Association between nucleoside analogues and risk of hepatitis B
virus-related hepatocellular carcinoma following liver resection
Short title: HBV, nucleoside analogues and HCC recurrence
Chun-Ying Wu, MD, PhD, MPH, LL.M.1-4, Yi-Ju Chen, MD, PhD1,5, Hsiu J. Ho, PhD6,
Yao-Chun Hsu, MD, MS3,7, Ken N. Kuo, MD8, 9, Ming-Shiang Wu, MD, PhD6,
and Jaw-Town Lin, MD, PhD6,7, 9,10
1Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei; 2Division of Gastroenterology, Taichung Veterans General Hospital, Taichung;
3Department of Public Health and Graduate Institute of Clinical Medicine, China Medical University,
Taichung;
4Department of Life Sciences, National Chung-Hsing University, Taichung; 5Department of Dermatology, Taichung Veterans General Hospital, Taichung; 6Division of Gastroenterology, National Taiwan University Hospital, Taipei, Taiwan; 7Department of Internal Medicine, E-Da Hospital/I-Shou University, Kaohsiung; 8College of Medicine, Taipei Medical College, Taipei;
9Institute of Population Health Sciences, National Health Research Institutes, Miaoli; 10School of Medicine, Fu Jen Catholic University, Taipei, all in Taiwan;
Abbreviations: Hepatitis B virus (HBV), hepatocellular carcinoma (HCC), National Health Insurance Research Database (NHIRD)
* Correspondence:
Chun-Ying Wu, MD, MPH, PhD
Faculty of Medicine, School of Medicine, National Yang-Ming University No. 155, Section 2, Linong Street, Taipei 11221, Taiwan
E-mail: chun@vghtc.gov.tw
Tel: +886-4-23592525 # 3304; Fax: +886-4-23741331 Jaw-Town Lin, MD, PhD, Chair Professor
School of Medicine, Fu Jen Catholic University
No. 510, Zhongzheng Rd., Xinzhuang Dist., New Taipei City, 24205, Taiwan E-mail: jawtown@gmail.com
Tel: +886-2-23562246; Fax: +886-2-23947899
Wu CY and Chen YJ contributed equally to this work as first authors; Wu CY and Lin JT contributed to this work as corresponding authors.
Date of the revision: August 18, 2012
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38
Word Count: 3514
Abstract word count: 295 Tables Count: 2
Figures Count: 3
Funding: Grant Support: This work was supported by Taiwan’s National Health Research
Institutes (PH-101-PP-23).
Competing interests: The authors have no conflicts of interest to report.
39 40 41 42 43 44 45
ABSTRACT
Context: Tumor recurrence is a major issue for patients with hepatocellular carcinoma (HCC)
following curative liver resection.
Objective: We aimed to investigate the association between nucleoside analogue use and risk
of tumor recurrence in patients with hepatitis B virus (HBV)-related HCC after curative surgery.
Design: A nationwide cohort study between October 2003 and September 2010. Setting: Data from the Taiwan National Health Insurance Research Database.
Patients: Among 100,938 newly diagnosed HCC patients, we identified 4,569 HBV-related
HCC patients who received curative liver resection for HCC between October 2003 and September 2010.
Main Outcome Measures: The risk of first tumor recurrence was compared between patients
not taking nucleoside analogues (untreated cohort, N=4,051) and patients taking nucleoside analogues (treated cohort, N=518). Cumulative incidences and hazard ratios were calculated after adjusting for competing mortality.
Results: Compared with untreated cohort, treated cohort had higher prevalence of liver
cirrhosis (38.7% vs. 48.6%, P<0.001), but lower risk of HCC recurrence (N=1,765, 43.6% vs. N=106, 20.5%, P<0.001) and lower overall death (N=1,145, 28.3% vs. N=55, 10.6%,
P<0.001). After adjusting for competing mortality, treated cohort had significantly lower 6-year HCC recurrence rate (untreated vs. treated: 54.6%; 95%CI, 52.5%-56.6% vs. 45.6%; 95%CI, 36.5%-54.6%; P<0.001). Six-year overall mortalities for untreated and treated cohorts were 42.4% (95%CI, 40.0%-44.7%) and 29.0% (95%CI, 20.0%-38.0%), respectively
(P<0.001). On modified Cox regression analysis, nucleoside analogue use (HR=0.67; 95%CI, 0.55-0.81, P<0.001), statins use (HR=0.68; 95%CI, 0.53-0.87, P=0.002), and NSAIDs or aspirin use (HR=0.80; 95%CI, 0.73-0.88, P<0.001) were independently associated with a reduced risk of HCC recurrence. Multivariable stratified analyses further verified the 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71
association in all subgroups of patients, including non-cirrhotic patients (HR=0.56; 95%CI, 0.42-0.76) and diabetic patients (HR=0.52; 95%CI, 0.31-0.89).
Conclusions: Nucleoside analogue use is associated with a lower risk of HCC recurrence
among patients with HBV-related HCC after liver resection. 72
73 74 75 76
INTRODUCTION
Surgery is considered the standard curative treatment option for hepatocellular carcinoma (HCC). However, long-term disease free survival after liver resection remains unsatisfactory due to persistent high incidences of HCC recurrence.1 Many factors affect HCC
recurrence risk after liver resection, including tumor size and stage, serum alfa-fetoprotein level, cirrhosis, HBeAg status and hepatitis B virus (HBV) viral load.2-4 Among these factors,
HBV viral load is the most clinically controllable.
