The allelochemicals of litchi leaf and its potential as natural
herbicide in weed control
C.M. WANG, Y.L. JHAN, L.S. YEN, Y.H. SU1, C.C. CHANG, Y.Y. WU2, C.I. CHANG3, S.Y. TSAI4 and C.H. CHOU*
Research Center for Biodiversity and Graduate Institute of Ecology and Evolutionary Biology, China Medical University, Taichung 40402, Taiwan.
E. Mail: choumasa@mail.cmu.edu.tw
(Received in revised form: August 20, 2013)
ABSTRACT
This study aimed to exploit the allelopathic potential of Litchi chinensis (Litchi) and its application as naturally occurring herbicide in the field. Aqueous leaf extracts of L. chinensis inhibited the radicle growth of test weeds. Allelopathic compounds presented in litchi leaves were identified as (-)-epicatechin, procyanidin A2, kaempferol-3-O-galactose and 4-hydroxybenzaldehyde using chromatography, mass analysis and NMR spectroscopic analysis. Field experiments in orchard and vegetable fields demonstrated that litchi leaf powder inhibited the growth of weeds including Bidens pilosa, Eleusine indica and Portulaca oleracea. Interestingly, the growth of weeds plant (Ipomoea sp.) in orchard and vegetable plant (Brassica
oleracea var. alboglabra) in vegetable fields was enhanced, indicating the beneficial
usage of litchi leaf in vegetable farms. Developing the natural herbicide to replace the traditional agrochemicals becomes an important subject because the use of synthetic agrochemicals has caused serious environment pollution due to their poor biodegradability. These results obtained from laboratory assays, greenhouse pots experiments and field trials concluded that the L. chinensis leaves exhibited potential as natural herbicides.
Keywords: Litchi chinensis, Bidens pilosa, Portulaca oleracea, Eleusine indica, Brassica oleracea var. alboglabra, natural herbicide, weed control.
INTRODUCTION
Synthetic agrochemicals, such as herbicides, fungicides, insecticides and nematocides, have been used intensively in current agricultural practice and many useful agrochemicals have been discovered during the last decade . The overuse of synthetic agrochemicals causes environmental hazards, an imbalance of soil microorganisms, nutrients deficiency and change the soil physicochemical properties, resulting in decreased crop productivity . Thus, developing naturally occurring agrochemicals to replace the traditional agrochemical becomes an important subject in sustainable agriculture .
Allelopathy was coined by Molish (34) and meant that a plant releases compounds to either stimulate or inhibit the growth of other plant sharing the same habitat in natural and agricultural ecosystem . The study of allelochemicals is an area of chemical ecology of great interest . Allelopathic compounds may play important roles in the determining the the plant productivity in agroecosystems . Such compounds are also released during the decomposition of crop residues in soil, resulting in the decreased crop productivity . This *Corresponding Author, 1Graduate Institute of Biotechnology, National Pingtung University of Science and
Technology, Pingtung 91201, Taiwan, 2AgNeeds Company, Taichung 41284, Taiwan, 3Department of
Biological Science and Technology, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan, 4School of Pharmacy, China Medical University, Taichung 40402, Taiwan.
process can be stimulated by acid rain and other abiotic factors . However, the allelopathic compounds can be used as naturally occurring herbicides , i.e. beneficial to agricultural productivity in sustainable agriculture . For example, the kikuyu grass was introduced into forest regeneration in Taiwan. It suppressed the growth of weeds, but did not harm the regeneration growth of conifer plants or seeding growth of other hardwood trees . This pasture-forest intercropping system not only completely avoids the use of herbicide and saving expensive manpower, but also enhances the forage material for livestock. In addition, the extracts of many dominant woody plants, such as Acacia confusa, Delonix
regia, Macaranga tanarius, Vigna radiata and endemic species Rhododendron formosanum contain various allelopathic compounds, including phenolic acids, alkaloids,
terpenoids and flavonoids . These compounds can be used as potential naturally occurring herbicides because these allelochemicals are involved in the environmental complex of managed or natural ecosystems. Incorporation of allelopathic substances into agricultural management may reduce the use of synthetic herbicides, fungicides and insecticides and lessen their environmental effects.
Litchi chinensis Sonn is a subtropical orchard crop belonging to the Sapindaceae
family. As an economic crop, the litchi plantation is mainly located in southern and central Taiwan. The yield and value of litchi in Taiwan are about 95,440 metric tons from over 12,000 ha area and US$97.3 million per annum, respectively . Based on high yields of litchi per year, the substantial amount of litchi waste (pericarp and leaves during harvesting) is good sources of naturally occurring agrochemicals due to high phytotoxicity potential of its leaves.
Previously, the natural agrochemicals isolated from neem plant (Melia azedarach) have been used as herbicide, fungicide, or nematicides in India . In addition, agrostemin was isolated from the corn cockle of Agrostemma githago, which has successfully been applied into agricultural practice as growth regulator in eastern European countries . According to our previous studies, many allelochemicals [4-hydroxybenzoic acid, mimosine and hydroquinone, etc. ] may offer potential lead compounds for the development of bio-agrochemical herbicides in future. In the past, little attention has been paid to the allelopathic interaction of the unique pattern of weed exclusion underneath the
L. chinensis trees except our laboratory. Various flavonoids with phytotoxic potential from
the pericap, but not from the leaves, of L. chinensis are reported . Extensive field observations demonstrated that few plant species were present on the ground below L.
chinensis, indicating the allelopathic potential of L. chinensis . In this study, the
allelopathic potential of L. chinensis from field to laboratory assays was investigated. Natural products present in the litchi leaves were isolated and identified. Furthermore, the leaf debris of litchi was applied to orchard and vegetable field to use as a natural herbicide. Overall, this study highlighted the allelochemicals of litchi leaves by using laboratory assays, greenhouse pot experiment and testing the possibility of its application as natural herbicide in the field.
MATERIALS AND METHODS
I. Field measurement of botanical composition and species coverage
The field experimental sites are located in litchi Farm, National Pintung University of Science and Technology (NPUST), Pingtung, southern Taiwan (N 22°38'26.35" and E 120°36'14.39") and Caotun township, Nantou county, central Taiwan (N 23°58'32.05" and E 120°41'21.33"). A transect experiment on the floor of litchi plants was conducted by setting three consecutive quadrats (1 m × 1 m each). The coverage of each species was measured from the trunk of litchi plant in each quadrats. Five replications were conducted. The same design was set at the grassland area adjacent to the litchi plantation as a control.
