, the effects of some operating parameters such as dosages of H2O2, Fe2+and initial carbofuran concentrations were observed during
carbofuran degradation by the ultrasound process, Fenton process and a combined ultrasound/Fenton process. The degradation kinetics of carbofuran was also examined based on the experimental data. The results show that more than 99% of the carbofuran was degraded by the ultrasound/Fenton process within short reaction time periods. Increased dosages of H2O2and Fe2+enhanced the degradation of
carbofu-ran in the ultrasound and Fenton oxidation processes, but initial carbofucarbofu-ran concentrations decreased carbofuran degradation in both the Fenton and ultrasound/Fenton processes. The degradation kinetics of carbofuran by the three oxidation processes was found to be in accordance with first-order reaction kinetics. The results provide fundamental information about the treatment of carbofuran wastewater and/or other pesticides by the ultrasound/Fenton oxidation process.
© 2010 Elsevier B.V. All rights reserved.
1. Introduction
Advanced oxidation processes (AOPs) are generally used to degrade hazardous and non-biodegradable organic materials and convert them into carbon dioxide by producing•OH radicals during the oxidation process[1,2]. Several kinds of AOPs (e.g. ultrasound [1,3,4], Fenton[5–9], ozone[10,11]and ultraviolet light[12,13]) have been effectively used in the treatment of wastewater con-taining pesticide, phenols, chlorophenols and azo dyes.
Carbofuran (2,3-dihydro-2,2-dimethylbenzofurarn-7-yl met-hylcarbamate, C12H15NO3) is a well-known methylcarbamate
pes-ticide used to inhibit insect activity in soybeans, rice, sugar cane, tobacco, maize, potatoes and vegetables. The use of carbofuran has aroused considerable concern not only due to its heavy rate of use (10.5% of the total pesticides in Taiwan, 2003–2007) but also due to its high oral toxicity[14]. Several biological and chemical treat-ment methods have been investigated to reduce the amount of carbofuran present in wastewater[9,12–19]. Chaudhry and Ali[15] isolated 15 bacteria from soil samples and used them as a biological method of degrading carbofuran. All isolates were gram negative and were oxidase- and catalase-positive rods, occurring singly or as short chains. The results indicated that the carbofuran is the bacte-ria’s sole source of nitrogen or carbon, and that they hydrolyze the carbofuran to carbofuran phenol. In addition, some of the bacteria
∗ Corresponding author. Tel.: +886 3 6102336; fax: +886 3 6102337. E-mail address:ysma0728@mail.ypu.edu.tw(Y.-S. Ma).
were observed to degrade the carbofuran rapidly, up to 40% being lost as14CO
2in a 1 h reaction. These results suggested that the
iso-lates degrade carbofuran by utilizing an oxidative pathway. Bano and Musarrat[18]isolated NJ-101, initially identifying it as Pseu-domonas sp. occurring in agricultural soil. The isolates exhibited efficient degradation of carbofuran at a constant rate of 0.035 day−1 in accordance with first-order rate kinetics. Their ability to perform several biological activities in tandem suggested that isolate NJ-101 was unique. However, isolation and cultivation of the bacterial well needed a long time. The chemical processes are considered in the degradation of carbofuran.
Other researchers[16]used a chemical UV/TiO2process to treat
wastewater containing 222 mg/L carbofuran at pH 2.8. Their results indicated that 90% of the carbofuran was oxidized within a 6 h reaction time, but complete mineralization of the carbofuran to car-bon dioxide by UV/TiO2process took 15 h. Hua and Thompson[17]
investigated the treatment efficiency of 30 mg/L carbofuran degra-dation by an ultrasonic process at an ultrasound energy output of 1800 W. These results indicated that 90% of the carbofuran was oxidized within a 30–60 min reaction period, and that increased ultrasonic energy output and decreased initial carbofuran concen-tration enhanced carbofuran degradation.
In another study, a combined O3/UV process was also used to
treat wastewater containing 100 mg/L carbofuran at pH 2; in this case, 90% of the carbofuran was oxidized within 50 min[12]. That study also investigated the effect of a range of other treatment methods, including photo-Fenton, O3/UV, UV/H2O2 and Fenton
processes. They found that the photo-Fenton method produced sig-0304-3894/$ – see front matter © 2010 Elsevier B.V. All rights reserved.
nificantly higher treatment efficiency than the other processes. It is evident from these results that a combination of several chemical processes is effective in enhancing the degradation of organic pollu-tants. Ma et al.[9]used the Fenton process to degrade carbofuran in aqueous systems; they found that more than 90% of the carbofuran was removed within 5 min by the Fenton reaction at 5 mg/L of Fe2+
and 100 mg/L of H2O2, and that increasing the Fe2+and/or H2O2
concentrations beyond 5 mg/L and 100 mg/L respectively removed all the carbofuran.
