www.elsevier.com / locate / chromb
P
urification of human plasma haptoglobin by hemoglobin-affinity
column chromatography
a a b a a ,
*
Chun Yi Liau , Tsai Mu Chang , Ju Pin Pan , Wen Liang Chen , Simon J.T. Mao
aResearch Institute of Biochemical Engineering, Department of Biological Science and Technology, National Chiao Tung University, 75 Po-Ai Street, Hsinchu, Taiwan
b
Division of Cardiology, Veterans General Hospital and Yang-Ming Medical College, Taipei, Taiwan
Abstract
Haptoglobin (Hp) is an acute-phase protein; its plasma levels increase consistently in response to infection and inflammation. The concentration of human plasma Hp is ranged between 1 and 1.5 mg / ml. Similar to blood type, individual human Hp is classified as Hp 1-1, 2-1, or 2-2. The structural and functional analysis of the Hp, however, has not been studied in detail due to its difficult isolation procedure. Previously, we reported a single step for the purification of porcine Hp. In this study, we established a purification method using a high capacity hemoglobin-affinity column. Briefly, DEAE-purified human hemoglobin was first coupled to Sepharose 4B to prepare an affinity column in a 15-ml bed volume. Following a flow through of human plasma and an extensive wash, the bound material was eluted with a solution of 0.15 M NaCl, pH 11 (adjusted by ammonium), to remove low-affinity bound proteins. The high-affinity bound Hp was then eluted with 0.15 M NaCl containing 5 M urea, pH 11, and collected in tubes containing 100 ml of 1 M Tris buffer, pH 7.0. The biological activity of dialyzed Hp was retained as it formed a complex with hemoglobin on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Using this procedure, approximately 10 mg of Hp 1-1, with homogeneity greater than 96%, was obtained from 15 ml of human plasma. Affinity purified Hp 2-1 or 2-2, however, contained trace amounts of apoA-I with the similar approach. The Hp could be further purified by HPLC using a Superose 12 gel-permeation chromatography, if desired, to achieve 100% purity. All the phenotypes of purified Hp consisted of a and
b chains on SDS–PAGE in the presence of a reducing reagent, further confirmed by a Western blot analysis. We conclude
that human hemoglobin-affinity column was most suitable for the isolation of Hp 1-1 in large quantities. Whereas, one additional step using a gel-permeation was necessary for that of Hp 2-1 and 2-2.
2003 Elsevier Science B.V. All rights reserved.
Keywords: Affinity adsorbents; Haptoglobin; Proteins; Glycoproteins
1
. Introduction pate in hemoglobin transport. The concentration of
Hp in human plasma is relatively high ranging from Haptoglobin (Hp), also known as an a-2 glycopro- 1.0 to 1.5 mg / ml [1,2], which may increase as an tein, is a hemoglobin-binding protein present in acute-phase protein in response to a variety of plasma of all vertebrates and is believed to partici- injuries and inflammatory disease states [3,4]. For this reason, Hp is useful as a diagnostic marker and as a clinical evaluation of many inflammatory
dis-*Corresponding author. Tel.: 1886-3-571-2121x56939 or
eases. Human Hp is a tetrameric structure linked by 56948; fax: 1886-3-572-9288.
E-mail address: mao1010@ms7.hinet.net(S.J.T. Mao). disulfide linkages among the two a and two b chains 1570-0232 / 03 / $ – see front matter 2003 Elsevier Science B.V. All rights reserved.