II. Sample collection
The leaves and stems of L. chinensis were collected consecutively in March, June, September and December of 2006, 2007, 2008, 2009 and 2011 at litchi Farm of NPUST and Caotun Farm. Leaves and stems were air-dried for further extraction. Dry leaves were grounded into powder for packing into small pellets (about 5 mm dia) with 3% carboxymethylcellulose for field experimental use.
III. Preparation of aqueous extracts
For bioassays, 1 to 5 % (5 g sample plus 95 ml double distilled water) concentrations of aqueous extracts of leaves or stems of L. chinensis were prepared. Their pH and osmotic concentrations measured with pH meter (Denver Instrument, UB-10, USA) and cryoscopic osmometer (Osmomat 030, Gonotec), respectively.
IV. Bioassays
The aqueous extracts of litchi leaves or stems were assayed by the standard sponge bioassay while the double distilled water was used as control. Tested seeds of
Lactuca sativa, Brassica chinensis, Ocimum basilicum, Amaranthus tricolor were
purchased from the Known-You seed company in Taiwan. In addition, seeds of weeds including Bidens pilosa and Ageratum houstonianum collected from the wild field were also used for bioassay. The length of radicle growth was measured 72 h after incubation. V. Isolation and identification of water-soluble phytotoxins
One hundred g air-dried leaves of L. chinensis were extracted with 1900 ml distilled water thrice. The aqueous extract was evaporated in vacuo to concentrate to dryness. The residue of extract was further partitioned with ethyl ether and the fraction was evaporated to dry in hood under the laboratory conditions. The dry residue was re-dissolved in methanol (1 mg/ml) and 50 μl of the aliquot was injected into HPLC. The analytical HPLC was run on a 4.6 × 250 mm Mightysil RP-18 GP column (Kanto Chemical Co. Inc.) eluted with a gradient consisting of water containing 0.1 % formic acid (A) and methanol (B) at a flow rate of 1.0 ml/min and monitored at 220 nm. The initial solvent composition of 5 % B in A was raised to 95 % B from 0 to 40 min and 95% B to 5 % B in A from 40 to 60 min. For preparative purpose, the aqueous extract was concentrated to give 395 g residue. After liquid-liquid partition with chloroform, ethyl acetate and butanol, 67g of ethyl acetate extract was subjected to pass through Silica gel
(Merck, USA), Sephadex LH-20 (Pharmacia, USA) and RP-18 (Merck, USA) column (2.5 cm × 92 cm) to obtain pure compound 1 (1.5 g), 2 (120.8 mg), 3 (7 mg) and 4 (5 mg). Purified compounds were subjected to spectroscopic identification by using 1H-NMR and 13C-NMR (Bruker Avance 400) and Mass (Bruker Daltonics Esquire HCT). Specific optical rotation was measured by polarimeter (Jasco P2000).
VI. Greenhouse pot experiments
Each pot (18 cm) was filled up with 150 g sandy loam soil and then mixed with either 1, 3, 5 and 10 g leaves powder of L. chinensis mixture. This soil without the leaves powder was served as control. Ten seeds of each B. pilosa, A. houstonianum and A.
tricolor were uniformly sown into each pot including control and irrigated with tap water
daily. The experiment was conducted in a greenhouse of the NPUST. Thirteen days after sowing, the seedlings of weeds were harvested to measure the radicle length and leaf area. VII. Field experiments
Two experimental sites were selected for field study. One was located in a peach orchard, Mingdao University (N 23°52'8.69" and E 120°29'38.39") in Changhua county, Taiwan. Another site was located in a vegetable field at Siluo township (N 23°46'15.30" and E 120°28'6.10") in the same county. Three quadrats (2.5 m × 2 m) were set for experimental and control of peach orchard. In the vegetable field at Siluo, the quadrats were set by 2 m × 2 m. To prolong the allelopathic activity in the wild field, the leaves of
L. chinensis was grounded into powder and mixed with 3% carboxymethylcellulose for
making into small pellets (5 mm size). One kilogram of pellets was sprayed onto the field uniformly and the same amount of powder was applied 2 weeks later. Twenty young sprouts of vegetable plants (Brassica oleracea var. alboglabra) were sown in the vegetable field. Twenty g Lactuca sativa seeds were also sprayed onto the field uniformly. The coverage of weeds was measured by using Image J (vision 1.45s) at 2 and 4 weeks after planting. Six weeks after treatment, the weeds and vegetable plants were harvested for biomass measurement. The field trial experiments were repeated three times during the period 2010 to 2011.
VIII. Statistical analysis
One-way and two-way ANOVA were carried out, depending on the cases with the values of the replicates (SPSS 13.0 software). Duncan’s multiple range test was used to evaluate the means at the α = 0.05 level.
RESULTS AND DISCUSSION
Field measurement of botanical composition in Litchi plantation area
There were poor understory species on the area immediately under the canopy of
Litchi chinesis plantation in both sites (Fig. 1). This pattern was common in litchi
plantation area and the senior author (CHC) has observed this pattern from 2002 to now. Thus, our field measurement was conducted at both study sites, particularly, the Caotun site was near our laboratory. Under the canopy of litchi plant, there was total lack of species growing in area in 1 m radius from the trunk of litchi plant. Beyond 1 m from the trunk, 6 weeds were observed and relative coverage in percentage is given in parenthesis,
namely Soliva anthemifolia (57.5 %), Gramineae sp. (10.8 %), Ixeris chinensis (9.9 %),
Callitriche palustris (9.5 %), Stellaria aquatic (7.4 %) and Kyllinga brevifolia (4.2 %). In
the control area, Bidens pilosa (65.5 %) as the dominant species, Digitaria sp. (16.9 %),
Mimosa pudica (7.4 %), Sida rhombifolia L. subsp. rhombifolia (5.2 %), Ipomoea obscura
(4.1 %) and Oxalis corymbosa (0.6 %) were found (Table 1). The pattern of botanical composition is relatively similar in both measurements of litchi understory and control area (Chou et al., unpublished data). A similar pattern was observed under the litchi plant although the habitats of both experimental sites were different, e.g. Caotun site was located in central Taiwan whereas the NPUST site was in southern Taiwan. Although these two sites are quite far from each other but the weeds appeared in litchi plantation and its control areas were quite common. Particularly, Bidens pilosa, a notorious weed hardly being controlled in Taiwan, is missing in litchi area but was abundant in control quadrats of both sites. This fact stimulated authors to further study the allelopathic potential of litchi plant.