The objectives of the present study were to investigate the degradation of carbofuran by the ultrasound, Fenton and a combined ultrasound/Fenton processes under different reaction conditions; carbofuran degradation rate constants were also inves-tigated using the first-order kinetic model.
2. Materials and methods
2.1. Materials
The chemical reagents used in this study were carbo-furan (C12H15NO3, purity > 98%), ferrous sulfate (FeSO4·7H2O,
purity > 99.5%), and an aqueous solution of hydrogen peroxide (H2O2, 30% (w/w) in water). During the analytical processes,
tita-nium sulfate solution (Ti(SO4)2), hydrogenophthalate potassium
(C8H5KO4) (KHP) and dichloromethane (CH2Cl2) were used in
determining H2O2concentration, total organic carbon (TOC)
con-centration and carbofuran concon-centration. These chemicals were the purest grade commercially available and were used without further purification.
2.2. Experimental apparatus
A schematic drawing of the reactor is shown inFig. 1. A 0–750 W sonicator (Microson VCX 750, USA) equipped with s sealed con-verter (Model CV 33, 63.5 mm in diameter and 183 mm in length) and titanium probe tip (Part. no. 630-0210, 25 mm in diameter and 122 mm in length) operated at 20 kHz was used in this study. The amplitude in the ultrasound and ultrasound/Fenton tests was adjusted at 40% (300 W power output) without pulse length setup. Reactions were performed in a cylindrical reactor (1 L working volume) with a cooling jacket and a circulating temperature con-troller to maintain the reaction temperature at 25◦C during the ultrasound, Fenton and ultrasound/Fenton tests. A magnetic mixer
Fig. 1. Schematic diagram of the experimental apparatus designed for carbofuran
decomposition.
Table 1
Design of reaction parameters in the decomposition of carbofuran by the ultrasound, Fenton and ultrasound/Fenton processes.
Process Parameters Range
Ultrasound Concentration of carbofuran (mg/L) 20
pH value 3
H2O2addition (mg/L) 0–500
Fenton Concentration of carbofuran (mg/L) 10–200
pH value 3
H2O2addition (mg/L) 100
Fe2+addition (mg/L) 1
Ultrasound/Fenton Concentration of carbofuran (mg/L) 50–200
pH value 3
H2O2addition (mg/L) 100
Fe2+addition (mg/L) 1–20
controlled the mixing speed of the solution at 100 rpm. Aeration inside the reactor was maintained at 0.2 L of air/min. Sensors for oxidation–reduction potential (ORP) and pH (Suntex PC-3200, Tai-wan) were placed in the solution; the ORP and pH profiles were monitored online. Prior to commencing the experiment, the ORP sensor was rechecked using a standard solution of 220 mV. 2.3. Experimental conditions and procedure
The value of the initial pH in all experiments was 3 attained by adding 1 N H2SO4as required. During the reactions, the pH values
varied freely without any hand correction. In the ultrasound pro-cess, the effect on carbofuran degradation of H2O2dosage ranging
from 0 mg/L to 500 mg/L was observed. In the Fenton process, the dosage of H2O2was 100 mg/L and the dosage of Fe2+was 1 mg/L.
The effect of initial carbofuran concentration (10–200 mg/L) on car-bofuran degradation by the Fenton process was studied.
The values of experimental variables such as H2O2, ferrous
iron and carbofuran concentrations were designed based on the other studies[13,14,16–18]and our preliminary study[9]. In the ultrasound/Fenton process, the dosage of H2O2was 100 mg/L. The
effects of initial carbofuran concentration (50–200 mg/L) and Fe2+
dosages (1–20 mg/L) were studied. The reaction parameters for the ultrasound, Fenton and ultrasound/Fenton processes are shown in Table 1.