Higher HBV viral load has been reported to be an independent risk factor for HCC recurrence in HBV-related HCC patients.5,6 Nucleoside analogues are effective in suppressing
HBV replication and in ameliorating HBV-related liver disease.7,8 They have been shown to
be associated with a lower risk of HCC and other cirrhosis-related complications in those with chronic hepatitis9,10 and cirrhosis.11 However, studies on the effectiveness of nucleoside
analogue use in HCC recurrence have been relatively limited and have yielded conflicting results.12-14 In Taiwan, under the National Health Insurance (NHI) program, reimbursement for
nucleoside analogues for HBV patients meeting certain criteria began on October 1, 2003 (eTable 1 and eTable 2).
The aim of this study was to examine the association between use of nucleoside
analogues and risk of HCC recurrence among HBV-related HCC patients after curative liver resection. We also examined this association among different subpopulations and calculated the number needed to be treated (NNT) for one less HCC recurrence.
77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96
METHODS
Study design
We conducted a nationwide cohort study by retrieving all patients receiving curative liver resection for hepatocellular carcinoma (HCC) from Taiwan’s National Health Insurance Research Database (NHIRD). The NHIRD has been described in detail in our previous studies.15-17 In brief, it consists of detailed health care data from more than 25 million
enrollees, representing more than 99% of Taiwan’s entire population. The accuracy of diagnosis of major diseases in the NHIRD, such as stroke and acute coronary syndrome, has been validated.17,18 This study has been approved by the ethical review board of the National
Health Research Institutes, Taiwan.
Study population
We identified all hospitalized patients who were admitted with a primary diagnosis of HCC (ICD-9 codes 155.0, 155.2) for the first time and who received curative liver resection between October 1, 2003 and September 30, 2010. The diagnostic accuracy of HCC was confirmed by both specific admission ICD-9 codes and inclusion in the Registry for Catastrophic Illness Patient Database (RCIPD), a subpart of the NHIRD.15,16 Surgical
pathological confirmation or typical image presentation of HCC is required for patients to be registered in the RCIPD.
Only HCC patients with HBV infection (ICD-9 codes: 070.2, 070.3, V02.61) were included in our study cohorts. Patients with hepatitis C (HCV) (ICD-9 codes: 070.41, 070.44, 070.51, 070.54, and V02.62), other viral hepatitis (ICD-9 code: V02.69), malignant tumor (ICD-9 codes: 104-208), or who had received antiviral treatments for more than 3 months before the index admission were excluded. Patients who received liver resection, transarterial chemoembolization (TACE), percutaneous ethanol injection (PEI), radiofrequency ablation (RFA) or liver transplantation before the index hospitalization were also excluded.
97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121
Study cohorts
Newly diagnosed HBV-related HCC patients who received curative liver resection were divided into 2 cohorts based on their use of nucleoside analogues. The untreated cohort was comprised of patients who had never received nucleoside analogues and the treated cohort was comprised of patients who had received nucleoside analogues for at least 90 days. Those receiving antiviral treatments for less than 90 days during the observation period or prior to the observation period were excluded. Information regarding medications was retrieved from the pharmacy prescription database. Reliability of the retrieved information was verified independently by two statisticians. Nucleoside analogues included lamivudine, entecavir, and telbivudine. For each nucleoside analogue prescription, the number of days of use was
calculated. Then, the numbers of days of use for each prescription were added together to determine the total number of days of nucleoside analogue use.
Main outcome measurements
HCC recurrence was defined as re-hospitalization with a primary diagnosis of HCC after the index admission date and a treatment modality for HCC recurrence, such as surgery, TACE, PEI, RFA or liver transplantation, during the study period. Patients with HCC recurrence in the first 3 months after the index hospitalization for liver resection were excluded. We utilized the incident user design with follow-up for each patient beginning on the date of first prescription of nucleoside analogue in the treated cohort.19,20 The follow-up
for untreated cohort began on the first day after the index hospitalization for liver resection. Both cohorts were followed up until the date of HCC recurrence, death or the end of 2010. Death was defined as withdrawal of the patient from the NHI program. Causes of death were defined according to the primary diagnosis of hospitalization in the three months preceding death.
Covariate assessment
Propensity score was calculated using logistic regression as proposed by Rosenbaum and 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147
Rubin21,22 to estimate the probabilities of assigning a patient to the treated cohort given the
background variables, including age, gender, extent of resection, liver cirrhosis, other comorbidities listed in Table 1, and use of statins, NSAIDs, and metformin. The mean and median propensity scores were compared between the two cohorts. Since the chance of HCC recurrence can be confounded by competing risk of mortality, we identified co-morbidities that may be associated with mortality based on diagnostic codes from both outpatient and inpatient datasets prior to the outcome of interest. All diseases included in the Charlson’s co-morbidity index were analyzed except for HIV, metastatic solid tumor and cancer because patients with these conditions were excluded from the present study23. Co-morbidities
included acute coronary syndrome (ICD-9 codes: 410-414), cerebrovascular accident (ICD-9 codes: 430-438), chronic obstructive pulmonary disease (ICD-9 codes: 490-496), diabetes mellitus (ICD-9 code: 250), liver cirrhosis (ICD-9 code: 571.5), liver failure (ICD-9 code: 570), renal failure (ICD-9 codes: 584-586), hypertension (ICD-9 codes: 401-405),
hyperlipidemia (ICD-9 codes: 272.0-272.2), and peptic ulcer disease (ICD-9 codes: 531-534). Certain drugs, including statins, aspirin, NSAIDs and metformin, which might alter the risk of recurrent HCC, were analyzed. Exposure to these drugs was defined as frequency of use of more than one tablet per month during the observation period. Liver resection
modalities for treatment of the original HCC, including major resection (extensive and partial hepatic lobectomy, with at least 3 segmental resections of liver parenchyma) and minor resection (extensive and partial hepatectomy, with 2 or fewer segmental resections of liver), were also analyzed.
