M A J O R A R T I C L E
Chronic Hepatitis C Virus Infection Increases
Mortality From Hepatic and Extrahepatic
Diseases: A Community-Based Long-Term
Prospective Study
Mei-Hsuan Lee,1Hwai-I. Yang,1,2,3Sheng-Nan Lu,4Chin-Lan Jen,1San-Lin You,1Li-Yu Wang,5Chih-Hao Wang,6 Wei J. Chen,7Chien-Jen Chen,1,7and for the R.E.V.E.A.L.-HCV Study Groupa
1
Genomics Research Center, Academia Sinica, Taipei;2Molecular and Genomic Epidemiology Center, China Medical University Hospital, Taichung;
3
Graduate Institute of Clinical Medical Science, China Medical University, Taichung;4Department of Gastroenterology, Kaohsiung Chang-Gung
Memorial Hospital, Kaohsiung;5MacKay Medical College, Taipei;6Department of Cardiology, Cardinal Tien Hospital, Taipei; and7Graduate Institute of
Epidemiology, College of Public Health, National Taiwan University, Taipei, Taiwan (See the editorial commentary by Nelson, on pages 461–3.)
Background. The study aimed to evaluate the risk of hepatitis C virus (HCV) infection on hepatic and extra-hepatic deaths.
Methods. A cohort of 23 820 adults aged 30–65 years old were enrolled during 1991–1992. The seromarkers hepatitis B surface antigen (HBsAg), anti-HCV, and serum HCV RNA levels at study entry were tested. The vital status was ascertained through computerized linkage with national death certification profiles from 1991 to 2008.
Results. There were 19 636 HBsAg-seronegatives, including 18 541 HCV seronegatives and 1095 anti-HCV seropositives. Among anti-anti-HCV seropositives, 69.4% had detectable serum anti-HCV RNA levels. There were 2394 deaths that occurred during an average follow-up period of 16.2 years. Compared with anti-HCV seronega-tives, anti-HCV seropositives had higher mortality from both hepatic and extrahepatic diseases, showing multivar-iate-adjusted hazard ratio (95% confidence interval) of 1.89 (1.66–2.15) for all causes of death; 12.48 (9.34–16.66) for hepatic diseases; 1.35 (1.15–1.57) for extrahepatic diseases; 1.50 (1.10–2.03) for circulatory diseases; 2.77 (1.49–5.15) for nephritis, nephrotic syndrome, and nephrosis; 4.08 (1.38–12.08) for esophageal cancer; 4.19 (1.18– 14.94) for prostate cancer; and 8.22 (1.36–49.66) for thyroid cancer. Anti-HCV seropositives with detectable HCV RNA levels had significantly higher mortality from hepatic and extrahepatic diseases than anti-HCV seropositives with undetectable HCV RNA.
Conclusions. Monitoring HCV RNA in anti-HCV seropositives is essential for the prediction of mortality associated with hepatitis C.
Hepatitis C virus (HCV) infects more than 170 mil-lions people worldwide [1]. There is a considerable
geographical variation in seroprevalence of antibodies against HCV (anti-HCV) throughout the world, with approximately 1.3% in developed countries and 2.6% in developing countries [2]. HCV is well recognized to cause fatal liver diseases, including liver cirrhosis and hepatocellular carcinoma. Individuals infected with HCV are often asymptomatic and not aware of their illness until severe and irreversible liver diseases occur. Several long-term follow-up studies examined the se-quelae associated with HCV infection were often limited to specific populations [3–5]. The impacts of HCV infections, particularly among those with viremia persistently, on the mortality of liver diseases
Received 22 October 2011; accepted 23 March 2012.
Correspondence: Chien-Jen Chen, ScD, Genomics Research Center, Academia Sinica, 128 Academia Rd Section 2, Nankang, Taipei 11529, Taiwan (cjchen@ntu. edu.tw).
a
Other members of the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis C Virus (R.E.V.E.A.L.-HCV) study are listed in the Appendix.
The Journal of Infectious Diseases 2012;206:469–77
© The Author 2012. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
DOI: 10.1093/infdis/jis385
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
have been less evaluated for the general population in the community.
In addition to hepatic diseases, HCV infection has also been found to be involved in a variety of extrahepatic diseases. Several clinical manifestations have been reported to be linked with HCV infection [6]. Negative-strand HCV RNA by strand-specific reverse transcriptase polymerase reaction, an evidence for viral replication, has been detected in extrahepatic tissues [7]. Antiviral therapy has been documented to decrease the rate offibrosis progression in patients with chronic HCV infection [8], suggesting extrahepatic diseases may become an important health burden in HCV-infected patients. However, the HCV-associated mortality from extrahepatic diseases has seldom been assessed in long-term follow-up studies on com-munity-based cohorts. The associations between the seroposi-tivity of HCV RNA and the mortality from extrahepatic diseases have never been evaluated.