2.4. Sample analysis
Concentrations of carbofuran were analyzed by gas chro-matography equipped with a mass spectrometry (GC–MS-QP2010, Shimadzu, Japan) using a DB-5MS column (length 30 m, thickness 0.25m, diameter 0.25 mm) in the GC oven. Before analysis, 5 mL water samples were collected at set time intervals and 1 mL of dichloromethane was added and mixed with the water sample for 30 min at a mixing speed of 150 rpm following the steps described
Table 2
Results of carbofuran degradation, TOC removal and first-order kinetic constants in the degradation of carbofuran by an ultrasonic process with different H2O2dosages.
Methods Factors Carbofuran degradation (%) TOC removal (%) First-order reaction kinetics
H2O2dosage (mg/L) K (×10−3min−1) R2 Ultrasound 0 22 3 2.4 0.814 10 34 6 2.7 0.810 50 36 10 3.0 0.899 100 39 14 3.9 0.986 200 44 20 4.6 0.972 300 14 5 1.0 0.909 400 12 5 0.9 0.868 500 12 4 0.8 0.805
in Wang and Lemley[14]. The GC oven temperature ranged from 80◦C (holding time 2 min) to 210◦C (holding time 3 min) at a ramp of 10◦C/min and from 210◦C to 305◦C at a ramp of 30◦C/min. The injector and detector temperatures were maintained at 220◦C and 250◦C respectively. High purity (99.99%) helium was used as a carrier gas (1.5 mL/min) and the sample was analyzed in splitless mode. Mass spectra were obtained by electron-impact (EI) at 70 eV using the full-scan mode.
Prior to sample analysis, a calibration curve was plotted with known concentrations of carbofuran (between 1 mg/L and 30 mg/L) and an area response with an R2of 0.995.Fig. 2shows the mass
spectrum of carbofuran determination in GC–MS analysis. Based on the appearance of mass spectrum including peak 131, 164 and 221 and comparison with the mass spectra library in GC–MS, it can be identified as the carbofuran where the similarity was greater than 99%. The hydrogen peroxide concentration was measured by the spectrophotometric determination using a 412 nm wavelength spectrophotometer (UV-2102, Unico TM, USA) and potassium tita-nium oxalate.
Mineralization of an organic compound is generally defined as its being readily oxidized to carbon dioxide; hence, in the present study, carbofuran mineralization was effected by removing all of the organic carbon. TOC concentrations were measured with the oxalate wet oxidation method using a TOC analyzer (TOC-500, Shimadzu, Japan) for which hydrogenophthalate potassium was adopted as the reference standard. Method detection limits (MDLs) and recovery of carbofuran, H2O2and TOC concentrations
are: carbofuran 0.08 mg/L, H2O20.11 mg/L and TOC 0.04 mg/L at
97.9± 2.8%, 94.9 ± 5.9% and 97.0 ± 3.6% recoveries respectively.
3. Results and discussion
3.1. Treatment of carbofuran by the ultrasound process
The ultrasound process was first used in waste treatment and medical research in 1927 and has been applied to wastewater treat-ment since 1956. Two reaction mechanisms have been proven to be effective in destroying the molecular structure of refractory organic matters: direct pyrolysis inside the cavitation bubbles produced by the passage of ultrasound waves through water; and indirect oxidation by hydroxyl (•OH) radical attack taking place in the ultra-sonic process. In the present study, the ultraultra-sonic process was used to treat the 20 mg/L carbofuran wastewater with different H2O2
concentrations at pH 3 for a reaction period of 120 min, where the sampling time intervals were 10 min, 20 min, 30 min, 60 min, 90 min and 120 min.