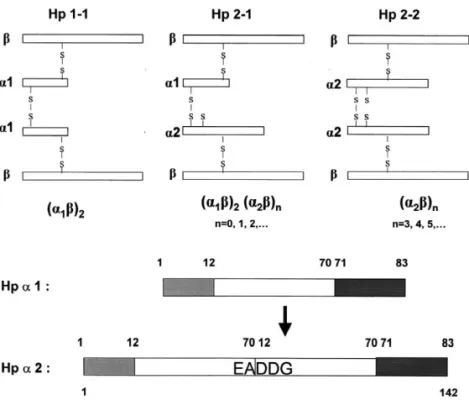
Fig. 1. Schematic drawing of the phenotype structure of human Hp 1-1, 2-1, and 2-2 (top). All three phenotypes share a common structure of b chains. The degree of polymerization within the inter-molecular arrangement is shown. The difference between a1 and a2 chains and their combinations determine the Hp phenotype (bottom). a2 is a duplicate of a1 with a repeat insert of residues 12–70. Making it simple, a2 (142 amino acids) contains two repeated domains showing a unique sequence of EADDG (residues 69–73) at the splicing site.
[5–7]. Based on the length of a chains, there are flammatory-related cardiovascular diseases and dia-three phenotypes of Hp in the population, Hp 1-1, betics than that with Hp 2-1 and 2-2 [13–15]. 2-1, and 2-2 (Fig. 1), which are similar to that of Although the protein has been well characterized blood types. All the phenotypes share the same two genetically, the exact physiological role and the b chains [each with about molecular mass (M )r biochemical mechanism by which Hp 1-1 is more 40 000 including 243 amino acids and approximate resistance to those inflammatory-related diseases are 30% (w / w) carbohydrate moiety] [5–7]. A typical not well understood. The later has been hampered by structure of homozygous Hp 1-1 is composed of two the availability of Hp, which is mainly due to the identical a 1 chains (each with about Mr 9000 considerably difficult procedures for Hp purification. including 83 amino acids). Whereas, Hp 2-2 is Commercially prepared Hp is not only expensive composed of two identical a 2 chains (142 amino lacking the biological activity, but also heteroge-acids) as compared to that of heterozygous Hp 2-1 neous containing the mixture of three phenotypes containing each a 2 and a 1 (Fig. 1). Likewise, the isolated from the plasma pools.
tetrameric arrangement is also found in other animal Currently, the most common procedures involved species such as rat, rabbit, and pig [3,8–10]. How- for the purification of human Hp are associated with ever, the two ab units joined by a non-covalent electrophoresis, affinity chromatography using a interaction, rather than a disulfide bridge, are found monoclonal antibody, and multiple high-performance in dog, cat, and bear [11,12]. liquid chromatography (HPLC) steps [16–20]. These Several functional differences between Hp methods are useful but are troublesome and time-phenotypes have been demonstrated, appearing to consuming, and the quantity of Hp obtained is have important biological and clinical consequences relatively small. Affinity column purification using [1,2,13,14]. For example, patients with phenotype chicken hemoglobin has been reported [19,21]. The Hp 1-1 are less prone to the development of in- binding affinity of chicken hemoglobin to human Hp,
however, is less than that of human hemoglobin The HPLC system (Waters) consisted of two [1,2]. The method [21] was satisfactory for Hp pumps, an automatic sample injector, a photodiode purification in species other than humans, but re- array detector, and an interface module [23,24]. A quired a few column-steps for human Hp [19]. Bio-Scale DEAE column (1031.5 cm) packed with Previously, we established a simple purification an anion-exchange Macro-Prep and equilibrated with method with high yield for porcine plasma Hp. The 20 mM phosphate buffer, pH 8.0, at a flow-rate of method, however, was not practical in the isolation 1 ml / min, was used for hemoglobin purification. of human samples due to the heterogeneity of human Partially purified ammonium sulfate fraction of Hp [22]. In the present report, we describe a hemoglobin (total 50 mg in 2 ml) was applied to the purification procedure for human Hp 1-1, 2-1, and column followed by an elution with the initial buffer 2-2 using an affinity column that was immobilized at a flow-rate of 1 ml / min. The chromatographic with highly purified human hemoglobin. Approxi- profile was monitored by a photodiode array detector mately 8–10 mg of human Hp can be obtained from and read at 280 nm.