Statistical analysis
Death prior to tumor recurrence was considered a competing risk event. The death-adjusted cumulative incidences of HCC recurrence were calculated using a two-step process and tested for equality among the study cohorts. Calculation and comparison of cumulative incidences in competing risk data ratios were conducted using modified Kaplan-Meier 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173
method24 and Gray's method25. We tested differences in the full time to event distributions
between the study groups using log-rank test.
To determine the independent risk factors for HCC recurrence, multivariable analyses and stratified analyses using hazard ratios were carried out with modified Cox proportional hazards models in the presence of competing risk event after adjusting for age, gender, resection modality, liver cirrhosis, diabetes, propensity score and use of statins, NSAIDs or aspirin, and metformin24. To assess the dose-dependent association of recurrence with
nucleoside analogue use, we further conducted multivariable analysis with nucleoside analogue use as a continuous variable. On multivariable stratified analyses, the association between nucleoside analogue use and the risk of HCC recurrence was examined in different subgroups. All subgroup comparisons were preplanned to control for potential confounding factors reported in previous studies.
NNT represented the number of patients that need to be treated for one less HCC
recurrence or mortality. NNT was calculated by the inverse of the absolute risk reduction. All results in the present study originated from a nationwide registry database. HCC is defined as a major disease and HCC patients can apply for a catastrophic illness certificate which grants exemption from co-payment. It is nearly impossible for these HCC patients to withdraw from the NHI program before death. Therefore, there were no missing data or loss of follow-up in our study population.
All data management was performed using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Calculations of cumulative incidences and Cox models in the competing risk analysis were carried out using the "cmprsk" package of R
(http://cran.r-project.org/web/packages/cmprsk/index.html). Calculated results were expressed as the estimated number together with 95% confidence interval (CI). Based on statistical power at 0.9, type I error rate at 0.05, and our case numbers in both cohorts, the detectable risk difference was estimated to be 0.02.
174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199
RESULTS
Demographic characteristics of HCC cohort
We first identified 100,938 potentially eligible HCC patients admitted for the first time and registered in the RCIPD. We excluded 78,948 patients who did not receive liver resection and 8,173 patients who received liver resection before October 1, 2003. In addition, 7,870 patients without HBV infection or co-infection with hepatitis C or other hepatitis were excluded. Those using antiviral agents 3 months before liver resection or for less than 3 months were also not enrolled. There were 1,019 patients who received other liver therapy or with another type of tumor before liver resection who were excluded. Another 359 patients with follow-up for less than three months were not included in the study. Finally, we enrolled 4,569 patients into our study cohorts (untreated: 4,051 patients; treated: 518 patients) (Figure 1). In the treated cohort, a total of 487 patients received only one nucleoside analogue, including 159 patients who received lamivudine, 292 patients who received entecavir, and 36 patients who received telbivudine. The remaining patients received more than one nucleoside analogue.
Demographic characteristics, confounding drugs, co-morbidities, and follow-up durations of the study cohorts are presented in Table 1. The mean follow-up durations were 2.18 (standard deviation, SD: 1.77) (median, 1.57; interquartile range, IQR, 0.77-3.15) and 2.64 (SD, 1.74) (median, 2.18; IQR, 1.21-3.69) years for untreated and treated cohorts, respectively. The mean interval to start of antiviral therapy after liver resection was 1.19 (SD, 1.38) (median, 0.66; IQR, 0.14-1.84) years. The mean duration of nucleoside analogue use in treated patients was 1.45 (SD, 1.38) (median, 0.95; IQR, 0.48-1.94) years. The mean
propensity scores for untreated and treated cohorts were 0.11 (SD, 0.03) (median, 0.11; IQR, 0.09-0.14) and 0.12 (SD, 0.03) (median, 0.12; IQR, 0.10-0.15), respectively. Treated cohort had significantly higher prevalence of liver cirrhosis (48.6%) when compared with untreated cohort (38.7%) (P<0.001). 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225
Six-year cumulative incidences of HCC recurrence and overall mortality
In the present study, we excluded 359 patients with follow-up of less than three months. Among these patients, there were 226 HCC recurrences and 93 deaths. During the observation period, 1,765 patients in the untreated cohort (43.6%) and 106 patients in the treated cohort (20.5%) developed HCC recurrence. Death before the recurrence of HCC was defined as competing mortality. Compared with untreated cohort (451 deaths, 11.1%), only 26 patients died before HCC recurrence in the treated cohort (5.0%). The major identifiable causes of competing mortality in untreated cohort were HCC or HCC treatment-related mortality (N=314), liver cirrhosis (N=14), pneumonia (12), sepsis (N=9), and gastrointestinal bleeding (N=7). The identifiable causes of competing mortality in treated cohort included HCC or HCC treatment-related mortality (N=20), pneumonia (N=2), liver cirrhosis (N=1), and sepsis (N=1). During the follow-up period, overall deaths for untreated and treated cohorts were 1,145 (28.3%) and 55 (10.6%), respectively. For overall mortality, the major identifiable causes of death in the untreated cohort were HCC or HCC treatment-related mortality (N=832), liver cirrhosis (N=47), sepsis (N=28), pneumonia (N=22), and gastrointestinal bleeding (N=21). The major identifiable causes of overall mortality in the treated cohort were HCC or HCC treatment-related mortality (N=38), liver cirrhosis (N=5), pneumonia (N=5), and sepsis (N=3).