Table 1. Comparison of floristic composition and relative coverage (%) of each understory species found on the floors of wild field and Litchi chinensis in the Caotun site
Species From trunk of litchi plant Grassland control
adjacent to litchi 0-1 m 1-2 m 2-3 m
Bidens pilosa -* - - 65.5±6.9
Digitaria sp. - - - 17.0±4.1
Mimosa pudica - - - 7.5±7.0
Sida rhombifolia L. subsp. rhombifolia - - - 5.2±9.0
Ipomoea obscura - - - 4.2±6.6 Oxalis corymbosa - - - 0.6±1.1 Soliva anthemifolia - 57.6±36.6 48.6±56.1 -Gramineae sp. - 10.8±21.5 2.4±4.8 -Ixeris chinensis - 9.9±10.4 47.8±53.8 -Callitriche palustris - 9.5±19.1 - -Stellaria aquatica - 7.4±14.8 0.4±0.9 -Kyllinga brevifolia - 4.3±6.7 - -* -: Not present
Phytotoxicity of aqueous extracts of litchi leaves, stems and decomposition of litchi leaves
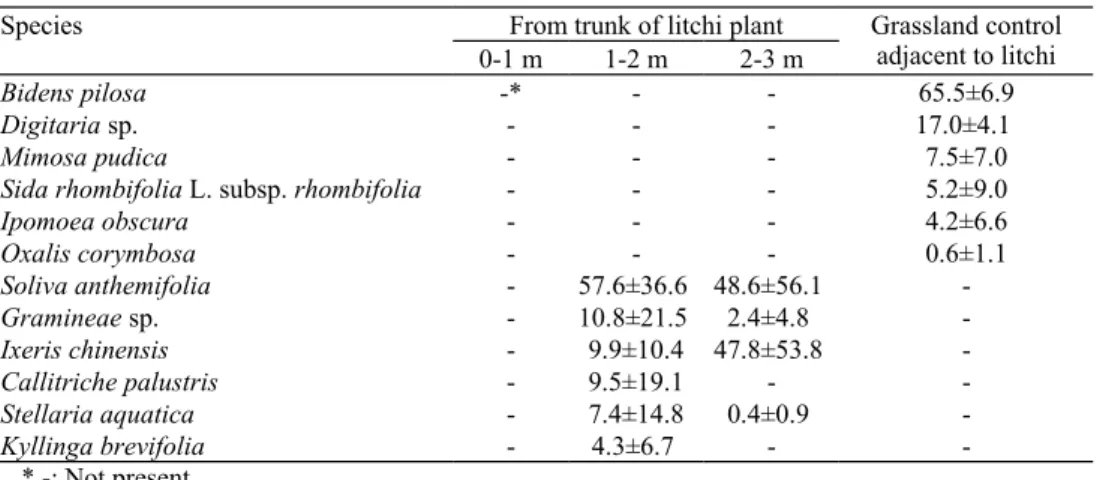
To avoid the misleading inhibition caused by pH and osmotic concentration, all extracts and leachates were subjected to such measurements. The pH of extracts ranged from 5.70 to 5.78, while the osmotic concentrations were 8 to 32 milliosmol (mosmol) (Fig. 2a). The pH and osmotic concentrations were in the threshold range and will not cause both pH and osmotic inhibition in bioassay .
To understand the phytotoxicity of litchi leaves, the aqueous extracts (1 to 5 %) of
Figure 1. An almost lacking understory species on the floor of litchi in NPUST site (a) and Caotun site (c) were observed as compared to its adjacent grassland area (b, d) where a luxuriant growth of weed species. The lack of understory species under the litchi canopy is about 2-3 m wide from the trunk in NPUST site (a) and that is about 1.5-2 m wide from the trunk in Caotun site (c)
1 % aqueous extracts inhibited the radicle growth of L. sativa and B. chinensis by 16.5 % and 46.4 %, respectively (Fig. 2b). Inhibition of growth of both plants was remarkably increased to 69.6 % and 62.0 % as the concentration increased. To understand the dynamics of phytotoxins produced from litchi leaves and stems during the year, the aqueous extracts of leaves and stems were obtained at 3-months intervals and the phytotoxicity was determined against 4 test plants. Radicle growth of L. sativa, B.
chinensis, Raphanus sativus and Medicago sativa was suppressed at 3 % extract
concentration of litchi leaves and stems (Fig. 2c, 2d). However, the radicle growth of R.
sativus was enhanced by the aqueous stem extracts collected in March (Fig. 2d).
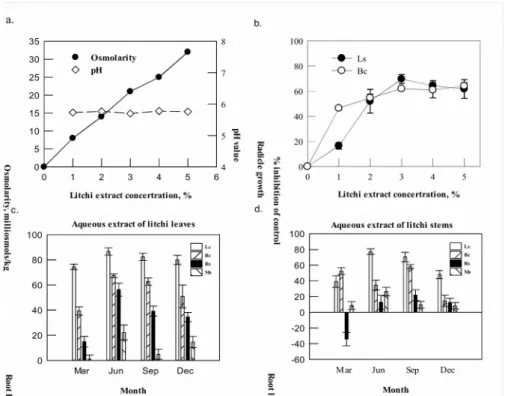
Moreover, the dynamics of phytotoxins produced during the decomposition of litchi leaves were also conducted. The aqueous extracts of decomposing leaves were obtained at one week intervals and its phytotoxicity was tested against 6 species. Radicle growth of L. sativa, B. pilosa, A. houstonianum and B. chinensis was suppressed by the 3 % extract of decomposing leaves in the first week. However, after four weeks, the allelochemicals of leaves from L. chinensis were degraded and the inhibitory activity of aqueous extract was dramatically decreased (Fig. 3). Among the test species common in litchi fields, B. pilosa and A. houstonianum were more sensitive to the aqueous extract of
Figure 2. The osmolarity, pH (a) and phytotoxicity of different aqueous extract (1 to 5%) from leaves of L. chinensis (b). Inhibition of the 3% aqueous leaf (c) and stem (d) extracts of L.
chinensis collected at 3 month interval on radicle growth of L. sativa (LS), B. chinensis
(BC), Raphanus sativus (RS) and Medicago sativa (MS). The inhibition was expressed by % inhibition of radicle growth over distilled water control. The negative values indicate the stimulation
L. chinensis leaves than A. tricolor and O. basilicum. It was concluded that the growth of
most abundant weeds in the litchi plantation as mentioned can be suppressed by the litchi leaves.