15 ml of plasma. The procedure can be easily scaled
up for Hp 1-1 purification. A simple hemoglobin 2 .3. Preparation of human hemoglobin-affinity isolation procedure using an isocratic DEAE HPLC column
system is also described.
DEAE-purified human hemoglobin was first cou-pled to CNBr-activated Sepharose-4B (Pharmacia, 2
. Experimental Uppsala, Sweden) according to the manufacturer’s
procedures. Briefly, 5 g of freeze–dried Sepharose 2
.1. Materials was swollen and suspended in 1 mM HCl and
immediately washed 33 within 15 min with the Goat anti-human haptoglobin was purchased from same solution on a glass filter [23,25]. The gel was Calbiochem-Novabiochem (San Diego, CA, USA). then washed with a coupling buffer containing 0.1 M All other chemicals were purchased from Sigma (St. NaHCO , and 0.5 M NaCl, pH 8.0, and subsequently3 Louis, MO, USA) and Merck (Darmstadt, Germany) degassed. A 2-ml volume of hemoglobin (25 mg / without any further purification. ml), pre-dialyzed in the coupling buffer, was slowly added to the gel (in 25 ml), while gently stirring for 2
.2. Preparation and purification of human 1 h at room temperature. After coupling, the gel was
hemoglobin washed 33 with the coupling buffer (200 ml) to
remove uncoupled hemoglobin via a glass filter. Fresh human blood collected in 0.1% EDTA was Finally, the gel was treated with 0.1 M Tris–HCl, pH immediately centrifuged at 3000 g for 25 min, after 8.0, for 2 h at room temperature to saturate the which time plasma was removed by aspiration. The remaining reactive sites of Sepharose. The coupling remaining red blood cells (RBCs) were washed five efficiency of hemoglobin to gel was approximately times with three volumes of phosphate-buffered of 98%. The degassed gel was then packed onto a saline (PBS) containing 0.12 M NaCl and 12 mM 2031.5 cm column and extensively washed with two phosphate, pH 7.2, and then lysed with two volumes cycles of PBS, pH 7.2, and 0.15 M NaCl, pH 11, of deionized water at 4 8C. Cell debris was removed which was adjusted by ammonium as previously by centrifugation at 3500 g for 30 min. The superna- described [25].
tant containing mostly hemoglobin was fractionated
by 50% saturated ammonium sulfate at 4 8C for 30 2 .4. Isolation of Hp by human hemoglobin-affinity min followed by a centrifugation at 4500 g for 40 column
min at 4 8C. The supernatant was dialyzed against
0.02 M sodium phosphate, pH 8.0, at 4 8C overnight Initially, 15 ml of human plasma was loaded onto followed by a filtration through a 0.45 mm nylon the hemoglobin-affinity column (15 ml in bed
fol-lowed by an extensive wash with 200 ml of PBS. a wash with TTBS for 3 min, the membrane was The bound materials were first eluted with three incubated with a primary antibody [1:2500 dilution volumes of 0.15 M NaCl, pH 11 (adjusted by in TTBS containing 1% (w / v) BSA] for 1 h at room ammonium), as fraction 1 [25] and then eluted with temperature and washed three times with TTBS. The three volumes of 5 M urea in 0.15 M NaCl, pH 11 membrane was then incubated with 1:5000 diluted (freshly prepared and filtered), as fraction 2. A 5-ml antiserum against goat immunoglobulin G (IgG) volume of each fraction was collected in a tube conjugated with horseradish peroxidase for 1 h in containing 0.1 ml of 1 M Tris–HCl, pH 7.0, to TTBS containing 1% (w / v) BSA. Finally, the mem-immediately neutralize the pH value. Pooled frac- brane was washed three times with TTBS and tions containing Hp were then dialyzed at 4 8C developed into a color immunoblot with 3,39-overnight with three changes of PBS. diaminobenzidine (DAB)-stabilized substrate for
horseradish peroxidase [22]. 2
.5. Gel electrophoresis and densitometry
Sodium dodecyl sulfate–polyacrylamide gel elec- 3 . Results trophoresis (SDS–PAGE) and native PAGE were
performed according to the Laemmli’s method [26] 3 .1. Purification of human hemoglobin with some modification as previously described [22].