In Figure 2A, cumulative incidences of HCC recurrence after adjustment for competing mortality are shown. The risk of HCC recurrence was significantly lower for patients in the treated cohort (6-year cumulative incidence: 45.6%, 95%CI 36.5%-54.6%) than for patients in the untreated cohort (54.6%, 95%CI 52.5%-56.6%) (P<0.001). The difference in 6-year cumulative incidence was 9%. The unadjusted NNT associated with one less HCC recurrence within 6 years was 12 (95% CI, 7.4-22.6). This implies that use of nucleoside analogues in 12 HCC patients after liver resection is associated with one less HCC recurrence within 6 years.
In eFigure 1, we stratified patients by liver cirrhosis and NSAIDs use. We found that 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251
nucleoside analogue use is associated with lower risk of HCC recurrence in non-cirrhotic patients, but not in cirrhotic patients. For both NSAIDs users and non-users, use of nucleoside analogues was associated with reduced risk of HCC recurrence.
Likewise, the risk of overall mortality was significantly lower in patients in treated cohort (6-year cumulative incidence, 29.0%; 95%CI, 20.0%-38.0%) than in patients in untreated cohort (42.4%; 95%CI, 40.0%-44.7%) (P<0.001). (Figure 2B) The difference in 6-year overall mortality was 13.4%. The unadjusted NNT associated with one less death within 6 years was 8 (95% CI, 5.7-11.0). This implies that use of nucleoside analogues in 8 HCC patients after liver resection is associated with one less death within 6 years.
Multivariable analysis
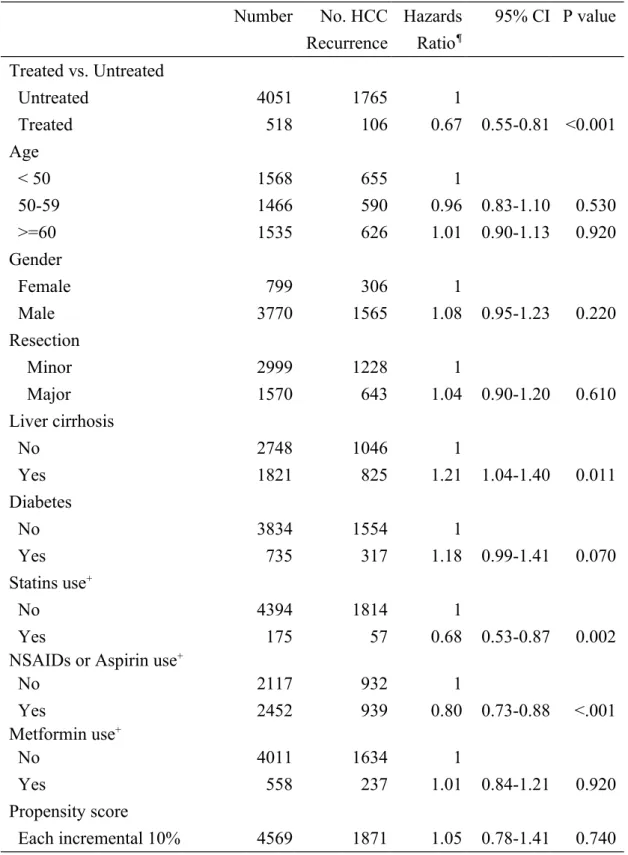
Compared with untreated cohort, treated cohort was associated with a significantly lower risk of HCC recurrence (HR: 0.67; 95%CI, 0.55-0.81, P<0.001). Use of statins (HR, 0.68; 95%CI, 0.53-0.87, P=0.002) and use of NSAIDs or aspirin (HR, 0.80; 95%CI, 0.73-0.88, P<0.001) were significantly associated with lower risk of tumor recurrence. Liver cirrhosis was found to be an independent risk factor for HCC recurrence (HR, 1.23; 95%CI, 1.12-1.35, P<0.001) (Table 2). Each incremental year of use of nucleoside analogues was associated with reduced risk of HCC recurrence (HR, 0.59; 95%CI, 0.51-0.68, P<0.001) (eTable 3).
Multivariable stratified analysis
Treated cohort was found to be associated with a reduced risk of HCC recurrence on all stratified analyses, including for non-cirrhotic patients (HR=0.56; 95%CI, 0.42-0.76) and diabetic patients (HR=0.52; 95%CI, 0.31-0.89) (Figure 3). These observations further confirmed the association between nucleoside analogue use and attenuated risk of HCC recurrence in HBV-related HCC patients after liver resection.