Identification of bioactive compounds from L. chinensis
By using the silica and Sephadex LH-20 gel column chromatography, several compounds were purified, structurally elucidated and identified by NMR (Tables 2-5). Spectroscopic data of 1H NMR and 13C NMR of compound I in CD
3OD (Table 2) were compared to known compound with literature . The identity of epicatechin was apparent from the 13C NMR spectrum which had a methylene carbon in the upfield region (δ 29.1) and two oxygenated methine carbons in the heterocyclic region (δ 67.3 and 79.7) which were characteristic of the pyran C-ring of flavanols. The upfield position of the C-2 carbon (δ 79.7) was characteristic of the epicatechin. The specific optical rotation was confirmed by polarimeter, thus, compound I was conformed as (-)-epicatechin.
Figure 3. Inhibition of the sequential 3% aqueous extracts at one week interval of decomposing L.
chinensis leaves on radicle growth of L. sativa (LS), B. pilosa , A. houstonianum (AH), B. chinensis (BC), A. tricolor (AT), and O. basilicum (OB). The negative values indicate the
stimulation effect
Procyanidin A2 (compound II) was isolated from the litchi leaves and its structure was compared to the procyanidin previously isolated from fruit pericarp of litchi . The characteristic ketal carbon at δ 100.1 indicated the presence of a doubly-linked subunit. No proton linked to the C-2 indicated that the location of the C2–O–C7 linkage occurred in the upper two units. The upfield signals from δ 29.1 to δ 81.6 (Table 3) were attributable to the carbons 2, 3 and 4 of each unit. The signals at δ 29.8 and δ 29.1 were attributed to C-4 carbons of upper and terminal units .
The structural elucidation by the data of 1H and 13C NMR demonstrated that compound III was a typical flavonol with a glycoside (Table 4). In the 1H spectrum, the signal exhibited ring-A (δ 6.20, δ 6.39) and ring-B (δ 6.87, δ 8.07) of kaempferol typically
Decomposition of L. chinensis leaves, week
R ad ic le g ro w th , % in hi bi ti on o f co nt ro l
Table 2. 1H NMR and 13C NMR data (δ, ppm) of compound 1(epicatechin) in CD 3OD compared with literature. Position 1H, ppm 1H (literature) 13C 13C (literature) 2 4.80(br s) 4.86(s) 79.7(d) 79.4(d) 3 4.16(ddd, J = 1.2, 2.8, 4.4) 4.21(m) 67.3(d) 66.8(d) 4 2.73(dd, J = 2.8, 16.8)-H-4β 2.86(dd, J = 4.4, 16.8)-H-4α 2.71(d, J = 16.6)-H-4β 2.85(dd, J = 4.5, 16.6)-H-4α 29.1(t) 28.8(t) 4a 100.1(s) 99.7(s) 5 157.3(s) 156.9(s) 6 5.93(d, J = 2.0) 5.93(d, J = 2.2) 96.4(d) 96.2(d) 7 157.7(s) 157.6(s) 8 5.95(d, J = 2.4) 6.02(d, J = 2.2) 95.9(d) 95.5(d) 8a 157.2(s) 157.6(s) 1' 132.1(s) 132.0(s) 2' 6.97(d, J = 1.6) 7.04(s) 115.2(d) 115.2(d) 3' 145.5(s) 145.2(s) 4' 145.7(s) 145.4(s) 5' 6.76(d, J = 8.4) 6.81(m) 115.3(d) 115.5(d) 6' 6.79(dd, 2.0, J = 8.4) 6.81(m) 115.9(d) 119.2(d) s = C, d = CH, t = CH2, q = CH3
Table 3. 1H NMR (400 MHz) and 13C NMR (100 MHz) data of compound 2 (procyanidin) A2 in CD 3OD
Position 1H, ppm 13C
Upper unit Terminal unit Upper unit Terminal unit
2 4.91 (br s) 100.1(s) 81.6(d) 3 4.07 (d, J = 3.6) 4.23 (m) 67.9(d) 66.8(d) 4 4.43 (d, J = 3.2) 2.76 (dd, J = 2.4, 17.2) 2.94 (dd, J = 4.8, 17.2) 29.8(d) 29.1(t) 4a 104.2(s) 102.4(s) 5 156.8(s) 156.4(s) 6 6.02 (d, J = 2.4) 6.11(s) 98.3(d) 96.5(d) 7 158.0(s) 152.1(s) 8 6.09 (d, J = 2.4) 96.6(d) 107.1(s) 8a 154.1(s) 152.0(s) 1' 132.3(s) 132.1(s) 2' 7.16 (d, J = 2.4) 7.17 (d, J = 2.4) 115.6(d) 115.7(d) 3' 146.2(s) 146.6(s) 4' 145.5(s) 145.8(s) 5' 6.81 (d, J = 8.4) 6.83 (d, J = 8.4) 116.1(d) 115.9(d) 6' 7.04 (dd, J = 2.0, 8.4) 6.97 (dd, J = 2.0, 8.4) 120.4(d) 119.8(d) s = C, d = CH, t = CH2, q = CH3
(Table 4). The 1H NMR data of galactose were also detected between δ 3.40 to δ 3.80. The signal of δ 5.12 (d, J=7.6 Hz) was identified as anomeric proton of galactose. All the 1H and 13C NMR spectra were identified by comparison with literature data . Thus, compound III was identified as kaempferol-3-O-galactose.
