Org. Chem. irradiation, the samples were placed in the dark because biacetyl absorbs visible light and may undergo photochemical reaction. The concentrations of the styrylamines were 3.5 mmol/dm3 (la) and 1.5 mmol/dm3 (lb) and those of biacetyl were 5-20 mmol/dm? The consumption of biacetyl during irradiation was negligible.
Stem-Volmer Plot for the Fluorescence of la or lb. The samples were purged with argon. The extinction coefficients of the styrylamines and biacetyl at the excitation wavelength (254 nm) are as follows: la, em = 5600; lb, em = 12 600; biacetyl, em
= 14.
Quantum Yield Determination. Hexanone actinometry was
used. Irradiation was performed with the Rayonet photochemical
reactor. The samples in quartz tubes were purged with argon. After irradiation the degree of reaction was determined by the NMR spectroscopy.
Acknowledgment. This work was supported by a Grant-in-Aid for Scientific Research (No. 02640379) from the Ministry of Education, Science and Culture of Japan. Supplementary Material Available: lH NMR spectra of l g and 2c (2 pages). This material is contained in many libraries on microfiche, immediately follows thie article in the m i c r o f i i version of the journal, and can be ordered from the ACS see any current masthead page for ordering information.
Correlation of Solvolytic Reactivities of l,l,l-Trifluoro-2-phenyl-2-propyl,
1-tert -Butyl-1-phenylmethyl, and Some Related Tosylatest
Kwang-Ting Liu,* Jye-Shane Yang, Shu-Mei Chang, Yan-Shyi Lin, Hun-Chang Sheu, and Meng-Lin Tsao
Department of Chemistry, National Taiwan University, Taipei, Taiwan 107, Republic of China Received August 29,1991
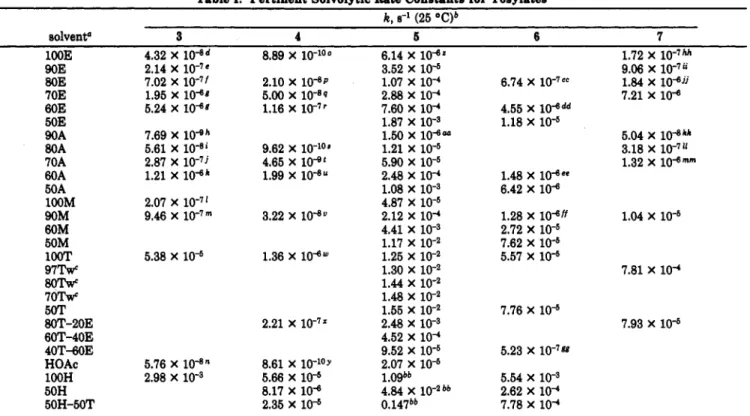
Solvolysis of l,l,l-trifluoro-2-phenyl-2-propyl tosylate (3), 2,2,2-trifluoro-l-(4'-methylphenyl)ethyl toeylate (4), 1-tert-butyl-1-phenylmethyl tosylate (S), l-tert-butyl-l-(3'-chlorophenyl)methyl toeylate (6), and 1-(3',5'- dichlorophenyl)-2,2,2-trifluoro-l-phenylethyl tosylate (7) in a variety of solventa were found to give log k
-
Ymplota with significant deviations from linearity ( R = 0.95-0.98). The data points for those measured in aqueous acetone always showed depression from the line, whereas those measured in nonnucleophilic solvents were on the line. On the other hand, 3,4,6, and 7 all showed excellent linear relationships (R
>
0.99) for log k againstlog k(5). Additional evidence for the limiting SN1 mechanism in the solvolysis of 5 was given. Similar to the previous work (refs 11-13) a new Y mscale based on the solvolysis rates of 5 for correlating the solvolytic reactivity of benzylic toeylates involving charge delocalizations to the aryl moiety in the cationic transition states was then proposed. The advantages of using this new Y scale of solvent ionizing powers were discussed. The importance of solvent nucleophilicities in the solvolysis of primary benzylic toeylate 8 and unhindered deactivating secondary benzylic tosylates 9 and 10 was confirmed.