252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274
DISCUSSION
The roles of HBV in HCC recurrence have been widely investigated. Kubo et al. first reported that high viral load is an independent risk factor for HCC recurrence after liver resection in HBV-related HCC patients.5 Hung et al. found that HBV viral load > 2,000
IU/mL is associated with an odds ratio as high as 22.3 for HCC recurrence after liver
resection.6 The association between serum HBV loads and risk of HCC recurrence after liver
resection or TAE has been confirmed in previous studies.3,26,27 In the present study, we did not
have data regarding patient’s HBV viral load or liver function. However, Taiwan’s NHI program has strict regulations regarding nucleoside analogues reimbursement.
Reimbursement is granted only to patients in high-risk populations (eTable 1). Under such regulations, patients in the treated cohort should have higher baseline HBV viral load, higher ALT level, or higher prevalence of liver decompensation than those in the untreated cohort to be eligible for reimbursement. In the present study, the treated cohort had higher prevalence of liver cirrhosis. If higher HBV viral load is associated with higher risk of HCC recurrence, higher baseline HBV viral load in the treated patients may have led to a more conservative estimation of the association in the present study.
Although it is generally accepted that HBV viral load plays an important role in HCC recurrence, studies regarding the effectiveness of nucleoside analogues in HCC recurrence have been very limited and have produced conflicting results.28 Based on a study of 14
patients receiving lamivudine and 10 controls, Kubo et al. found a lower 5-year HCC disease-free survival rate after surgery in the lamivudine-treated patients.13 Chan et al. reported that
the 5-year tumor free survival rates in lamivudine or entecavir-treated group (N=42, 51.4%) were significantly higher than in control group (N=94, 33.8%).14 In contrast, Li et al. found no
significant differences in the 1-year tumor-free survival after curative hepatectomy between lamivudine (N=43, 23.3%) and control (N=36, 8.3%) groups.29 Kuzuya et al. demonstrated
that 5-year cumulative recurrence rates of HCC do not differ in a study of 16 patients 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300
receiving lamivudine and 33 controls.12 In the present study, we confirmed the association
between nucleoside analogue use and reduced risk of HCC recurrence in HBV patients after liver resection based on a nationwide database. However, reimbursement for oral antiviral agents for HBV infection is strictly limited to specified indications in Taiwan and HCC does not qualify as an indication. Therefore, most of the HBV-infected HCC patients in the present study did not fulfill the NHI criteria for oral antiviral therapy.
In previous studies, HCC with cirrhosis has been associated with decreased overall survival compared to HCC without cirrhosis after curative liver resection.2 However, the
outcome of patients with HBV-related compensated cirrhosis has not been shown to be worse than that of non-cirrhotic patients.4 In the present study, nucleoside analogue use was not only
associated with improved disease-free survival, but also with improved overall survival in HBV-related HCC after liver resection. The higher prevalence of cirrhosis in the treated cohort was the main reason that the absolute difference in HCC recurrence rates (54.6% vs. 45.6%) was much smaller than the hazard ratio (0.67) observed on multivariable analysis.
On multivariable analysis, we found that statin and NSAIDS or aspirin are associated with a lower risk of HCC recurrence. The protective role of statins in HBV-infected HCC has been reported in a recent study.30 The potential mechanisms involve AMPK, p21 expression,
ER stress and autophagy.31 The association between the use of NSAIDs or aspirin and a lower
risk of HCC recurrence is a novel finding. Aspirin has been reported to induce cell cycle arrest and apoptosis, mediated by increased metabolic and oxidative stress.32 An in vivo study
showed that aspirin results in tumor growth inhibition.33 More recently, Sitia et al.