The spectroscopic data of 1H NMR and 13C NMR of compound IV in CDCl 3 are in Table 5. The typical signals of benzene ring were detected at δ 6.97 and δ 7.82,
Table 4. 1H NMR and 13C NMR data (δ, ppm) of compound 3 (kaempferol-3-O-galactose) in CD 3OD
compared with literature
Position 1H 1H (literature) 13C 13C (literature)
2 159.0(s) 158.0(s) 3 135.6(s) 134.6(s) 4 179.6(s) 179.8(s) 4a 105.0(s) 104.6(s) 5 163.1(s) 162.1(s) 6 6.20(d, J = 2.0) 6.21(d) 100.0(d) 98.8(d) 7 166.4(s) 165.0(s) 8 6.39(d, J = 1.6) 6.42(d) 94.8(d) 93.5(d) 8a 158.5(s) 157.5(s) 1' 122.7(s) 121.5(s) 2' 8.07(d, J = 8.8) 8.09(d) 116.1(d) 114.9(d) 3' 6.87(d, J = 8.8) 6.88(d) 132.3(d) 131.1(d) 4' 161.6(s) 160.6(s) 5' 6.87(d, J = 8.8) 6.88(d) 132.3(d) 131.1(d) 6' 8.07(d, J = 8.8) 8.09(d) 116.1(d) 114.9(d) 1" 5.12(d, J = 7.6) 5.20(d) 100.0(d) 103.7(d) 2" 3.77(dd, J = 8.0, 9.6) 3.78(dd) 73.0(d) 71.7(d) 3" 3.52(m) 3.50(m) 75.0(d) 73.7(d) 4" 3.80(dd, J = 2.0, 2.8) 3.81(dd) 70.0(d) 68.7(d) 5" 3.43(dd, J = 6.0, 6.0) 3.43(ddd) 77.1(d) 75.8(d) 6" 3.50(dd, J = 6.0, 10.8) 3.62(dd, J = 6.0, 10.8) 3.50(m)3.62(dd) 62.0(t) 60.6(t) s = C, d = CH, t = CH2
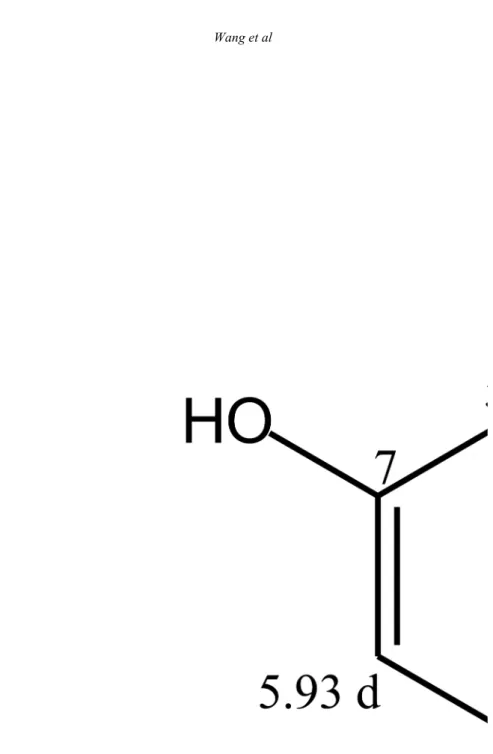
Table 5. 1H NMR and 13C NMR data (δ, ppm) of compound 4 (4-hydroxybenzaldehyde) in CDCl 3
compared with literature
Position 1H, ppm 1H (literature) 13C 13C (literature)
1 129.7(s) 130.3(s) 2 7.82 (d, J= 8.5) 7.81(d, J= 8.6) 132.5(d) 133.4(d) 3 6.98(d, J= 8.5) 6.95(d, J= 8.6) 116.0(d) 116.9(d) 4 161.8(s) 165.2(s) 5 6.98(d, J= 8.5) 6.95(d, J= 8.6) 116.0(d) 116.9(d) 6 7.82 (d, J= 8.5) 7.81(d, J= 8.6) 132.5(d) 133.4(d) 7 9.86(s) 9.87(s) 191.3(d) 192.8(d) s = C, d = CH
respectively. The signal of δ 5.12 (d, J=7.6 Hz) was identified as aldehyde proton of hydroxybenzaldehyde. Overall, the 1H and 13C NMR data of compound IV (Table 5) were in agreement with that of 4-hydroxybenzaldehyde previously identified and described . At very low concentration of 1 µM, 4-hydroxybenzaldehyde had little phytotoxicity, while, the remaining 4 compounds [epicatechin, procyanidin A2, kaempferol-3-O-galactose and 4-hydroxybenzoic acid] stimulated the radicle growth (Table 6). Most of the allelopathic compounds stimulates the plant growth at very low concentration due to hormetic effect . However, the compounds, except kaempferol-3-O-galactose and 4-hydroxybenzaldehyde, reveal inhibitory activity when the
Table 6. Phytotoxic effects of major compounds isolated from the leaf of L. chinensis at different concentration on the radicle growth of L. sativa.
Compounds Inhibition (%) over control 1 (µM) 10 (µM) 100 (µM) Epicatechin *-8.3 + 2.0 6.8 + 3.4 6.8 + 5.9 Procyanidin A2 -3.7 + 4.9 0.7 + 2.2 24.0 + 6.6 Kaempferol-3-O-galactose -11.9 + 2.9 -4.3 + 9.2 2.2 + 9.6 4-Hydroxybenzaldehyde 2.5 + 1.7 -1.6 + 0.8 1.5 + 0.4 4-Hydroxybenzoic acid -1.5 + 0.6 7.6 + 1.8 22.8 + 1.7 *-: Negative values indicate % stimulation over control
concentration increases to 10 µM. When the concentration increases to 100 µM, all the listed compounds were inhibitory, particularly, procyanidin A2 and 4-hydroxybenzoic acid.
Several flavonoids have been isolated and identified from pericarps of litchi, such as epicatechin and procyanidins A2 . In this study, these two compounds were the major flavonoids in the litchi leaves. Flavonoids are one category of the many secondary metabolites implicated in plant allelopathy and have been observed in both natural and managed ecosystems, where they cause a number of ecological and economic problems, such as declines in crop yield due to soil sickness, regeneration failure of natural forests and replanting problems in orchards . (-)-Epicatechin is the most abundant monomeric flavanol (1.5/67g) in the leaf of litchi. Although (-)-epicatechin did not exhibit dramatic phytotoxic effects on radicle growth of L. sativa, the catechin isomer of epicatechin, was identified as an allelochemical of invasive knapweed in northern America .
In addition, kaempferol-3-O-galactopyranoside is present in many edible plants (e.g. tea, broccoli, cabbage, kale, beans, endive, leek, tomato, strawberries and grapes). This is the first report on isolation and identification of the kaempferol-3-O-galactopyranoside from the litchi leaves. Numerous studies reported that kaempferol and some glycosides of kaempferol have wide range of pharmacological activities [including antioxidant, anti-inflammatory, antimicrobial and anticancer activities ]. Little is known for the allelopathic potential of kaempferol-3-O- -galactopyranoside. It is known that glycosylation of the active site of flavonoid increase the structural stability but decrease the pharmacological activity . After leaching by rains, the kaempferol-3-O-galactopyranoside was released into soil and may be degraded into glucose and kaempferol by soil microorganisms. Thus, the active aglycone, kaempferol would contribute to the allelopathic potential of litchi.