In 1948 the Grunwald-Winatein equation (1) was stated
to define the first scale of empirical solvent ionizing power
(Y)
based on the solvolytic rate constants of tert-butyl chloride.' Multiparameter equations, such as (2): werelog (k/k,) = mY (1)
then introduced to accommodate the effect of the solvent nucleophilicity. Later studies indicated the use of 1- or 2-adamantyl toeylate as a better reference for defining the solvent ionizing power?*' Moreover, the necessity of using different
Yx
scales to correlate the solvolytic reactivity of substrates containing different leaving groups(X)
was firstshown by Bentley and co-workers? A variety of Y x scales based on 1- or 2-substituted adamantanes have thus been developed6 and have been generally considered as an im- portant set of solvent parameters for the understanding of the correlation of solvolytic reactivities.' The obser- vation of dispersed log k
-
Y x plota due to the rate con- stanta measured in nonnucleophilic solventa, such as those that have been noted in the cases of tett-butyl halides5ss and 2-(trifluoromethyl)-2-propyl triflate? has been con- sidered aa an useful probe for detecting nucleophilic sol- vent assistance.10+Dedicated to Profaor Herbert C. Brown on t h e occasion of his
80th birthday.
0022-32631921 1957-3041$03.00/0
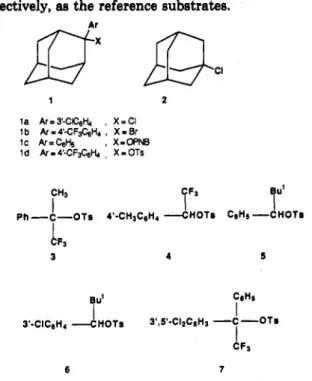
On the other hand, we recently demonstrated the ad- vantage of using a new YBncl scale based on 2-aryl-2- chloroadamantane la'' over the Ycl based on l-chloro-
adamantane (2)5 to describe the solvent ionizing power of a large number of solvents for correlating the solvolytic reactivity of tertiary benzylic chlorides by eq 1. A similar
approach
has
also been found to be successful for benzylic bromides12 and p-nitrobenzoate~'~ by employing the cor- responding 2-aryl-2-adamantyl derivatives l b andIC,
re-(1) Grunwald, E.; Winstein,
s.
J. Am. Chem. SOC. 1948, 70,846. (2) Winstein, S.; Grunwald, E.; Jones, H. W. Ibid. 1961, 73, 2700.(3) Fry, J. L.; Lancelot, C. J.; Lam, L. K. M.; Harris, J. M.; Raber, D.
(4) Kevill, D. N.; Kolwyck, K. C.; Weitl, F. L. Ibid. 1970,7300.
(5) Bentley, T. W.; Cartar, G. E. J. Am. Chem. SOC. 1982,104,6741.
(6) Bentley, T. W.; Llewellyn, G. Pmgr. Phys. Org. Chem 1990,17,121.
(7) For examples of some popular advanced textbooks: (a) Lowry, T.
H.; Richardson, K. S. Mechanism and Theory in Organic Chemistry, 3rd
ed.; Harper & Row: New York, 1987; pp 335-340. (b) Ritchie, C. D.
Physical Organic Chemistry; Maecel Dekker: New York and Baael, 1990;
pp 66-69.
(8) Bentleu, T. W.; Brown, C. T.: Parker, W.: Watt, C. I. F. J. Am. J.; Hall, R. E.; Schleyer, P. v. R. Ibid. 1970, 2538.
Chem. SOC. 1979, 101, 2486.
(9) Jansen, M. P.; Koehy, K. M.; Mangru, N. N.; Tidwell, T. T. Ibid.
(10) Bentlev. T. W.: Roberta. K. J. Chem. SOC.. Perkin Trans. 2 1989. 1981,103, 3863.
- .
1055.