The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer (R.E.V.E.A.L.)-HCV study is a prospec-tive community-based cohort study in Taiwan, which provides a large number of participants for investigations of natural history and long-term disease burden of chronic hepatitis C [9]. In this analysis, we compared both hepatic and extrahe-patic mortality rates predicted by seromarkers of HCV infec-tion, including anti-HCV and HCV RNA at study entry. METHODS
Study Cohort Enrollment
This community-based cohort study invited 89 293 (47 079 male and 42 214 female) residents aged 30–65 years to partici-pate in 1991–1992. They were living in 7 townships located in Taiwan. Initial invitations were made by mailed letters, and follow-up telephone calls were subsequently made to those who did not respond to the initial invitation. Individuals willing to participate were personally interviewed to provide detail information to assure their complete understanding of the informed consent. A total of 23 820 (11 973 men and 11 847 women) agreed to participate with written informed consent. The demographic characteristics such as sex and age of participants were quite similar to those who did not partici-pate [10]. All participants received health examinations at en-rollment, and those with abnormal findings of serological or biochemical tests were referred to hospitals or clinics for prompt managements. All participants were regularly followed up until 31 December 2008. A detailed description of the study population and data collection has been documented previously [11]. A total of 19 636 participants seronegative for hepatitis B surface antigen (HBsAg) were included in this analysis. This study was approved by the institutional review board of the College of Public Health, National Taiwan Uni-versity in Taipei.
Questionnaire Interview and Blood Collection
All participants were personally interviewed by public health nurses with structured questionnaires. The collected information included demographic characteristics, habits of cigarette smoking and alcohol consumption, and personal history of major diseases. Each participant provided a 10 mL blood sample with a standard sterile syringe for various serological and biochemical tests. Blood samples were separated on the day of collection and kept at−70°C until assay.
Laboratory Examinations
Serological markers, including hepatitis B surface antigen (HBsAg) and anti-HCV, were tested by commercial assays as described previously [11]. Samples seropositive for anti-HCV were further examined for HCV RNA by polymerase chain reaction using the COBAS TaqMan HCV test, v2.0 (Roche Di-agnostics, Indianapolis, NJ). The detection limit for COBAS TaqMan HCV test was 25 IU/mL.
Ascertainment of Causes of Death
In Taiwan, it is mandatory to register deaths of all citizens in a computerized database. The National Death Certification Registry profile contains the information on the date and causes of death, which has been used for several significant outcome-based research studies [10,12]. By law, certificates
must be registered within 1 month after death in Taiwan. All death certificates were coded and reviewed by medical regis-trars in the central office. The death certification system keeps updated and complete information on the vital status and causes of death of all inhabitants in Taiwan. The national identification number, date at birth, and sex were used as the linking variables to double-check the vital status and causes of death of study participants from the national death certifica-tion system. The Internacertifica-tional Classification of Diseases, Version 9 (ICD-9) codes were identified and utilized for subse-quent analyses. All deaths occurring between study entry and 31 December 2008 were included.
Statistical Analysis
Mortality rates of both hepatic and extrahepatic diseases were evaluated systematically by stratifying anti-HCV serostatus. The ICD-9 codes were identified and grouped according to anatomic sites for statistical analyses. However, some specific causes of death with small numbers of deaths (<3) were not tested for their associations with HCV infection and were not listed in the tables (except thyroid gland cancer, which was previously reported to be associated with HCV) [13]. The person-years of follow-up were calculated for each participant as the time from the enrollment date either to the date at death or to 31 December 2008 for those who were still alive then. Mortality rates of specific cause were expressed per 100 000 person-years.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
The cumulative risk of dying from specific cause of death in anti-HCV seronegatives and seropositives was estimated by the Kaplan–Meier method and the statistical significance of the dif-ference was examined by log-rank test. Cox proportional hazard models were used to estimate age-sex–adjusted and multivari-ate-adjusted hazard ratio (HR) with 95 percent confidence inter-vals (95% CIs) of specific cause of deaths for HCV infection. Statistical significance levels were determined by a 2-sided P value of .05. All analyses were performed using the SAS statisti-cal software package (release 9.1; SAS Institute Inc, Cary, NC). RESULTS
There were 1095 HCV seropositives and 18 541 anti-HCV seronegatives in this study. The mean age in this cohorts was 47.6 years old at study entry (47.4 years in anti-HCV sero-negatives and 50.8 years in anti-HCV seropositives; P < .01). There were 9060 (48.9%) males in anti-HCV seronegatives and 465 (42.5%) in anti-HCV seropositives (P < .01).