Phenolic compounds are most important and common plant allelochemicals in the ecosystem. The principal compound, 4-hydroxybenzoic acid (4-HBA), was abundant in the aqueous extracts of Delonix regia , in the rhizosphere soil of Ageratum conyzoides and in leaf litter leachates of Eucalyptus tereticornis, E. camaldulensis, E. polycarpa and E.
microtheca . 4-HBA suppressed the growth of lettuce and Chinese cabbage . In addition,
the phytotoxicity of 4-HBA was tested in laboratory bioassay and in field experiments. The phytotoxicity of 4-hydroxybenzoic acid and 4-hydroxybenzaldehyde is similar at lower concentration. However, the solubility of 4-hydroxybenzoic acid in water (5000 mg/ml) is much greater than 4-hydroxybenzaldehyde (13.8 mg/ml) . Water solubility is a considerable factor that affects the mode of action in allelopathic effects.
Greenhouse pot experiment
The seeds of weeds (Bidens pilosa and Ageratum houstonianum) and vegetable (Amaranthus tricolor) were sown in pots containing different amount of leaves powder of
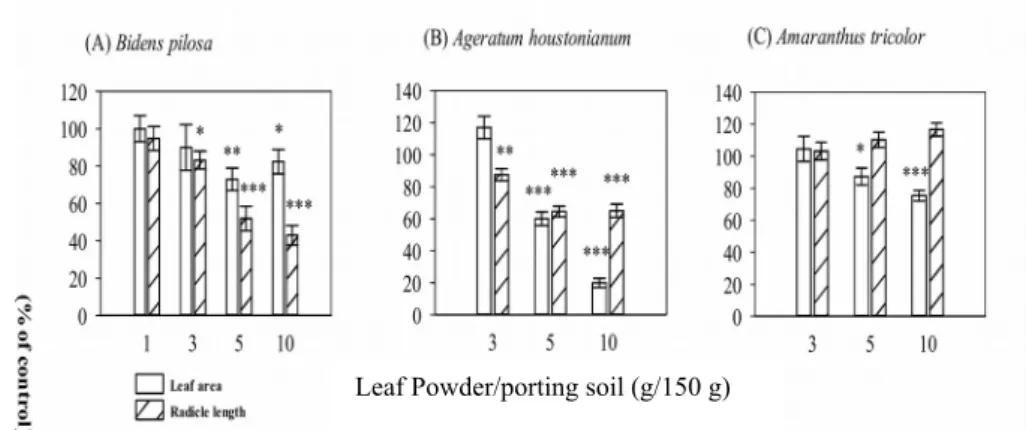
planting, the coverage of leaves was measured and further analyzed by Image J software and dry weight of weeds and vegetables were measured. The inhibition of radicle growth of weeds was generally increased with increase in dose of litchi powder mixed in pot soil (Fig. 4). The inhibition of radicle growth of B. pilosa was 5.1 % at 1:150 ratio and increased to 57 % at 1:15 ratio (Fig. 4a). In addition, the radicle length and leaf area of
Ageratum houstonianum were inhibited (P < 0.001) at concentration of litchi-soil ratio
from 1:30 to 1:15 (Fig. 4b). However, the radicle growth of vegetable seedling (Amaranthus tricolor) was not affected by leaf extracts but the leaf coverage was reduced at higher concentration of 1:15 ratio (Fig. 4c), indicating that the suppression of radicle growth was more pronounced in weeds than on vegetable plants. Presumably, the litchi leaves may possibly be used as naturally occurring herbicide to control weed growth in the vegetable field.
Figure 4. Effect of leaf powder in a serial concentration of L. chinensis on the radicle length and leaf area in greenhouse pot experiments. Asterisk indicates significant difference between control and treatment: * p<0.05, ** p<0.01, *** p< 0.001
The effects of litchi leaf powder on peach orchard and vegetable crops
To apply the L. chinensis as a bio-agrochemical, two different experimental sites were selected to test the herbicidal effects of leaf debris of L. chinensis. The first experimental site was located at a peach orchard field at the Mingdao University, where
Bidens pilosa was abundant. Second experimental site was set at the vegetable field at
Siluo dominated by Portulaca oleracea. The field trial showed that the growth of Bidens
pilosa was suppressed (P < 0.01) in peach orchard, while biomass of Ipomoea species was
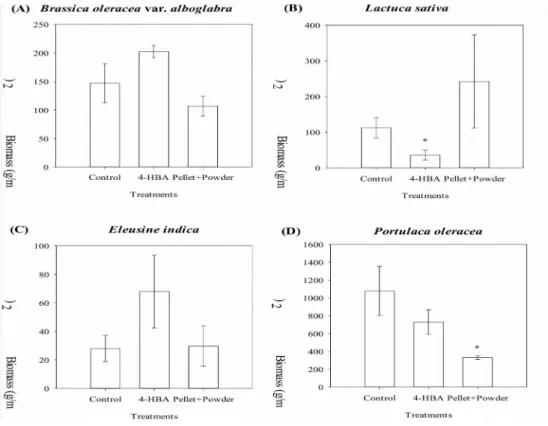
increased (P < 0.05) by application of litchi leaf powder than control (without litchi leaf powder) 4-weeks after planting (Fig. 5). In the vegetable field at Siluo, trial was conducted separately by applying either litchi leaf pellet and powder or the 4-hydroxybenzoic acid solution, which was prepared in distilled water to make into a final concentration of 1 mM. Fields were sprayed with 10 L of 1 mM 4-hydroxybenzoic acid and spread another 10 L
Figure 5. Mean biomass of weeds within orchard fields harvested 4 weeks after treatments of the leaf powder from L. chinensis. The abbreviations of species names are: Bidens pilosa , Poaceae spp. (Poa), Ipomoea spp. (Ipo). Asterisk indicates significant difference between control and treatment: * p<0.05, *** p< 0.001
Figure 6. Relative coverage of weeds (Eleusine
indica and Portulace oleracea) in vegetable field
as measured by Image J software 2 and 4 weeks after treatments of 4-hydroxybenzoic acid and leaf pellets from L. chinensis. Asterisk indicates significant difference between control and treatment: * p<0.05
Figure 7. Effect of 4-hydroxybenzoic acid (4-HBA) and leaf pellets of L. chinensis on the biomass of vegetable crops (A) B. oleracea and (B) L. sative and on the biomass of weeds (C) E. indica and (D) P.