Several authors have used different treatment methods to degrade carbofuran, all of them finding that the degradation of carbofuran followed a first-order kinetics process [12,13,18]. Accordingly, first-order kinetics was used in the present study to determine the rate constant (K) of carbofuran degradation. The results of carbofuran degradation, TOC removal and rate constants inTable 2show that degradation of the carbofuran increased from 22% to 44% with increasing H2O2 dosages of 0–200 mg/L within
120 min. Dosages greater than 300 mg/L led to a decrease in car-bofuran degradation to around 12–14%. Sun et al.[20]investigated the effect of H2O2dosage on the degradation of AB1 dyes by the
ultrasound/Fenton process and found that the reaction was sig-nificantly influenced by H2O2 dosage: the decoloration efficiency
Table 3
Results of carbofuran degradation, TOC removal and first-order kinetic constants in the degradation of carbofuran by the Fenton process for different carbofuran concentrations. Methods Factors Carbofuran degradation (%) TOC removal (%) First-order reaction kinetics
K (×10−3min−1) R2
Fentona Carbofuran concentration (mg/L)
10 81 10 25.7 0.987 20 42 8 16.3 0.991 50 40 7 12.5 0.954 100 39 6 11.9 0.912 200 15 6 4.9 0.984 Ultrasound/Fenton Fe2+dosage (mg/L) 1b 52 8 17.4 0.971 5 100 12 –c – 10 100 40 – – 20 100 46 – – Carbofuran concentration (mg/L) 50d 100 14 102.7 0.973 100 63 10 41.3 0.922 200 38 9 37.1 0.866 aDosages of Fe2+and H
2O2were 1 mg/L and 100 mg/L, respectively. bInitial carbofuran concentration and dosage of H
2O2were 20 mg/L and 200 mg/L, respectively. c The concentration of carbofuran was lower than MDL in the first collected sample (reaction time 1 min). dDosages of Fe2+and H
increased from 67% to 92.39% as a consequence of increasing H2O2
dosage from 0.5 mM to 8.0 mM after 10 min. Further increase of the H2O2dosage from 8.0 mM to 32.0 mM, however, slowed the
degra-dation rate of AB1. This might be due to the increase in•OH radical formation when H2O2is first added, but at higher H2O2
concentra-tions•OH might be consumed by various mechanisms, including the scavenging effects of H2O2and the recombination of•OH
rad-icals[21]. This might explain the results of the present study, in which it was observed that continued increase of H2O2dosage led
to a decrease in the carbofuran degradation. Comparable results for TOC removal and the first-order rate constants are shown inTable 2: TOC removal gradually increased from 3% to 20% within 30 min and the first-order rate constant increased from 2.4× 10−3min−1to 4.6× 10−3min−1with H2O2dosages from 0 mg/L to 200 mg/L, after
which H2O2dosages greater than 200 mg/L resulted in a decline in
both TOC removal and first-order rate constant. 3.2. Treatment of carbofuran by the Fenton process
Ma et al.[9]applied the Fenton process to the degradation of carbofuran in an aqueous system. Batch experiments at pH 3 were designed using the central composite approach for the two inde-pendent variables Fe2+and H
2O2. Experimental results indicated
that more than 90% of the carbofuran was removed from an initial carbofuran concentration of 10 mg/L within 5 min using 5 mg/L of Fe2+and 100 mg/L of H
2O2. Increases in Fe2+and/or H2O2dosages
beyond 5 mg/L and 100 mg/L, respectively, produced 100% carbo-furan removal.
In the present study, the Fenton process was carried out using Fe2+ and H
2O2 concentrations of 1 mg/L and 100 mg/L,
respec-tively, added to initial carbofuran concentrations around 10 mg/L to 200 mg/L and a reaction time of 30 min where the sampling time intervals are 1 min, 2 min, 5 min, 10 min, 20 min and 30 min. The resulting carbofuran degradation, TOC removal and first-order rate constants are given inTable 3. It was observed that the carbofuran degradation decreased from 81% to 15% when the initial carbofu-ran concentration increased from 10 mg/L to 200 mg/L. In addition, 59% of the carbofuran was degraded within 1 min when the initial carbofuran concentration was 10 mg/L (data not shown). This indi-cates that adding the Fenton reagents Fe2+and H
2O2produces•OH
radicals in aqueous solution, leading to rapid degradation of carbo-furan. When the initial carbofuran concentration was increased to 200 mg/L, however, further addition of Fenton reagents was insuf-ficient to effectively degrade the carbofuran; the degradation of carbofuran was seen to decrease to 15% (Table 3).
Mineralization of carbofuran by the Fenton process at different initial carbofuran concentrations is also shown inTable 3, where the TOC removal fell slightly from 10% to 6% as the initial carbofuran concentration increased from 10 mg/L to 200 mg/L, indicating that the traditional Fenton process can effectively degrade and transfer the carbofuran to other products or intermediates but is ineffective in mineralizing the carbofuran to carbon dioxide. Several authors [6,8,22,23]have reported similar results.
Comparing the carbofuran degradation rate constants (Table 3), the rate constant decreased from 25.7× 10−3min−1 (carbofuran
10 mg/L) to 4.9× 10−3min−1(carbofuran 200 mg/L); that is, lower
initial carbofuran concentration yields greater carbofuran degrada-tion.