The average follow-up period was 16.2 years. A total of 2394 deaths occurred during 317 742 person-years follow-up, giving an overall mortality of 753.4 per 100 000 person-years among
HBsAg-seronegative participants. Table 1 shows the mortality rates and multivariate-adjusted HRs with 95% CIs of specific causes of death by serostatus of anti-HCV at study entry. Liver cancer and chronic liver diseases and cirrhosis contributed to most of the hepatic diseases. The proportionality assumption (nonchanging HRs over time) of Cox models was examined, and the assumption was not violated. Participants seropositive for anti-HCV had an increased risk of dying from hepatic dis-eases with a multivariate-adjusted HR (95% CI) of 12.48 (9.34– 16.66). The mortality rate of extrahepatic diseases per 100 000 person-years was 671.6 for anti-HCV seronegatives and 1054.8 for anti-HCV seropositives. Among the extrahepatic causes of death, 1383 (68.5%) and 124 (69.3%) were noncancer deaths for participants seronegative and seropositive for anti-HCV, respec-tively. The mortality rates per 100 000 person-years for extrahe-patic noncancer causes were 459.8 for anti-HCV seronegatives and 730.7 for anti-HCV seropositives with an multivariate-ad-justed HR (95% CI) of 1.38 (1.15–1.16). Participants seropositive for anti-HCV had a higher risk of dying from circulatory diseas-es and renal diseasdiseas-es with a multivariate-adjusted HR (95% CI) of 1.50 (1.10–2.03) and 2.77 (1.49–5.15), respectively, compared with anti-HCV–seronegative participants.
Table 1. Mortality Rates (Per 100 000 Person-Years) and Crude and Adjusted Hazard Ratios of Specific Causes of Death by Serostatus of Antibodies Against Hepatitis C Virus (Anti-HCV) at Study Entry
Anti-HCV (−), N = 18 541 (300 772 person-years) Anti-HCV (+), N = 1095 (16 970 person-years)
Causes of Death (ICD-9 Codes) Death No. Mortality Rate Death No. Mortality Rate
Crude Hazard Ratio (95% CI) Age-Sex–Adjusted Hazard Ratio (95% CI) Multivariate-Adjusted Hazard Ratioa(95% CI) All causes 2132 708.8 262 1543.9 2.21 (1.95–2.51) 1.87 (1.64–2.12) 1.89 (1.66–2.15) Hepatic diseases (155, 570–573) 112 37.2 83 489.1 13.35 (10.05–17.73) 11.83 (8.88–15.76) 12.48 (9.34–16.66) Liver cancer (155) 50 16.6 65 383.0 23.52 (16.27–34.01) 20.56 (14.17–29.84) 21.63 (14.83–31.54)
Chronic liver diseases
and cirrhosis (571–572)
58 19.3 18 106.1 5.57 (3.28–9.46) 5.06 (2.97–8.63) 5.38 (3.15–9.19)
Other disorders of liver (570, 573) 4 1.3 0 0.0 – – – Extrahepatic diseases 2020 671.6 179 1054.8 1.59 (1.37–1.86) 1.34 (1.15–1.56) 1.35 (1.15–1.57) Cancers (140–239 except 155) 637 211.8 55 324.1 1.56 (1.18–2.05) 1.31 (1.00–1.73) 1.32 (1.00–1.74) Diabetes mellitus (250) 183 60.8 18 106.1 1.78 (1.10–2.89) 1.37 (.84–2.23) 1.49 (.91–2.42) Circulatory diseases (390–459) 477 158.6 46 271.1 1.73 (1.28–2.34) 1.42 (1.05–1.93) 1.50 (1.10–2.03) Respiratory diseases (460–519) 165 54.9 8 47.1 0.87 (.43–1.78) 0.73 (.36–1.48) 0.71 (.35–1.44) Nephritis, nephrotic syndromes and nephrosis (580–589) 69 22.9 12 70.7 3.13 (1.70–5.78) 2.61 (1.41–4.83) 2.77 (1.49–5.15)
Abbreviation: CI, confidence interval. a
All hazard ratios were adjusted for age, sex, cigarette smoking, alcohol drinking, betel nuts chewing, and central obesity; hazard ratios for all causes of death, hepatic diseases, extrahepatic diseases, and nephritis, nephritic syndromes and nephrosis were additionally adjusted for personal history of diseases (diabetes, hypertension, heart diseases, cerebrovascular disease); hazard ratios for diabetes mellitus and circulatory diseases were additionally adjusted for personal history of diseases and baseline serum levels of cholesterol and triglycerides.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
Table2shows the mortality rates of cancers in the partici-pants. Anti-HCV–seropositive participants had a higher mor-tality from esophagus cancer, prostate cancer, and thyroid cancer than anti-HCV–seronegative ones, showing a multivar-iate-adjusted HR (95% CI) of 4.08 (1.38–12.08), 4.19 (1.18– 14.94), and 8.22 (1.36–49.66), respectively.