demonstrated that aspirin diminishes the number of intrahepatic HBV-specific CD8(+) T cells and HBV-nonspecific inflammatory cells, the severity of liver fibrosis, and the development of HCC in an HBV transgenic mouse model.34 Based on prior studies and our results, we
postulated that NSAIDs including aspirin may be beneficial for reducing tumor recurrence in HCC patients. Future randomized controlled trial studies are necessary to clarify this issue. 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326
There are several limitations to the present study. First, it is difficult to infer causation between a drug of interest and risk of HCC recurrence based on an observational study, without a random assignment of treatments. Confounding by indication may exist and account for differences in outcomes. The patients in the study cohorts may differ in many measured and unmeasured ways. We did not have personal information for our patients such as lifestyle, family history of malignant diseases, body mass index, or laboratory parameters, including HBV DNA viral load, which may contribute to tumor recurrence risk. To avoid these biases, we selected only patients receiving curative liver resection as resectable patients are
comparable in terms of disease extent and remnant liver function. We analyzed propensity scores and Charlson’s scores to examine the comparability of these two cohorts. Multivariable analysis was performed to adjust for potential confounders. Furthermore, we conducted multivariable stratified analysis to examine the risk of HCC recurrence after liver resection for the study cohorts in different strata. Although unmeasured confounders may still exist, we believe the methodology used in the present study is solid and robust. Second, coding error is possible in a database. We were unable to check the accuracy of nucleoside analogue use in the NHIRD. However, the information regarding insurance-paid nucleoside analogues was accurate as every prescription is strictly regulated and only patients fitting specific criteria are eligible to receive reimbursement. Third, some patients may have used self-paid nucleoside analogues and thus may have been inappropriately classified into the untreated cohort. On the other hand, nucleoside analogue users in the treated cohort may have poor compliance. These potential misclassifications may have led to an underestimation of the association. Fourth, HCC recurrence was defined as re-hospitalization with a primary diagnosis of HCC and further HCC therapy. It is possible that some incidence of HCC recurrence might have been missed, for example if a patient did not receive therapy for the recurrence. Since all enrolled participants were able to undergo curative resection, the majority of them were unlikely to give up therapy for a recurrence. Furthermore, such misclassification would have
327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342 343 344 345 346 347 348 349 350 351 352
underestimated the incidence of recurrent HCC in both cohorts and biased the results toward null difference. Fifth, information about adverse events of nucleoside analogues was not available from the NHIRD. However, abundant data have demonstrated excellent short-term safety profiles for the available nucleoside analogues for chronic hepatitis B35. Therefore,
adverse events associated with oral anti-HBV therapy were very unlikely to substantially influence clinical outcomes in the present study. Finally, we did not treat nucleoside analogue use as a time dependent variable in the Cox model. Instead, we used the incident user design in this study, in which exposure time begins with the start of new antiviral agents. This minimizes the immortal person-time bias prior to treatment exposure, which may result in a downward trend toward underestimation of the risk rate ratios.
In conclusion, nucleoside analogue use is associated with a lower risk of HCC recurrence among patients with HBV-related HCC after liver resection.
353 354 355 356 357 358 359 360 361 362 363 364 365 366
Acknowledgments: This study is based on data from the National Health Insurance Research
Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes, Taiwan. This work was supported by the National Health Research Institutes (Grant number: PH-101-PP-23). The National Health Research Institutes provided access to the NHIRD and financial support for the hiring of a research assistant. The National Health Research Institutes did not have any other role in this work. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or the National Health Research Institutes. Citation of URL: National Health Insurance Research Database, Taiwan.
http://www.nhri.org.tw/nhird/en/index.htm
Chun-Ying Wu has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
367 368 369 370 371 372 373 374 375 376 377 378 379
REFERENCES
1. Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol. Sep 2011;26(9):1354-1360.
2. Grazi GL, Cescon M, Ravaioli M, et al. Liver resection for hepatocellular carcinoma in cirrhotics and noncirrhotics. Evaluation of clinicopathologic features and comparison of risk factors for long-term survival and tumour recurrence in a single centre.
Alimentary pharmacology & therapeutics. Jun 2003;17 Suppl 2:119-129.
3. Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. Journal of hepatology. Nov 2009;51(5):890-897.
4. Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. Journal of
clinical oncology: official journal of the American Society of Clinical Oncology. Mar
2000;18(5):1094-1101.
5. Kubo S, Hirohashi K, Tanaka H, et al. Effect of viral status on recurrence after liver resection for patients with hepatitis B virus-related hepatocellular carcinoma. Cancer. Mar 1 2000;88(5):1016-1024.
6. Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection.
The American journal of gastroenterology. Jul 2008;103(7):1663-1673.
7. Liaw YF. Antiviral therapy of chronic hepatitis B: opportunities and challenges in Asia.
Journal of hepatology. Aug 2009;51(2):403-410.
8. Yuen MF, Lai CL. Treatment of chronic hepatitis B: evolution over two decades. J
Gastroenterol Hepatol. Jan 2011;26 Suppl 1:138-143.
9. Tong MJ, Sun SC, Schaeffer BT, Chang NK, Lo KJ, Peters RL. Hepatitis-associated antigen and hepatocellular carcinoma in Taiwan. Annals of internal medicine. Nov 1971;75(5):687-691.
10. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. Oct 7 2004;351(15):1521-1531.
11. Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA: the journal of the American
Medical Association. Jan 4 2006;295(1):65-73.
12. Kuzuya T, Katano Y, Kumada T, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J
Gastroenterol Hepatol. Nov 2007;22(11):1929-1935.
13. Kubo S, Tanaka H, Takemura S, et al. Effects of lamivudine on outcome after liver resection for hepatocellular carcinoma in patients with active replication of hepatitis B virus. Hepatology research. Feb 2007;37(2):94-100.
380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399 400 401 402 403 404 405 406 407 408 409 410 411 412 413 414 415 416 417
14. Chan AC, Chok KS, Yuen WK, et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. Jun 2011;146(6):675-681.
15. Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Effective reduction of gastric cancer risk with regular use of nonsteroidal anti-inflammatory drugs in Helicobacter pylori-infected patients. Journal of clinical oncology. Jun 20 2010;28(18):2952-2957.
16. Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease.
Gastroenterology. Nov 2009;137(5):1641-1648 e1641-1642.
17. Wu CY, Chan FK, Wu MS, et al. Histamine2-receptor antagonists are an alternative to proton pump inhibitor in patients receiving clopidogrel. Gastroenterology. Oct 2010;139(4):1165-1171.
18. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiology and
drug safety. Mar 2011;20(3):236-242.
19. Suissa S. Immortal time bias in pharmaco-epidemiology. American journal of
epidemiology. Feb 15 2008;167(4):492-499.