Samples (typically 10 mg) for SDS–PAGE were A typical HPLC profile for the purification of preheated at 100 8C for 10–15 min in an SDS ammonium sulfate fractionated hemoglobin is shown loading buffer [50 mM Tris–HCl, 2% (w / v) SDS, in Fig. 2. Both SDS–PAGE and native-PAGE analy-100 mM 2-mercaptoethanol, pH 6.8]. For molecular ses show that the homogeneity of purified hemoglo-mass calibration, a subset of the following standards bin was greater than 96% (Fig. 3).
was included in each gel: b-galactosidase (116 000),
phosphorylase B (97 000), bovine serum albumin 3 .2. Preparation of hemoglobin-affinity column (BSA, 66 000), ovalbumin (45 000), carbonic
anhy-drase (31 000), soybean trypsin inhibitor (21 500), In theory, the purity of human hemoglobin ob-lysozyme (14 400), and aprotinin (6500). The sam- tained from ammonium sulfate fractionation was ples were run for 0.5 to 1 h at 120 V and stained by a
Coomassie brilliant blue G-250. Densitomertic anal-ysis of SDS–PAGE gel was performed using a Molecular Dynamics densitometer for data acquisi-tion and Image Quant software for integraacquisi-tion and analysis.
2
.6. Immunoblot analysis
Following the separation of proteins by SDS– PAGE, the gel was soaked in a transfer buffer containing 50 mM Tris–HCl, 50 mM boric acid, and
1 mM EDTA, pH 8.2, for 30 min. The gel was then Fig. 2. Typical purification profile of human hemoglobin on transferred to a nitrocellulose membrane (Pharmacia) DEAE HPLC. About 5 mg of 50% saturated ammonium sulfate at 100 mA for 1 h in a semi-dry transfer cell top fraction was applied to a DEAE column (1031.5 cm) pre-equilibrated with 20 mM phosphate, pH 8.0. A mobile phase (Bio-Rad) containing a transfer buffer. The
mem-containing the same buffer was run through for 10 min at a brane was immersed in 1% BSA, Tween-containing
flow-rate of 1 ml / min. A linear gradient was produced from 0 to Tris-buffered saline (TTBS) [20 mM Tris–HCl, 50 0.3 M of NaCl to regenerate the DEAE resin. The same procedure mM NaCl, 0.05% (w / v) Tween 20, pH 7.4] for 1 h was used for the purification of hemoglobin in large scale (total of with gentle shaking at room temperature. Following 50 mg in 2 ml) as described in the Experimental section.
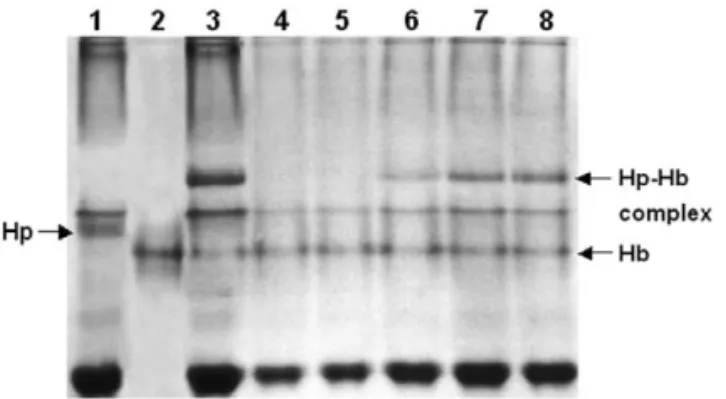
Fig. 4. Evaluation of binding capacity of hemoglobin-conjugated Sepharose to Hp in plasma. Briefly, plasma containing Hp was passed through 1 ml of conjugated Sepharose column. The pass-through fraction was collected and mixed with free hemoglobin. The unbound or remaining Hp, if any, was able to form a Fig. 3. Analyses of purified human hemoglobin using SDS–PAGE
Hp–hemoglobin complex displaying an extra band in a 7% native-(A) and native-PAGE (B). native-(A) Lane M represents the molecular