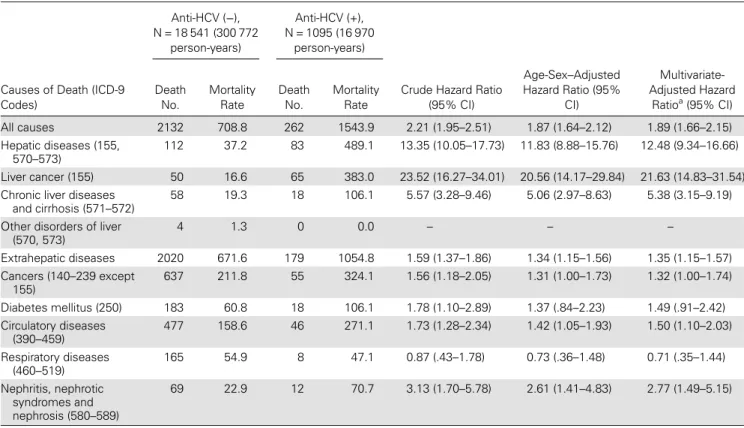
In this study, 975 anti-HCV seropositives had retrievable samples for serum HCV RNA test. Among them, there were 298 (30.6%) undetectable and 677 (69.4%) detectable for HCV RNA. Figure1shows the cumulative mortality from all causes, hepatic diseases, and extrahepatic diseases by seropositivity of anti-HCV and HCV RNA. Anti-HCV seropositives with detect-able serum HCV RNA levels had a significantly higher risk of dying from all causes of death, hepatic diseases, and extrahepat-ic diseases than anti-HCV seropositives with undetectable serum HCV RNA and anti-HCV seronegatives (P < .001).
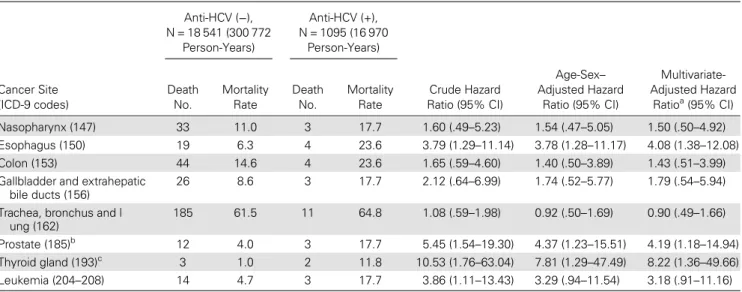
Figure2shows the cumulative mortality from liver cancer and chronic liver diseases and cirrhosis by seropositivity of anti-HCV and HCV RNA. After 18 years of follow-up, the cumulative liver cancer mortality was 0.3%, 1.6%, and 10.4% for anti-HCV sero-negatives, anti-HCV seropositives with undetectable serum HCV RNA levels, and anti-HCV seropositives with detectable serum HCV RNA, respectively (P < .001). There was no case that died from chronic liver diseases and cirrhosis among participants seropositive for anti-HCV with undetectable serum HCV RNA. The cumulative mortality of chronic liver diseases and cirrhosis
with detectable serum HCV RNA was 0.3% for anti-HCV seronegatives and 2.8% for anti-HCV seropositives.
Figure 3 shows the cumulative mortality from circulatory and renal diseases by seropositivity of anti-HCV and HCV RNA. The cumulative mortality from circulatory diseases was 2.9%, 3.5%, and 5.0% for anti-HCV seronegatives, anti-HCV seropositives with undetectable serum HCV RNA, and anti-HCV seropositives with detectable serum anti-HCV RNA, respec-tively (P < .01). The corresponding cumulative mortality from nephritis, nephrotic syndrome, and nephrosis was 0.47%, 0.92%, and 1.48%, respectively (P < .01).
Table 3 shows multivariate-adjusted HRs (95% CIs) of dying from selected causes of death by serostatus of anti-HCV and HCV RNA at study entry. There was a significantly in-creasing trend in mortality from anti-HCV seronegatives, anti-HCV seronegatives with undetectable serum HCV RNA, to anti-HCV seronegatives with detectable serum HCV RNA for most of the diseases. There was no death from chronic liver disease and cirrhosis, esophagus cancer, prostate cancer, and thyroid cancer among anti-HCV seropositives with unde-tectable serum HCV RNA at study entry.