3.3. Treatment of carbofuran by the ultrasound/Fenton process From the above, it is clear that carbofuran mineralization is insignificant over short reaction periods for aqueous systems when either the ultrasound process, or the Fenton process. Therefore, in the present study we have examined the effect of combining the ultrasound and Fenton processes (termed the ultrasound/Fenton
process) for the degradation of carbofuran. In addition, the effect of Fe2+dosage and initial carbofuran concentration on the
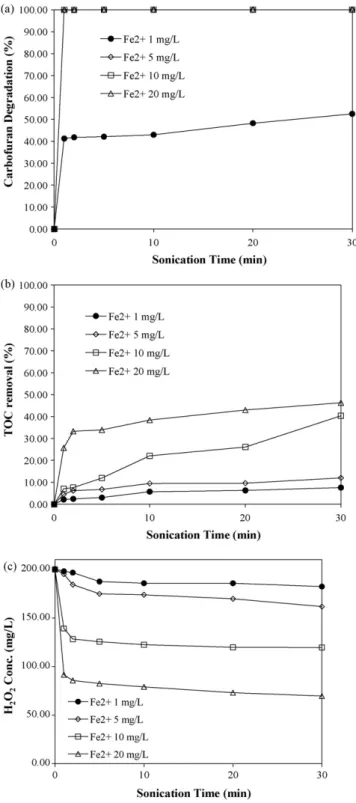
degra-dation and mineralization of carbofuran have been investigated. Fig. 3shows the results for carbofuran degradation, removal of TOC and consumption of H2O2in the degradation of carbofuran by
the combined ultrasound/Fenton process. In this case, the initial carbofuran concentration and the dosages of H2O2were 20 mg/L
and 200 mg/L and the dosages of Fe2+were 1 mg/L, 5 mg/L, 10 mg/L
and 20 mg/L. InFig. 3(a), the carbofuran degradation reached 52% within 30 min at the Fe2+dosage of 1 mg/L. It is also seen inFig. 3(a)
Fig. 3. Effect of different Fe2+dosages on carbofuran degradation in the
ultra-sound/Fenton process (a) degradation of carbofuran, (b) removal of TOC, and (c) residual of H2O2.
Fig. 4. Effect of initial carbofuran concentrations on the decomposition of
carbofu-ran in the ultrasound/Fenton process (a) degradation of carbofucarbofu-ran and (b) removal of TOC (Fe2+/H
2O2= 10/100 mg/L).
that the residual carbofuran concentrations since the first sampling time were all lower than the MDL of carbofuran for the Fe2+dosages
of 5 mg/L, 10 mg/L and 20 mg/L. Therefore, these three experimen-tal lines were overlapped. Calculation of the rate constants was not necessary because no carbofuran was present in the aqueous solu-tion after the ultrasound/Fenton process had been carried out for 1 min (Table 3).
Other researchers have reported comparable results: Liang et al.[2]found that the ultrasound/Fenton process decomposed more than 99% of 4-chlorophenol within a reaction time of 2 min; Sun et al.[20]combined the ultrasound and Fenton processes to treat azo dye acid black 1 wastewater, achieving 99% removal efficiency in a very short time at the ultrasound frequency of 40 kHz and adding 0.025 mM Fe2+and 8.0 mM H
2O2.
In the present study,Fig. 3(b) shows the results of TOC removal at different Fe2+ dosages using the ultrasound/Fenton process.
Removal of TOC was 8% for Fe2+dosage of 1 mg/L, increasing to 46%
at 20 mg/L Fe2+within 30 min.Fig. 3(c) shows residual H
2O2for an
initial H2O2 dosage of 200 mg/L. Consumption of H2O2 increased
from 9% to 65% when the Fe2+dosage was increased from 1 mg/L
to 20 mg/L. Higher consumption of H2O2led to higher production
of•OH radicals and oxidation potential in the ultrasound/Fenton process, enhancing degradation and mineralization of carbofuran.