27 PAGE. Lanes: 15human plasma prior to the affinity column; mass markers (molecular masses 310 ). Lanes 1–3 represent
25purified hemoglobin; 35plasma spiked with purified hemoglo-hemoglobin obtained from the RBC lysate, top fraction of 50%
bin showing a Hp–hemoglobin complex; 4–85samples of 0.25, saturated ammonium sulfate precipitation, and DEAE HPLC,
0.5, 0.75, 1.0, and 2.0 ml plasma passed through a hemoglobin-respectively. Approximately 15 mg of each protein was loaded on
Sepharose containing 1 mg of hemoglobin, respectively. No Hp an 18% SDS–PAGE in the presence of a reducing reagent. (B)
was detected in lanes 4 and 5 when 0.25–0.5 ml of plasma was Lanes 1–3 represent hemoglobin obtained from RBC lysate, top
applied onto the conjugated Sepharose. According to our calcula-fraction of 50% saturated ammonium sulfate precipitation, and
tion, 1 mg of hemoglobin coupled on Sepharose could bind about DEAE HPLC, respectively. Each protein was loaded on a 10%
0.75 to 1.13 mg Hp (lanes 5 and 6). native-PAGE.
adequate in preparing affinity column for Hp purifi- 1-1 was applied to the column followed by an cation as that described using chicken hemoglobin extensive wash (Fig. 5); the bound protein was first [19]. However in a preliminary application, we found eluted with 0.15 M NaCl, pH 11 (fraction 1) to that column immobilized with ammonium sulfate remove the low-affinity binding proteins such and fraction of hemoglobin could produce significant apoA-I. The column was then eluted with 0.15 M plasma clots and subsequently demolished the chro- NaCl containing 5 M urea, pH 11 (fraction 2) for matography (data not shown). This clotting effect, high-affinity binding Hp. Each eluent was immedi-however, was not observed when DEAE-purified ately neutralized in the tube containing 100 ml of hemoglobin was employed for affinity column. 1 M Tris–HCl, pH 7.0 (Fig. 5). SDS–PAGE analysis Using native-PAGE to evaluate the binding capacity on fraction 1 revealed that it contained mostly high-of Sepharose 4B immobilized with human hemoglo- molecular-mass proteins and apoA-I (Figs. 6 and 7), bin, the capacity we estimated was approximately but not in fraction 2. The purity of Hp 1-1 in fraction between 0.75 and 1.13 mg of Hp 1-1 per mg of 2 was approximately 96%. The recovery of Hp in hemoglobin (Fig. 4). This binding capacity was 20- fraction 2 was approximately 45.5% from the plasma times greater than that reported using chicken hemo- with a final of 77-fold purification (Table 1). Under
globin [19]. the same condition, however, some apoA-I was
found to be co-eluted in the fraction 2 of Hp 2-1 and 3