DISCUSSION
An increasing HCV-related mortality from 1.09 to 2.40 per 100 000 person-years has been reported in the United States
Table 2. Mortality Rates (Per 100 000 Person-Years) and Crude and Adjusted Hazard Ratios of Extrahepatic Cancers by Serostatus of Antibodies Against Hepatitis C Virus (Anti-HCV) at Study Entry
Anti-HCV (−), N = 18 541 (300 772 Person-Years) Anti-HCV (+), N = 1095 (16 970 Person-Years) Cancer Site (ICD-9 codes) Death No. Mortality Rate Death No. Mortality Rate Crude Hazard Ratio (95% CI) Age-Sex– Adjusted Hazard Ratio (95% CI) Multivariate-Adjusted Hazard Ratioa(95% CI) Nasopharynx (147) 33 11.0 3 17.7 1.60 (.49–5.23) 1.54 (.47–5.05) 1.50 (.50–4.92) Esophagus (150) 19 6.3 4 23.6 3.79 (1.29–11.14) 3.78 (1.28–11.17) 4.08 (1.38–12.08) Colon (153) 44 14.6 4 23.6 1.65 (.59–4.60) 1.40 (.50–3.89) 1.43 (.51–3.99)
Gallbladder and extrahepatic bile ducts (156)
26 8.6 3 17.7 2.12 (.64–6.99) 1.74 (.52–5.77) 1.79 (.54–5.94)
Trachea, bronchus and l ung (162) 185 61.5 11 64.8 1.08 (.59–1.98) 0.92 (.50–1.69) 0.90 (.49–1.66) Prostate (185)b 12 4.0 3 17.7 5.45 (1.54 –19.30) 4.37 (1.23–15.51) 4.19 (1.18–14.94) Thyroid gland (193)c 3 1.0 2 11.8 10.53 (1.76 –63.04) 7.81 (1.29–47.49) 8.22 (1.36–49.66) Leukemia (204–208) 14 4.7 3 17.7 3.86 (1.11–13.43) 3.29 (.94–11.54) 3.18 (.91–11.16)
Abbreviation: CI, confidence interval. a
Hazard ratios for nasopharynx cancer and esophagus cancer were adjusted for age, sex, cigarette smoking, alcohol drinking and betel nuts chewing; hazard ratios for colon cancer, gallbladder and extrahepatic bile ducts cancer, and trachea, bronchus, and lung cancer were adjusted for age, sex, cigarette smoking, alcohol drinking, betel nuts chewing, and central obesity; hazard ratio for prostate cancer was adjusted for age, cigarette smoking, alcohol drinking, betel nuts chewing and central obesity; hazard ratio for thyroid cancer was adjusted for age and central obesity; and hazard ratio for leukemia was adjusted for age, sex, and central obesity.
b Men only. c
All cases were women.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
from 1995 to 2004 [14]. The predicted mortality over a 20-year period is expected to continue to rise [15], suggesting the health burden related to HCV infection will be substantially considerable in the foreseeable future.
The detectable serum HCV RNA level is a marker for active replication of HCV, and 52%–80% of serum samples seropos-itive for anti-HCV were found to have detectable serum levels of HCV RNA in previous reports [16–18]. We found that anti-HCV seropositives with detectable serum HCV RNA had an increased risk of dying from all causes of death, whereas the risk for anti-HCV seropositives with negative HCV RNA was similar to the risk for anti-HCV seronegatives. The results implied that chronic hepatitis C patients with active virus in-fection may benefit from antiviral treatment to reduce their overall mortality. This finding was in line with another pro-spective study conducted in Japan, which showed that 28.0%
Figure 1. Cumulative mortality from all causes of death (A), hepatic dis-eases (B), and extrahepatic diseases (C) by serostatus of antibodies against hepatitis C virus (anti-HCV) and serum HCV RNA level at study entry.
Figure 2. Cumulative mortality from liver cancers (A) and chronic liver diseases and cirrhosis (B) by serostatus of antibodies against hepatitis C virus (anti-HCV) and serum HCV RNA level at study entry.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
anti-HCV seropositives with HCV viremia (detectable HCV core antigen or HCV RNA in serum) and 17.9% anti-HCV seropositives without HCV viremia died from any cause during an average follow-up period of 8.2 years [19]. However, the cumulative mortality for anti-HCV seropositives without viremia in the Japanese study was 5% higher than that in the R.E.V.E.A.L.-HCV study. This may have resulted from differences in the age and sex composition and clinical characteristics of HCV-infected participants between the 2 studies. Possibly, the different detection limit of the HCV RNA assays might also have contributed to the discrepancy.
In this study, the cumulative hepatic disease mortality 18 years after enrollment was as high as 9.3% for anti-HCV sero-positives. It has been reported that the cumulative mortality from hepatic diseases among anti-HCV seropositives was
0.35%–5% after 10–25 years of follow-up [3–5,20]. However, most previous long-term follow-up studies enrolled relatively young and healthy populations [3,5]. More notably, anti-HCV seropositives with detectable serum HCV RNA had a signi fi-cantly higher risk of dying from any hepatic disease than anti-HCV seropositives with undetectable serum anti-HCV RNA. Our previous report also documented an elevated incidence of hepatocellular carcinoma in anti-HCV seropositives when de-tectable serum HCV RNA levels were compared to those with undetectable levels [9]. The consistent findings suggest active infection (seropositive for HCV RNA) rather than prior infec-tion (seropositive for anti-HCV) is more important in predict-ing the long-term risk of mortality from liver diseases.