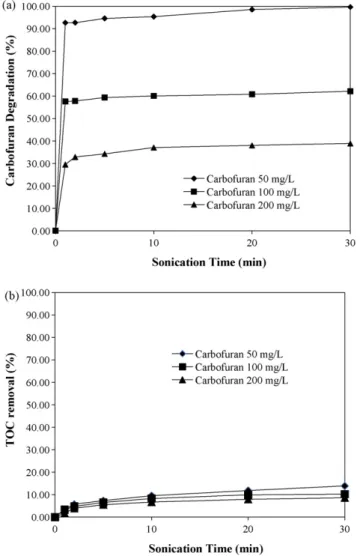
Fig. 4shows the results of carbofuran degradation and TOC removal by the ultrasound/Fenton process for different initial
car-Fig. 5. Effect of initial carbofuran concentration on the degradation of carbofuran by
the ultrasound/Fenton process. (Fe2+: 10 mg/L, H
2O2: 100 mg/L; (a) Fenton process;
(b) ultrasound/Fenton process; carbofuran concentration I: 20 mg/L, II: 100 mg/L, III: 200 mg/L.)
bofuran concentrations. Dosages of Fe2+and H
2O2 were 10 mg/L
and 100 mg/L and the initial carbofuran concentrations ranged from 50 mg/L to 200 mg/L. It is evident fromFig. 4(a) that approximately 90% of the carbofuran was immediately degraded when the ini-tial carbofuran concentration was 50 mg/L; degradation gradually increased to almost 100% after 30 min. When the initial carbofu-ran concentration was increased to 100 mg/L or more, however, degradation after 30 min significantly decreased to 60% (carbofu-ran 100 mg/L) and 35% (carbofu(carbofu-ran 200 mg/L).Fig. 4(b) shows that 14% of TOC was removed after 30 min, by which time the 50 mg/L carbofuran solution was oxidized. As the carbofuran concentration was increased to 100 mg/L and 200 mg/L, TOC removal fell further to 10%.
Fig. 5compares the effect of initial carbofuran concentrations on the degradation of carbofuran by both the ultrasound/Fenton process and the Fenton process with the Fe2+and H
2O2dosages
of 10 mg/L and 100 mg/L, respectively. Phases I–III represent the initial carbofuran concentrations of 20, 100 mg/L and 200 mg/L, respectively. Degradation efficiencies were 60%, 42% and 25% for the Fenton process alone after the reaction time of 30 min. When the ultrasound and Fenton process were combined, carbofu-ran degradation efficiencies rose to 99%, 63% and 39% for initial carbofuran concentrations of 20 mg/L, 100 mg/L and 200 mg/L, respectively. Table 3shows that more than 99% of the carbofu-ran was degraded by the combined ultrasound/Fenton process for the initial carbofuran concentration of 50 mg/L; the rate con-stant was 102.7× 10−3min−1, much higher than for the other
reaction conditions. As the initial carbofuran concentrations increased to 100 mg/L and 200 mg/L, the rate constants dropped to 41.3× 10−3min−1and 37.1× 10−3min−1.
4. Conclusion
The degradation of carbofuran in aqueous solution was investi-gated by ultrasonic, Fenton and ultrasound/Fenton processes under different experimental conditions, including different dosages of H2O2 and Fe2+ and a range of initial carbofuran concentrations.
More than 99% carbofuran degradation efficiency combined with 46% mineralization was achieved after 30 min reaction time for the initial carbofuran concentration of 20 mg/L and H2O2and Fe2+
dosages of 100 mg/L and 20 mg/L, respectively, all at pH 3. Degradation efficiency was enhanced by an increase in the Fen-ton reagents H2O2 and Fe2+, but an increase in initial carbofuran
study indicated that the degradation kinetics of carbofuran closely followed the first-order kinetics model, with the effects of the experimental parameters on the degradation of carbofuran result-ing in comparable reaction rate constants.
References
[1] R.A. Torres, F. Abdelmalek, E. Combet, C. Petrier, C. Pulgarin, A compar-ative study of ultrasonic cavitation and Fenton’s reagent for bisphenol: a degradation in deionised and natural waters, J. Hazard. Mater. 146 (2007) 546–551.
[2] J. Liang, S. Komarov, N. Hayashi, E. Kasai, Improvement in sonochemical degra-dation of 4-chlorophenol by combined use of Fenton-like reagents, Ultrason. Sonochem. 14 (2007) 201–207.
[3] M.H. Enterzari, M. Masoud, S.Y. Ali, A combination of ultrasound and bio-catalyst: removal of 2-chlorophenol from aqueous solution, Ultrason. Sonochem. 13 (2006) 37–41.
[4] H. Zhang, J. Zhang, C. Zhang, F. Liu, D. Zhang, Degradation of C.I. acid orange 7 by the advanced Fenton process in combination with ultrasonic irradiation, Ultrason. Sonochem. 16 (2009) 325–330.