.3. Isolation of human Hp by hemoglobin-affinity 2-2 (Figs. 6 and 7). The contaminated apoA-I could
column chromatography be further removed (data not shown) using a single
step on HPLC Superose 12 as previously described Fig. 5 shows a typical chromatography using an by us [22]. A typical Western blot analysis showing affinity column conjugated with highly purified three isolated phenotypes of Hp is depicted in Fig. 7. human hemoglobin. Initially, 15 ml of plasma of Hp The presence of apoA-I in Hp 2-1 and 2-2 was
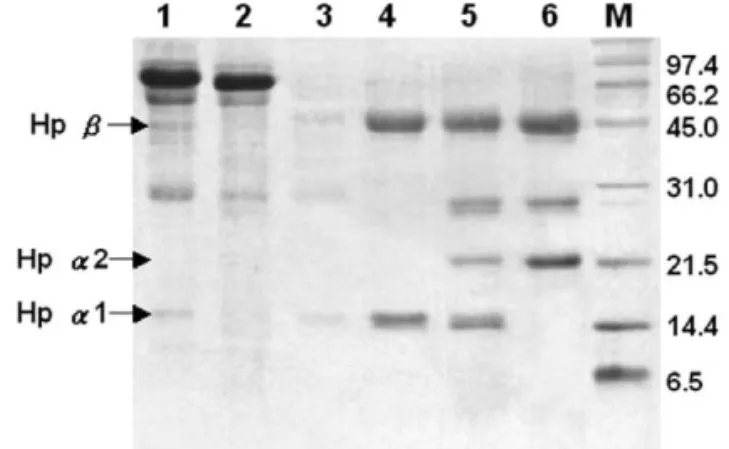
Fig. 6. Analyses of isolated Hp from affinity column on 15% SDS–PAGE. Lanes: M5molecular mass markers, 15plasma of Hp 1-1, 25a typical pass-through fraction (Hp 1-1 plasma) from hemoglobin-affinity chromatography, 35a typical sample from fraction 1 (Hp 1-1) contaminated mostly apoA-I and proteins with large molecular mass. Lanes 4 and 6 represent fraction 2 of isolated Hp 1-1, 2-1, and 2-2, respectively. Notably, apoA-I is co-eluted in Hp 2-1 and 2-2.
4
. Discussion
The acute phase serum protein, Hp, response to infection, inflammation, and trauma has been iden-tified in a number of species. Methods designed for large isolation of human Hp have been complicated and time-consuming. We have recently described a single-step purification procedure for porcine Hp Fig. 5. Typical elution profile of hemoglobin-affinity column
chromatography of plasma containing Hp 1-1 (A), Hp 2-1 (B), and Hp 2-2 (C). Initially, 15 ml of human plasma was applied to the hemoglobin-affinity column followed by an extensive wash with 200 ml of PBS. The bound materials were first eluted with three volumes of 0.15 M NaCl, pH 11 (adjusted by ammonium), as fraction 1 and then eluted with three volumes of freshly prepared and filtered 5 M urea in 0.15 M NaCl, pH 11, as fraction 2. A 5-ml volume of each fraction was collected in a tube containing 0.1 ml of 1 M Tris–HCl, pH 7.0, to immediately neutralize the pH value.
unavoidable using hemoglobin-based affinity column Fig. 7. Western blot analyses on affinity-purified human Hp 1-1, and was confirmed by a monoclonal antibody pre- 2-1, and 2-2 by a goat antibody prepared against human haptog-lobin. Lanes: 15Hp standard purified from a human plasma pool, pared against apoA-I (Fig. 7). Nevertheless, the
2–45affinity-purified Hp 1-1, 2-1, and 2-2, respectively, 5 and major isolation procedure was simple and can be
65the apoA-I co-eluted in affinity-purified Hp 2-1 and 2-2 achieved within a few hours. This procedure should fractions by a mouse monoclonal antibody prepared against be widely used for the purification of Hp and human apoA-I. Purified Hp 1-1 did not reveal immunoreactive particularly for 1-1 phenotype. apoA-I (data not shown).