20. Suissa S. Immortal time bias in observational studies of drug effects.
Pharmacoepidemiology and drug safety. Mar 2007;16(3):241-249.
21. Rosenbaum PR, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika, 1983;70(1):41-55.
22. Rosenbaum PR, Rubin D. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American Statistical
Association. 1984;79(387): 516-524.
23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying
prognostic comorbidity in longitudinal studies: development and validation. Journal
of chronic diseases. 1987;40(5):373-383.
24. Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Long-term peptic ulcer rebleeding risk estimation in patients undergoing haemodialysis: a 10-year nationwide cohort study. Gut. Aug 2011;60(8):1038-1042.
25. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16(3):1141-1154.
26. Kim BK, Park JY, Kim do Y, et al. Persistent hepatitis B viral replication affects
recurrence of hepatocellular carcinoma after curative resection. Liver international. Mar 2008;28(3):393-401.
27. Jang JW, Choi JY, Bae SH, et al. The impact of hepatitis B viral load on recurrence after complete necrosis in patients with hepatocellular carcinoma who receive transarterial chemolipiodolization: implications for viral suppression to reduce the risk of cancer recurrence. Cancer. Oct 15 2007;110(8):1760-1767.
418 419 420 421 422 423 424 425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440 441 442 443 444 445 446 447 448 449 450 451 452 453 454 455 456
28. Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology
international. 2010;4(2):439-474.
29. Li N, Lai EC, Shi J, et al. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection.
Annals of surgical oncology. Jan 2010;17(1):179-185.
30. Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. Journal of clinical oncology. Feb 20
2012;30(6):623-630.
31. Yang PM, Liu YL, Lin YC, Shun CT, Wu MS, Chen CC. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer research. Oct 1 2010;70(19):7699-7709.
32. Raza H, John A, Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells.
European journal of pharmacology. Oct 1 2011;668(1-2):15-24.
33. Hossain MA, Kim DH, Jang JY, et al. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. International journal of oncology. Apr 2012;40(4):1298-1304.
34. Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B.
Proceedings of the National Academy of Sciences of the United States of America. Jul
2 2012.
35. Kwon H, Lok AS. Hepatitis B therapy. Nature reviews. Gastroenterology & hepatology. May 2011;8(5):275-284. 457 458 459 460 461 462 463 464 465 466 467 468 469 470 471 472 473 474 475 476 477 478 479 480 481 482
FIGURE LEGENDS:
Figure 1. Study patients selection flow diagram. *A case may be excluded due to more
than one criterion. Therefore, the total excluded cases in each step may outnumber the sum of case numbers excluded by individual criterion.
Figure 2. Cumulative incidences of HCC recurrence and overall mortality following liver resection after adjustment for competing mortality. In part A, calculation and
comparison of cumulative incidences in competing risk data ratios were conducted using modified Kaplan-Meier method and Gray's method. In part B, Kaplan-Meier method was used. Recurrences (part A) and deaths (part B) during the first three months were excluded. Abbreviations: Untreated: HBV patients not receiving nucleoside analogues; Treated: HBV patients receiving nucleoside analogues.
Figure 3. Multivariable stratified analyses for the association between nucleoside analogue use and HCC recurrence. Among HBV-related HCC patients, nucleoside
analogue use (treated cohort) was found to be associated with reduced risk of HCC recurrence on nearly all analyses stratified according to age, gender, extent of liver resection, liver cirrhosis, diabetes, and use of statins, NSAIDs, and metformin. Some hazard ratios were not statistically significant due to a small number of cases. Recurrences during the first three months were excluded.
Abbreviations: Untreated: HBV patients not receiving nucleoside analogues; Treated: HBV patients receiving nucleoside analogues; N, number; Rec, recurrence; Major resection, extensive and partial hepatic lobectomy with at least 3 segmental resections of liver
parenchyma; Minor resection, extensive and partial hepatectomy with 2 or fewer segmental resections of liver.
eFigure 1. Cumulative incidences of HCC recurrence following liver resection in untreated cohort and treated cohort after adjustment for competing mortality. Part A: non-cirrhotic patients; part B; cirrhotic patients; part C: NSAIDs or aspirin 483 484 485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500 501 502 503 504 505 506 507
users; part D: non-NSAIDs or aspirin users. Abbreviations: Untreated: HBV patients not receiving nucleoside analogues; Treated: HBV patients receiving nucleoside analogues.