[5] R. Oliveira, M.F. Almeida, L. Santos, L.M. Madeira, Experimental design of 2,4-dichlorophenol oxidation by Fenton’s reaction, Ind. Eng. Chem. Res. 45 (2006) 1266–1276.
[6] E.C. Catalkaya, F. Kargi, Effects of operating parameters on advanced oxidation of diuron by the Fenton’s reagent: a statistical design approach, Chemosphere 69 (2007) 485–492.
[7] G. Cravotto, S.D. Carlo, M. Curini, V. Tamiatti, C. Roggero, Decontamination of soil containing POPs by the combined action of solid Fenton-like reagents and microwaves, Chemosphere 69 (2007) 1326–1329.
[8] M.R.A. Silva, A.G. Trovo, R.F.P. Nogueira, Degradation of the herbicide tebuthiuron using solar photo-Fenton process and ferric citrate com-plex at circumneutral pH, J. Photochem. Photobiol. A Chem. 91 (2007) 187–192.
[9] Y.S. Ma, M. Kumar, J.G. Lin, Degradation of carbofuran-contaminated water by Fenton process, J. Environ. Sci. Health A: Tox. Hazard. Subst. Environ. Eng. 44 (2009) 914–920.
[10] V.O. Abramov, O.V. Abramov, A.E. Gekhman, V.M. Kuznetsov, G.J. Price, Ultrasonic intensification of ozone and electrochemical destruction of 1,3-dinitrobenzene and 2,4-dinitrotoluene, Ultrason. Sonochem. 13 (2006) 303–307.
[11] H. Zhang, L. Duan, D. Zhang, Decolorization of methyl orange by ozonation in combination with ultrasonic irradiation, J. Hazard. Mater. B 138 (2006) 53–59. [12] F.J. Benitez, J.L. Acero, F.J. Real, Degradation of carbofuran by using ozone, UV radiation and advanced oxidation processes, J. Hazard. Mater. B 89 (2002) 51–65.
[13] H. Katsumata, K. Matsuba, S. Kaneco, T. Suzuki, K. Ohta, Y. Yobiko, Degrada-tion of carbofuran in aqueous soluDegrada-tion by Fe (III) aquacomplexes as effective photocatalysts, J. Photochem. Photobiol. A: Chem. 170 (2005) 239–245. [14] Q. Wang, A.T. Lemley, Oxidative degradation and detoxification of aqueous
car-bofuran by membrane anodic Fenton treatment, J. Hazard. Mater. 98 (2003) 241–255.
[15] G.R. Chaudhry, A.N. Ali, Bacterial metabolism of carbofuran, Appl. Environ. Microbiol. 54 (1998) 1414–1419.
[16] K. Tennakone, C.T.K. Tilakaratne, I.R.M. Kottegoda, Photomineralization of car-bofuran by TiO2-supported catalyst, Water Res. 31 (1997) 1909–1912.
[17] I. Hua, U.P. Thompson, Ultrasonic irradiation of carbofuran: decomposition kinetics and reactor characterization, Water Res. 35 (2001) 1445–1452. [18] N. Bano, J. Musarrat, Characterization of a novel carbofuran degrading
Pseu-domonas sp. with collateral biocontrol and plant growth promoting potential, FEMS Microbiol. Lett. 231 (2004) 13–17.
[19] M. Mahalakshmi, B. Arabindoo, M. Palanichamy, V. Murugesan, Photocatalytic degradation of carbofuran using semiconductor oxides, J. Hazard. Mater. 143 (2007) 240–245.
[20] H.J. Sun, S.P. Sun, J.Y. Sun, R.Z. Sun, L.P. Qiao, H.Q. Gua, M.H. Fan, Degradation of azo dye acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation, Ultrason. Sonochem. 14 (2007) 761–766.
[21] C.G. Joseph, G.L. Puma, A. Bono, D. Krishnaiah, Sonophotocatalysis in advanced oxidation process: a short review, Ultrason. Sonochem. 16 (2009) 583–589. [22] M. Perez, F. Torrades, J.A. Garcıa-Hortal, X. Domenech, J. Peral, Removal of
organic contaminants in paper pulp treatment effluents under Fenton and photo-Fenton conditions, Appl. Catal. B: Environ. 36 (2002) 63–74.
[23] Y. Segura, R. Molina, F. Martinez, J.A. Melero, Integrated heterogeneous sono-photo Fenton processes for the degradation of phenol aqueous solutions, Ultrason. Sonochem. 16 (2009) 417–424.