Table 1
Analytical recovery of haptoglobin 1-1 purified from 15 ml human plasma
Total protein Theoretical amount Total Hp Final yield Purity Fold
from plasma (mg) of Hp (mg) yield (mg) (%) (%) purification
1755 22 10 45.5 .96 77
using HPLC gel-permeation chromatography in the Third, the pH of each eluted fraction was immedi-presence of 5 M urea [22]. The procedure, however, ately neutralized by a 1 M Tris buffer, pH 7.0, to could not be reproduced in human Hp isolation. restore the biological activity (complex formation Presumably, the human Hp structure is more compli- between Hp and hemoglobin). It is worth mentioning cated in its polymerization nature (Fig. 1) than that that ammonium, rather than a high-capacity buffer of pig. Thus, the purification for human Hp has been solution, was used for adjusting the final pH of the hampered by its structural diversity as each Hp 1-1, saline solution (pH 11) in eluting the Hp; this was 2-1, and 2-2 has average molecular masses of because the eluent could be easily neutralized by a 100 000, 220 000, and 400 000, respectively (Fig. 1). Tris buffer. A similar procedure was employed Although the procedure using a salting-out of plasma previously in our laboratory [25]. Fourth, the most proteins followed by anion-exchange chromatog- contaminants of proteins that bound weakly or non-raphy has been recommended, the reproducibility specifically to the affinity column were differentially (including the yield) is rather poor due to the removed using pH 11 saline solution (Fig. 5, fraction heterogeneity of its polymerization form of Hp 2-1 1). Fifth, unlike phenotypes Hp 2-1 and 2-2, Hp 1-1 and 2-2 [20,27,28]. It is almost not feasible to isolate could be isolated without apoA-I contaminant, as Hp 2-2 as a pool and to study its biochemical confirmed by a Western blot analysis (Fig. 7).
properties. In the present study, about 8–10 mg of Hp 1-1
An immunoaffinity chromatography method to could be isolated from 15 ml of human plasma in purify human Hp had been developed using a two- one isolation. A similar yield of Hp 2-1 and 2-2 was monoclonal antibody system [20], in which the obtained, but it required a further gel-filtration to phenotypes and the final purity of Hp were not remove apoA-I. The mechanism by which the affini-specified. The yield, on the other hand, is limited and ty column favored the Hp 1-1 purification is not utilized only for the preparation of antigen and readily clear. Since Hp 2-1 and 2-2 molecules are polyclonal antibodies [20]. largely polymerized by disulfide linkages with mo-With respect to hemoglobin-affinity column, lecular weights ranging from 153 000 to 1 200 000 Rademacher and Steele [19] have reported use of [6], these polymers may more accessibly ‘‘trap’’ the Sepharose immobilized with chicken hemoglobin. apoA-I than that of monomeric Hp 1-1. To address However, an attempt using human hemoglobin for this assumption, we applied purified-apoA-I [25] the purification of human Hp was unsuccessful [19]. directly to the affinity column. There was no apoA-I The method we employed was different from that of binding to the column suggesting that apoA-I did not chicken hemoglobin-Sepharose chromatography. interact with hemoglobin in the absence of Hp (data First, our human hemoglobin-Sepharose had a bind- not shown). On the other hand, apoA-I may weakly ing capacity 0.75–1.00 mg Hp / mg hemoglobin that bind to Hp and therefore was co-eluted with Hp was about 20 times greater than that of using chicken during the purification. Regardless, the apoA-I de-hemoglobin (Fig. 4). Second, our results demon- ficient plasma, which can be easily obtained by a strated that highly purified hemoglobin via DEAE simple ultra-centrifugation for the removal of high-chromatography should be used for the affinity density lipoproteins [25], may be ultimately consid-column rather than a crude extract of hemoglobin ered for the purification of all Hp phenotypes. This from ammonium sulfate fraction described previous- experimental procedure is currently in progress in ly [19]. Under this condition, the formation of our laboratory.
con-[6] D. Patzelt, G. Geserick, H. Schroder, Electrophoresis 9 veniently isolated in large quantities by ammonium
(1988) 393. sulfate fractionation followed by a HPLC DEAE
[7] J. Javid, Curr. Top. Hematol. 1 (1978) 151.
column. Immobilized human hemoglobin had a [8] I.H. Fraser, D.B. Smith, Can. J. Biochem. 49 (1971) 141. binding capacity about 20-times greater than that of [9] H. Baumann, G.P. Jahreis, J. Cell Biol. 97 (1983) 728. chicken hemoglobin and could be more suitable for [10] V. Chow, A. Kurosky, R.K. Murrary, J. Biol. Chem. 258
(1983) 7858. the purification of phenotype Hp 1-1. Accordingly,
[11] A. Kurosky, R.E. Hay, B.H. Bowman, Comp. Biochem. the procedure described in this report can be simply
Physiol. 62B (1978) 339.