In this prospective study, HCV infection was associated with an increased mortality from extrahepatic diseases, includ-ing circulatory diseases and renal diseases. Chronic HCV in-fection was associated with an increased (1.4-fold) mortality from circulatory diseases, which was consistent with other reports in Western countries [3,20]. We have reported that HCV infection was associated with cerebrovascular death after considering for conventional risk factors. The dose–response relationship between serum HCV RNA level and the risk of cerebrovascular death further strengthened the causal associa-tion of HCV infecassocia-tion and atherosclerosis [21]. HCV infection may play as a stimulus for atherothrombosis by triggering a cascade of immune and inflammatory responses, either locally within vascular tissue or systematically through inflammatory mediators [22].
Anti-HCV seropositives, particularly anti-HCV seroposi-tives with positive HCV RNA, had an increased risk of dying from renal diseases compared with anti-HCV seronegatives. A large cohort of veterans in the United States found that HCV-infected participants had an increased risk of developing end-stage renal diseases treated with dialysis or renal trans-plantation [23]. The pathogenesis of HCV-associated renal disease might have resulted from the deposition of circulating immune complexes in the mesangium and subendothelium, which activate the complement system with the proliferation and infiltration of mononuclear phagocytes, enabling the release of protease and oxidants to alter the glomerular permeability [24].
In addition to hepatocellular carcinoma, this study found significant associations between HCV infection and increased mortality from cancers of the esophagus, prostate, and thyroid. A case-control study found an association between HCV and thyroid cancer with a significant odds ratio of 3.3 [13]. Yet, other large-scale prospective studies failed to find the associations [20,25]. The associations with HCV infection for prostate and esophagus cancer have never been reported previously and need further studies to confirm. Interestingly, all participants who died from these cancers had detectable serum HCV RNA, suggesting that active HCV infection might
Figure 3. Cumulative mortality from circulatory diseases (A) and ne-phritis, nephrotic syndrome, and nephrosis (B) by serostatus of antibodies against hepatitis C virus (anti-HCV) and serum HCV RNA level at study entry.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
play a role. By computerized linkage with national cancer reg-istration profiles, we also found that participants with HCV infection had an increased incidence of esophagus, prostate, and thyroid cancers (data not shown). A large veteran cohort indicated that HCV infection conferred a 20%–30% increased risk of non-Hodgkin lymphoma [25]. In this cohort, only 2 cases died from non-Hodgkin lymphoma and no cases died from Hodgkin’s lymphoma among anti-HCV seropositives. It was difficult to evaluate the association between HCV infec-tion and lymphoma in this study.
Ourfindings indicate that anti-HCV seropositives with de-tectable serum HCV RNA had an elevated mortality from several extrahepatic diseases, whereas the risk for anti-HCV seropositives with undetectable HCV RNA had mortality rates much similar to those seronegative for anti-HCV. This sug-gests that not only hepatic deaths but also extrahepatic deaths could be decreased in anti-HCV seropositives by clearing the virus with efficient antiviral therapy. Our results strengthen the importance of including an HCV RNA test for anti-HCV seropositives in clinical practice. Anti-HCV seropositives, particularly those with detectable serum HCV RNA, should be encouraged to modify health behaviors, including weight reduction, tobacco cessation, or eating a balanced diet, in
order to decrease the risk of cancers, circulatory diseases, and renal diseases.
The strength of this study is its generalizability for relatively healthy individuals with chronic HCV infection, particularly for those who acquired HCV via iatrogenic exposures in devel-oping countries. Unlike most Western countries, the most im-portant risk factor of HCV infection in our study population was iatrogenic factors [26–28]. The epidemiological character-istics of HCV infection in Taiwan were similar to those in Japan, Korea, Italy, India, and developing countries [16,19,
29,30]. People acquired HCV infection when they received medical or dental procedures, blood transfusion, medical in-jections, hemodialysis, acupuncture, and similar procedures. Although our study population has a limited generalizability to Western countries where most individuals infected with HCV were drug abusers, the findings that active HCV infec-tion ( positive for HCV RNA) was associated with an in-creased risk for either hepatic or extrahepatic diseases are still applicable to Western populations. Drug users should be edu-cated to not share injection equipment to avoid HCV reinfec-tions, and they should be encouraged to receive antiviral treatment. The liver-related mortality reported in this study is considered to be a critical parameter for evaluating the efficacy