Figure 8. 1H and 13C assignment of four allelochemicals isolated from litchi leaf. Compound (I): epicatechin; compound (II): procyanidin A2; compound (III): kaempferol-3-O-galactose; compound (IV): 4-hydroxybenzaldehyde
CONCLUSIONS
Litchi (Litchi chinensis, Sapindaceae) is a tropical fruit originating from China having a red bright attractive pericarp. The production of litchi makes the debris of pericarp and leaves a great resource for bio-agrochemicals production. In this study, the allelopathic potential for L. chinensis was demonstrated in laboratory assays and greenhouse pot experiments, as well as in the vegetable field. Field observations demonstrated that the L. chinensis could inhibit the growth of Bidens pilosa and Digitaria sp. but not Soliva anthemifolia and Ixeris chinensis. In greenhouse pot experiments, the aqueous extracts of litchi leaves suppressed the growth of weeds, including B. pilosa and
A. houstonianum. In Taiwan, several important weeds, such as B. pilosa, A. houstonianum, E. indica and Portulaca oleracea are distributed commonly in wild fields. Based on these
findings, the leaves of L. chinensis had allelopathic substances which are also present in the decomposing leaves residues. At least four potential allelopathic compounds were identified in litchi, but more allelopathic compounds may be isolated in further studies. It is, thus, concluded that the debris of L. chinensis leaves may act as natural herbicides due to the presence of allelopathic compounds.
ACKNOWLEDGEMENTS
This study was funded by grants from the National Science Council of Taiwan (NSC99-2622-B-039-003-CC3, NSC100-2811-B-039-006, NSC101-2811-B-039-013 and NSC102-2811-B-039-005) to C. H. Chou. We thank associate Prof. Chung Chen at the Department of Post Modern Agriculture of the Mingdao University for the field studies.
REFERENCES
1. Akihisa, T., Pan, X., Nakamura, Y., Kikuchi, T., Takahashi, N., Matsumoto, M., Ogihara, E., Fukatsu, M., Koike, K. and Tokuda, H. (2013). Limonoids from the fruits of Melia azedarach and their cytotoxic activities. Phytochemistry 89: 59-70.
2. Allard, R. (1965). Genetic systems associated with colonizing ability in predominantly self-pollinated species. in The Genetics of Colonizing Species (H. Baker and G. Stobbins, Eds.), Academic Press, New York.
3. Anthony, R.G. and Hussey, P.J. (1999). Double mutation in eleusine indica alpha-tubulin increases the resistance of transgenic maize calli to dinitroaniline and phosphorothioamidate herbicides. The
Plant journal : for cell and molecular biology 18: 669-674.
4. Bais, H.P., Walker, T.S., Stermitz, F.R., Hufbauer, R.A. and Vivanco, J. M. (2002). Enantiomeric-dependent phytotoxic and antimicrobial activity of (+/-)-catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiology 128: 1173-1179.
5. Belz, R.G. and Piepho, H.P. (2012). Modeling effective dosages in hormetic dose-response studies. PLoS
One 7: e33432.
6. Calderon-Montano, J.M., Burgos-Moron, E., Perez-Guerrero, C. and Lopez-Lazaro, M. (2011). A review on the dietary flavonoid kaempferol. Mini Reviews in Medicinal Chemistry 11: 298-344.
7. Cantrell, C.L., Dayan, F.E. and Duke, S.O. (2012). Natural products as sources for new pesticides. Journal of
Natural Products 75: 1231-1242.
8. Chen, Y. ., Lin, J., Liu, S.C., Lu, P.S. and Yang, D.J. (2011). Composition of flavonoids and phenolic acids in Lychee (Litchi Chinensis Sonn.) flower extracts and their antioxidant capacities estimated with human LDL, erythrocyte, and blood models. Journal of Food Science 76: C724-728.
9. Chou, C.H. (1995). Allelopathy and sustainable agriculture. In: Allelopathy: organisms, processes, and
Chemical Society, Washington, USA.
10. Chou, C.H. (1999). Methodologies for allelopathic research: from fields to laboratory. In: Recent Advances
in Allelopathy, Vol. 1: A Science for the Future (Eds., F. A. Macías, Galindo, J.C.G., Molinillo,
J.M.G., and Cutler, H.G. ), pp 3-24, International Allelopathy Society. Servicio De Publicaciones Universidad de Càdiz, Spain.
11. Chou, C.H. (2010). Role of allelopathy in sustainable agriculture: Use of allelochemicals as naturally occurring bio-agrochemicals. Allelopathy Journal 25: 3-16.
12. Chou, C.H. (1993). The role of allelopathy in the diversity of plant-communities in Taiwan. Botanical
Bulletin of Academia Sinica 34: 211-221.
13. Chou, C H., Chang, S.J., Cheng, C.M., Wang, Y.C., Hsu, F.H. and Den, W.H. (1989). The selective allelopathic interaction of a pasture-forest intercropping in Taiwan. 2. Interactions between Kikuyu grass and 3 hardwood plants. Plant and Soil 116: 207-215.
14. Chou, C.H., Fu, C.Y., Li, S.Y. and Wang, Y.F. (1998). Allelopathic potential of Acacia confusa and related species in Taiwan. Journal of Chemical Ecology 24: 2131-2150.
15. Chou, C.H. and Leu, L.L. (1992). Allelopathic substances and interactions of Delonix regia (Boj) Raf.
Journal of Chemical Ecology 18: 2285-2303.
16. Chou, C.H. and Lin, H.J. (1976). Autointoxication mechanism of Oryza sativa, I. Phytotoxic effects of decomposing rice residues in soil. Journal of Chemical Ecology 2: 353-367.
17. Chou, C.H. and Muller, C.H. (1972). Allelopathic mechanisms of Arctostaphylos glandulosa var. zacaensis.
The American Midland Naturalist Journal 88: 324-347.
18. Chou, C.H. and Waller, G.R. (1983). Allelochemicals and pheromones. Monograph Vol. 5, Institute of Botany, Academia Sinica, Taipei, Taiwan.
19. Chou, C.H., Waller, G.R., Cheng, C.S., Yang, C.F. and Kim, D. (1995). Allelochemical activity of naturally-occurring compounds from mungbean (Vigna radiata L) plants and their surrounding soil.
Botanical Bulletin of Academia Sinica 36: 9-18.