508 509
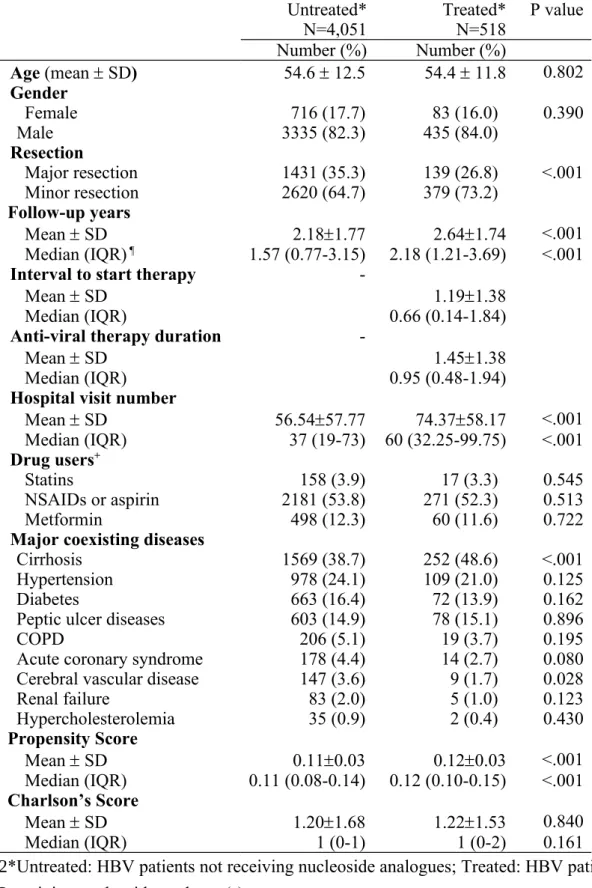
Table 1. Baseline and demographic characteristics of study cohorts following liver resection
Untreated* Treated* P value
N=4,051 N=518 Number (%) Number (%) Age (mean SD) 54.6 12.5 54.4 11.8 0.802 Gender Female 716 (17.7) 83 (16.0) 0.390 Male 3335 (82.3) 435 (84.0) Resection Major resection 1431 (35.3) 139 (26.8) <.001 Minor resection 2620 (64.7) 379 (73.2) Follow-up years Mean SD 2.181.77 2.641.74 <.001 Median (IQR) ¶ 1.57 (0.77-3.15) 2.18 (1.21-3.69) <.001
Interval to start therapy
-Mean SD 1.191.38
Median (IQR) 0.66 (0.14-1.84)
Anti-viral therapy duration
-Mean SD 1.451.38
Median (IQR) 0.95 (0.48-1.94)
Hospital visit number
Mean SD 56.5457.77 74.3758.17 <.001 Median (IQR) 37 (19-73) 60 (32.25-99.75) <.001 Drug users+ Statins 158 (3.9) 17 (3.3) 0.545 NSAIDs or aspirin 2181 (53.8) 271 (52.3) 0.513 Metformin 498 (12.3) 60 (11.6) 0.722
Major coexisting diseases
Cirrhosis 1569 (38.7) 252 (48.6) <.001
Hypertension 978 (24.1) 109 (21.0) 0.125
Diabetes 663 (16.4) 72 (13.9) 0.162
Peptic ulcer diseases 603 (14.9) 78 (15.1) 0.896
COPD 206 (5.1) 19 (3.7) 0.195
Acute coronary syndrome 178 (4.4) 14 (2.7) 0.080
Cerebral vascular disease 147 (3.6) 9 (1.7) 0.028
Renal failure 83 (2.0) 5 (1.0) 0.123 Hypercholesterolemia 35 (0.9) 2 (0.4) 0.430 Propensity Score Mean SD 0.110.03 0.120.03 <.001 Median (IQR) 0.11 (0.08-0.14) 0.12 (0.10-0.15) <.001 Charlson’s Score Mean SD 1.201.68 1.221.53 0.840 Median (IQR) 1 (0-1) 1 (0-2) 0.161
*Untreated: HBV patients not receiving nucleoside analogues; Treated: HBV patients receiving nucleoside analogue(s).
¶ IQR: interquartile range
+ Drug users indicate patients using drugs at least one day per month on average. 510 511 512 513 514 515
Abbreviations: N, number; COPD, chronic obstructive pulmonary disease; NSAIDs, non-steroidal anti-inflammatory drugs
Charlson’s Score represents degree of health. A higher score indicates a worse health condition.
516 517 518 519
Table 2. Multivariable Cox proportional hazards model analysis for risk of HCC recurrence
after adjusting for competing mortality
Number No. HCC Recurrence Hazards Ratio¶ 95% CI P value Treated vs. Untreated Untreated 4051 1765 1 Treated 518 106 0.67 0.55-0.81 <0.001 Age < 50 1568 655 1 50-59 1466 590 0.96 0.83-1.10 0.530 >=60 1535 626 1.01 0.90-1.13 0.920 Gender Female 799 306 1 Male 3770 1565 1.08 0.95-1.23 0.220 Resection Minor 2999 1228 1 Major 1570 643 1.04 0.90-1.20 0.610 Liver cirrhosis No 2748 1046 1 Yes 1821 825 1.21 1.04-1.40 0.011 Diabetes No 3834 1554 1 Yes 735 317 1.18 0.99-1.41 0.070 Statins use+ No 4394 1814 1 Yes 175 57 0.68 0.53-0.87 0.002
NSAIDs or Aspirin use+
No 2117 932 1 Yes 2452 939 0.80 0.73-0.88 <.001 Metformin use+ No 4011 1634 1 Yes 558 237 1.01 0.84-1.21 0.920 Propensity score Each incremental 10% 4569 1871 1.05 0.78-1.41 0.740
*Untreated: HBV patients not receiving nucleoside analogues; Treated: HBV patients receiving nucleoside analogues.
+ Drug users indicate patients using a drug at least one day per month on average. ¶Adjusted for covariate factors, including age, gender, resection extent, liver cirrhosis,
520 521 522 523 524 525
diabetes, and use of statins, NSAIDs or aspirin and metformin. 526