Table 3. Multivariate-Adjusted Hazard Ratios of Dying From Selected Causes of Death by Serostatus of Antibodies Against Hepatitis C Virus (Anti-HCV) and Serum HCV RNA Level at Study Entry
Multivariate-adjusted Hazard Ratioa(95% CI)
Causes of Death
Anti-HCV Seronegative
Anti-HCV Seropositive With Undetectable Serum HCV
RNA Level
Anti-HCV Seropositive With Detectable Serum HCV RNA
level
P Value (For Trend)
All causes 1.00 (referent) 0.97 (.70–1.35) 2.20 (1.90–2.55) <.0001
Hepatic diseases 1.00 (referent) 2.19 (.81–5.97) 16.36 (12.09–22.13) <.0001
Liver cancer 1.00 (referent) 4.70 (1.68–13.11) 28.02 (18.96–41.41) <.0001
Chronic liver disease and
cirrhosisb
1.00 (referent) — 7.37 (4.22–12.87) <.0001
Extrahepatic diseases 1.00 (referent) 0.90 (.64–1.28) 1.47 (1.23–1.77) .0002
Circulatory diseases 1.00 (referent) 1.16 (.62–2.17) 1.53 (1.05–2.23) .026
Nephritis, nephrotic syndrome, and nephrosis
1.00 (referent) 1.66 (.40–6.81) 2.98 (1.43–6.22) .0032
Esophagus cancerb 1.00 (referent)
— 5.86 (1.98–17.35) .0014
Prostate cancerb 1.00 (referent)
— 5.83 (1.64–20.77 .0065
Thyroid cancerb 1.00 (referent)
— 7.07 (.73–68.35) .09
Abbreviation: CI, confidence interval. a
Hazard ratios for all causes of death, extrahepatic deaths, and nephritis, nephrotic syndromes and nephrosis were adjusted for age, sex, cigarette smoking, alcohol drinking, betel nuts chewing, central obesity, personal history of disease (diabetes, hypertension, heart disease, cerebrovascular disease); hazard ratio for hepatic diseases (including liver cancer, chronic liver diseases, and cirrhosis) was adjusted for age, sex, cigarette smoking, alcohol drinking, betel nuts chewing, central obesity, and personal history of diabetes; hazard ratio for circulatory diseases was adjusted for age, sex, cigarette smoking, alcohol drinking, betel nuts chewing, central obesity, personal history of disease (diabetes, hypertension, heart disease, cerebrovascular disease), and baseline serum triglycerides and cholesterol levels; hazard ratio for esophagus cancer was adjusted for age, sex, cigarette smoking, alcohol drinking, and betel nuts chewing; hazard ratio for prostate cancer was adjusted for age, cigarette smoking, alcohol drinking, and central obesity; hazard ratio for thyroid cancer was adjusted for age and central obesity.
b
There was no death from chronic liver disease and cirrhosis, esophagus cancer, prostate cancer, and thyroid cancer among anti-HCV seropositives with undetectable serum HCV RNA levels (<25 IU/mL) at study entry.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
and effectiveness of chronic hepatitis C management. In addi-tion, the associations between HCV infection and extrahepatic diseases provide insights for future investigations. We classi-fied the risk of hepatic and extrahepatic mortalities for anti-HCV seropositives by including anti-HCV RNA testing, and we also considered other conventional risk factors that have been reported be associated with the diseases. However, some dis-eases were too rare to derive precise risk estimates associated with HCV infection. A collaborative study with an enlarged sample size is needed to further elucidate the association between HCV infection and rare diseases.
In this community-based cohort study, HCV infection was found to be associated with deaths from hepatic and extrahe-patic diseases, particularly for those with detectable serum HCV RNA. It is implied that anti-HCV seropositives should be consulted regarding their elevated risks of both hepatic and extrahepatic diseases. It is also suggested that a serum HCV RNA test with appropriate assay may be helpful to triage HCV-infected patients who need intensive care.
Notes
Acknowledgments. The R.E.V.E.A.L.-HCV study was funded by:
De-partment of Health, Executive Yuan, Taipei, Taiwan; Bristol-Myers Squibb Co, United States; Academia Sinica, Taipei, Taiwan; and the National Health Research Institutes (NHRI-EX98-9806PI), Chunan, Taiwan. We thank all the patients for their cooperation in this study. C. -J. Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: C. J. Chen, M. H. Lee. Acquisition of data: C. J. Chen, M. -H. Lee, -H. -I. Yang, C. -L. Jen, S. -L. You. Analysis and interpretation of data: C.J. Chen, M. H. Lee, H. I. Yang. Drafting of the manuscript: M. -H. Lee. Critical revision of the manuscript for important intellectual content: C. -J. Chen, M. -H. Lee, H. -I. Yang, S. -N. Lu, C. -L. Jen, S. -L. You, L. -Y. Wang, C. -H. Wang, W. J. Chen. Statistical analysis: M. -H. Lee. Obtained funding: C. -J. Chen. Study supervision: C. -J. Chen.
Financial support. This work was supported by research grants from
Department of Health, Executive Yuan, Taipei, Taiwan; Bristol-Myers Squibb Co, United States; Academia Sinica, Taipei, Taiwan; and National Health Research Institutes (NHRI-EX98-9806PI), Chunan, Taiwan.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
1. Lauer GM, Walker BD. Hepatitis C virus infection.[see comment]. N Engl J Med 2001; 345:41–52.
2. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118:3030–44.
3. Guiltinan AM, Kaidarova Z, Custer B, et al. Increased all-cause, liver, and cardiac mortality among hepatitis C virus–seropositive blood donors. Am J Epidemiol 2008; 167:743–50.