20. Chou, C.H. and Young, C.C. (1974). Effect of osmotic concentration and pH on plant growth. Taiwania 19: 157-165.
21. Chou, S.C., Huang, C.H., Hsu, T.W., Wu, C.C. and Chou, C.H. (2010). Allelopathic potential of
Rhododendron formosanum Hemsl in Taiwan. Allelopathy Journal 25: 73-91.
22. Dayan, F.E., Cantrell, C.L. and Duke, S.O. (2009). Natural products in crop protection. Bioorganic &
Medicinal Chemistry 17: 4022-4034.
23. Dayan, F. E. and Duke, S.O. (2009). Biological activity of allelochemicals. In: Plant-derived Natural
Products: Synthesis, Function, and Application (Eds., A. E. Osbourn and V. Lanzotti), pp
361-384, Springer, New York, NY.
24. Duke, S.O. (2010). Allelopathy: Current status of research and future of the discipline: A Commentary.
Allelopathy Journal 25: 17-29.
25. Gajic, D. (1966). Interaction between wheat and cron cockle on brown soil and smonitsa. Research Journal
of Agriculture and Biological Sciences 19: 63-96.
26. Goto, A., Miyamoto, H., Salomon, M., Goto, R., Fukuda, H., Konigsberger, E. and Konigsberger, L.C. (2011). IUPAC-NIST Solubility Data Series. 90. Hydroxybenzoic acid derivatives in binary, ternary, and multicomponent systems. Part I. Hydroxybenzoic acids, hydroxybenzoates, and hydroxybenzoic acid salts in water and aqueous systems. Journal of Physical and Chemical
Reference Data 40.
27. Holm, L.G., Plucknett, D.L., Pancho, J.V. and Herberger, J.P. (1977). The World's worst weeds : distribution
and biology, Published for the East-West Center by the University Press of Hawaii, Honolulu.
28. Inderjit, Wardle, D.A., Karban, R. and Callaway, R.M. (2011). The ecosystem and evolutionary contexts of allelopathy. Trends in Ecology and Evolution 26: 655-662.
29. Jiang, G.X., Lin, S., Wen, L.R., Jiang, Y.M., Zhao, M.M., Chen, F., Prasad, K.N., Duan, X.W. and Yang, B. (2013). Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chemistry 136: 563-568.
30. Kaur, S., Singh, H.P., Batish, D.R. and Kohli, R.K. (2012). Phytotoxicity of decomposing below-ground residues of Ageratum conyzoides: nature and dynamics of release of phytotoxins. Acta
Physiologiae Plantarum 34: 1075-1081.
31. Li, Z.H., Wang, Q., Ruan, X., Pan, C.D. and Jiang, D.A. (2010). Phenolics and plant allelopathy. Molecules
15: 8933-8952.
32. Liu, L., Xie, B.J., Cao, S.Q., Yang, E.N., Xu, X.Y. and Guo, S.S. (2007). A-type procyanidins from Litchi
chinensis pericarp with antioxidant activity. Food Chemistry 105: 1446-1451.
Chemistry 59: 187-194.
34. Mallik, M.A.B. and Williams, R.D. (2005). Allelopathic growth stimulation of plants and microorganisms.
Allelopathy Journal 16: 175-198.
35. Markham, K.R., Ternai, B., Stanley, R., Geiger, H. and Mabry, T.J. (1978). 13C NMR studies of flavonoids.
III. Naturally occuring flavonoid glycosides and their acylated derivatives. Tetrahedron 34: 1389-1397.
36. Molish, H. (1937). Der Einfluss einer Pflanze auf die andere Allelopathie., Fischer, Jena.
37. Muller, C.H. (1974). Allelopathy in the environmental complex. In: Handbook of Vegetation Science PartVI:
Vegetation and Environment (Eds., B.R. Strain and W.D. Billings), pp 73-85. Dr W. Junk BV
Publisher, The Hague.
38. Palacios, S.M., Carpinella, M.C., Napal, G.N.D., Vaccarini, C.E. and Ferrayoli, C.G. (2009). Phytotoxic effects of Melia azedarach L. (Meliaceae) fruit extract on weeds and crops. Allelopathy Journal
24: 389-396.
39. Patrick, Z.A. (1971). Phytotoxic substances associated with the decomposition of certain plant residues in soil. Soil Science 111: 13-18.
40. Sarni-Manchado, P., Le Roux, E., Le Guerneve, C., Lozano, Y. and Cheynier, V. (2000). Phenolic composition of litchi fruit pericarp. Journal of Agricultural and Food Chemistry 48: 5995-6002. 41. Sasikumar, K., Vijayalakshmi, C. and Parthiban, K.T. (2002). Allelopathic effects of Eucalyptus on
blackgram (Phaseolus mungo L.). Allelopathy Journal 9: 205-214.
42. Schmitt, B. and Schneider, B. (1999). Dihydrocinnamic acids are involved in the biosynthesis of phenylphenalenones in Anigozanthos preissii. Phytochemistry 52: 45-53.
43. Shimada, A., Takeuchi, S., Nakajima, A., Tanaka, S., Kawano, T. and Kimura, Y. (2000). Phytotoxicity of indole-3-acetic acid produced by the fungus, Pythium aphanidermatum. Bioscience
Biotechnology and Biochemistry 64: 187-189.
44. Su, Y.H. (2007). Allelopathic potential of Litchi chinensis Sonn. In: Graduate Institute of Biotechnology, p 70, National Pingtung University of Science and Technology, Pingtung, Taiwan.
45. Teng, Y. (2005). Litchi production in Taiwan. Acta Horticulturae 665: 43-46.
46. Tseng, M.H., Kuo, Y.H., Chen, Y.M. and Chou, C.H. (2003). Allelopathic potential of Macaranga tanarius (L.) Muell.-Arg. Journal of Chemical Ecology 29: 1269-1286.
47. Veitch, N.C. and Grayer, R.E.J. (2008). Flavonoids and their glycosides, including anthocyanins. Natural
Product Reports 25: 555-611.
48. Wang, R.L., Staehelin, C., Dayan, F.E., Song, Y.Y., Su, Y.J. and Zeng, R.S. (2012). Simulated acid rain accelerates litter decomposition and enhances the allelopathic potential of the invasive plant
Wedelia trilobata (creeping daisy). Weed Science 60: 462-467.
49. Yalkowsky, S.H. and He, Y. (2003). An extensive compilation of aqueous solubility data for organic compounds extracted from the AQUASOL database. In: Handbook of Aqueous Solubility Data (Eds., S.H. Yalkowsky and Y. He), p 377. CRC Press LLC, Boca Raton, FL.