scaled up using a 100-ml bed affinity column for [12] K. Mominoki, N.N. Tosa, M. Morimatsu, B. Syuto, M. Saito, even larger Hp purification. This Hp purification Comp. Biochem. Physiol. 110B (1995) 785.
procedure is currently used in our laboratory; the [13] A. Roguin, F. Ribichini, V. Ferrero, G. Matullo, P. Herer, W. Wijns, A.P. Levy, Am. J. Cardiol. 89 (2002) 806. resulting Hp has been utilized in studying the
[14] I. Hochberg, A. Roguin, E. Nikolsky, P.V. Chanderashekhar, structural and functional relationship and preparing
S. Cohen, A.P. Levy, Atherosclerosis 161 (2002) 441. polyclonal and monoclonal antibodies. [15] D.D. Bacquer, G.D. Backer, M. Langlois, J. Delanghe, H.
Kesteloot, M. Kornitzer, Atherosclerosis 157 (2001) 161. [16] N. Tosa, M. Morimatsu, M. Nakagawa, F. Miyoshi, E.
Uchida, M. Niiyama, B. Syuto, M. Saito, J. Vet. Med. Sci. 55
A
cknowledgements
(1993) 27.
[17] B.H. Bowman, D.R. Barnett, J.B. Lum, F. Yang, Methods This work was supported by grants NHRI-EX92- Enzymol. 163 (1988) 452.
9229SI (S.J.T.M.) from the National Health Research [18] M.K. O’Bryan, J. Grima, D. Mruk, C.Y. Cheng, J. Androl. 18 Institute, and NSC 89-2313-B-009-001-A20 (1997) 637.
[19] B. Rademacher, W.J. Steele, Anal. Biochem. 160 (1987) 119. (S.J.T.M.) and NSC 90-2314-B-075-099 (J.P.P.) from
[20] I. Katnik, J. Jadach, Arch. Immunol. Ther. Exp. 41 (1993) the National Science Council, Taiwan. The authors
303.
thank Ms. Yu-Chi Pong for her dedicated administra- [21] F. Delers, C. Lombart, M. Domingo, S. Musquera, Anal.
tive assistance. Biochem. 118 (1981) 353.
[22] S.J. Yang, S.J.T. Mao, J. Chromatogr. B 731 (1999) 395. [23] S.J.T. Mao, M.T. Yates, T.J. Owen, J.L. Krstenansky,
Biochemistry 27 (1988) 8170.
R
eferences
[24] L.F. Chu, W.C. Lee, P.C. Yang, R. Chu, T.Y. Huang, S.J.T. Mao, Protein Expr. Purif. 10 (1997) 180.
[1] W. Dobryszycka, Eur. J. Clin. Chem. Clin. Biochem. 35 [25] S.J.T. Mao, J.P. Miller, A.M. Gotto Jr., J.T. Sparrow, J. Biol.
(1997) 647. Chem. 255 (1980) 3448.
[2] M.R. Langlois, J.R. Delanghe, Clin. Chem. 42 (1996) 1589. [26] U.K. Laemmli, Nature 227 (1970) 680.
[3] J.M. Hanley, T.H. Haugen, E.C. Heath, J. Biol. Chem. 258 [27] W. Dobryszycka, E. Lisowska, Biochim. Biophys. Acta 121
(1983) 7858. (1966) 42.
[4] M. Morimatsu, B. Syuto, N. Shimada, T. Fujinaga, S. [28] M. Morimatus, B. Syuto, N. Shimada, T. Fujinaga, S. Yamamoto, M. Saito, M. Naiki, J. Biol. Chem. 266 (1991) Yamamoto, M. Saito, M. Naiki, J. Biol. Chem. 266 (1991)
11833. 11833.