4. Seeff LB, Buskell-Bales Z, Wright EC, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med 1992; 327:1906–11.
5. Wiese M, Grungreiff K, Guthoff W, Lafrenz M, Oesen U, Porst H. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany—a 25-year multicenter study. J Hepatol 2005; 43:590–8.
6. Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahe-patic manifestations. Cleve Clin J Med 2005; 72:1005–8.
7. Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequenc-es. Hepatology 2006; 44:15–22.
8. Poynard T, McHutchison J, Manns M, et al. Impact of pegylated
in-terferon alfa-2b and ribavirin on liverfibrosis in patients with chronic
hepatitis C. Gastroenterology 2002; 122:1303–13.
9. Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and sub-sequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol 2010; 28:4587–93. 10. Iloeje UH, Yang HI, Jen CL, et al. Risk and predictors of mortality
associated with chronic hepatitis B infection. Clin Gastro Hepatol 2007; 5:921–31.
11. Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
12. Fwu CW, Chien YC, Nelson KE, et al. Mortality after chronic hepatitis B virus infection: a linkage study involving 2 million parous women from Taiwan. J Infect Dis 2010; 201:1016–23.
13. Montella M, Pezzullo L, Crispo A, et al. Risk of thyroid cancer and high prevalence of hepatitis C virus. Oncol Rep 2003; 10:133–6. 14. Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in
hepatitis C–related mortality in the United States, 1995–2004. Hepa-tology 2008; 47:1128–35.
15. Lehman EM, Wilson ML. Epidemic hepatitis C virus infection in Egypt: estimates of past incidence and future morbidity and mortality. J Viral Hepat 2009; 16:650–8.
16. Chowdhury A, Santra A, Chaudhuri S, et al. Hepatitis C virus infec-tion in the general populainfec-tion: a community-based study in West Bengal, India. Hepatology 2003; 37:802–9.
17. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. [see comment]. N Engl J Med 1999; 341:556–62.
18. McMahon BJ, Hennessy TW, Christensen C, et al. Epidemiology and risk factors for hepatitis C in Alaska Natives. Hepatology 2004; 39:325–32.
19. Uto H, Stuver SO, Hayashi K, et al. Increased rate of death related to presence of viremia among hepatitis C virus antibody–positive sub-jects in a community-based cohort study. Hepatology 2009; 50:393–9. 20. Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Causes of death
after diagnosis of hepatitis B or hepatitis C infection: a large commu-nity-based linkage study. Lancet 2006; 368:938–45.
21. Lee MH, Yang HI, Wang CH, et al. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke 2010; 41:2894–900. 22. Hansson GK. Mechanisms of disease—inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med 2005; 352:1685–95. 23. Tsui JI, Vittinghoff E, Shlipak MG, et al. Association of hepatitis C
seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med 2007; 167:1271–6.
24. Daghestani L, Pomeroy C. Renal manifestations of hepatitis C infec-tion. Am J Med 1999; 106:347–54.
25. Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 2007; 297:2010–7.
26. Sun CA, Chen HC, Lu CF, et al. Transmission of hepatitis C virus in Taiwan: prevalence and risk factors based on a nationwide survey. J Med Virol 1999; 59:290–6.
27. Sun CA, Chen HC, Lu SN, et al. Persistent hyperendemicity of hepati-tis C virus infection in Taiwan: the important role of iatrogenic risk factors. J Med Virol 2001; 65:30–4.
28. Lee MH, Yang HI, Jen CL, et al. Community and personal risk factors for hepatitis C virus infection: a survey of 23,820 residents in Taiwan in 1991–2. Gut 2011; 60:688–94.
29. Shin HR, Kim JY, Kim JI, et al. Hepatitis B and C virus prevalence in a rural area of South Korea: the role of acupuncture. Br J Cancer 2002; 87:314–18.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/
30. Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differ-ences and temporal trends. Semin Liver Dis 2000; 20:1–16.
Appendix
Other members of the R.E.V.E.A.L.-HCV study group: National Taiwan University Hospital: C. Y.: Hsieh, H. S. Lee, P. M. Yang, C. H. Chen, J. D. Chen, S. P. Huang, C. F. Jan. National Taiwan University: T. H. H. Chen. National Defense Medical Center:
C. A. Sun. Taipei City Psychiatric Center: M. H. Wu. Tzu Chi University: S. Y. Chen. Shin Kong Wu Ho-Su Memorial Hospi-tal: K. E. Chu. Huhsi Health Center, Penghu County: S. C. Ho, T. G. Lu. Provincial Penghu Hospital: W. P. Wu, T. Y. Ou. Sanchi Health Center, Taipei County: C. G. Lin. Provincial Chutung Hospital: K. C. Shih. Provincial Potzu Hospital: W. S. Chung, C. Li. Kaohsu Health Center, Pingtung County: C. C. Chen. Paihsa Health Center, Penghu County: W. C. How.
at China Medical University Library on August 7, 2012
http://jid.oxfordjournals.org/