(11) (a) Liu, K.-T.; Sheu, H.-C.; Chiu, P.-F.; Hu, C.-R. Tetrahedron
Lett. 1990,25,3611. (b) Liu, K.-T.; Sheu, H.-C. J. Org. Chem. 1991,56,
3021.
(12) Liu, K.-T.; Sheu, H.-C. J. Chin. Chem. SOC. (Taipei) 1991,38,29.
(13) Liu, K.-T.; Chen, H.-I.; Chin., C.-P. J . Phys. Org. Chem. 1991,4,
463.
0 1992 American Chemical Society
Downloaded by NATIONAL TAIWAN UNIV on August 31, 2009 | http://pubs.acs.org
- 6 - - 4 -8 70E u oo
'
r
5
IOOE 8 'a 0 0 BOE BOA -- 0 70A EO* ,,?HOAcspectively, as the reference substrates. b.
1 2
la Ar=3'-Cc& , X=CI
l b Ar=4'-CF&H4 , X=Br
1c Ar=c& , x-OPNB
l d k = 4 ' - C F & & , X-OTS
3 4 5 Con5
'i"
I
3'-CICEH, A H O T s 3',5'-ClzC6H3 -C-OTsI
CF3 6 7Tosylates are among the most commonly employed substrates in solvolytic studies. The solvolysis of l,l,l-
trifluoro-2-phenyl-2-propyl tosylate (3) was considered to be a limiting SN1 process from the linear log
k
-
Y m
relationship realized in alcoholic and acidic solvents and from other kinetic evidence."Js The same conclusion was also reached in the case of 2,2,2-trifluoro-l-(4/-methyl-pheny1)ethyl tosylate (4).16 Extended study on the sol- volytic behavior of tosylates 3 and 4 in additional solventrs is thus desirable to examine the applicability of Ym8 or
the necessity of developing a new YBS scale in correlating
log ks for benzylic tosylates.
Preliminary study on the solvolysis of 3 in aqueous acetone revealed deviations from the original linear log k
-
Ym8 plot.17 The tertiary 2-aryl-2-adamantyl tosylateId would be expected to be unstable and too reactive to
define a "YBnmn scale for benzylic tosylates from acculzLte kinetic measurements. Other tosylates will thus
used.
The solvolysis of 1-aryl-1-tert-butylmethyl tosylates has beenshown to proceed with
k,
mechanism without nucleophilic solvent assistance or methyl participation.18Jg We now wish to report the results of our independent studies on the Solvolysis of 1-tert-butyl-1-phenylmethyl tosylate (5) and some other representative tosylates. The Yms scale was found to be not an appropriate one for the correlation of reactivities. A new YBnm scale17b is proposed, and ita advantages over Yms will also be discussed.Results
l,l,l-Triiuoro-2-phenyl-2-propyl tosylate (3), 2,2,2-tri- fluoro- 1
-
(4/-methylphenyl) ethyl tosylate (41, 1-
tert-
bu-(14) Allen, A. D.; Jamen, M. P.; Koehy, K. M.; Mangru, N. N.; Tidwell,
T. T. J. Am. Chem. SOC. 1982,104,207.
(15) Liu, K.-T.; Kuo, M.-Y.; Shu, C.-F. Zbid. 1982, 104, 211. (16) Allen, D. A.; Ambidge, I. C.; Che, C.; Michael, H.; Muir, R. J.; Tidwell, T. T. Ibid. 1981,106,2343.
(17) (a) Lm, Y.4. M.S. Thesis, National Taiwan University, June
1988. (b) A preliminary account of the work on the development of
Y B . ~ was presented a t the 198th National Meeting of the American Chemical Society, Miami Beach, FL, Sept 10-15, 1989; Abstracts of Papers, ORGN 281.
(18) Winstein, S.; Morse, B. K. J. Am. Chem. SOC. 1952, 74, 1133. (19) (a) Tsuji, Y.; Fujio, M.; Truno, Y. Bull. Chem. Soc. Jpn. 1990,63, 856. (b) Tsuji, Y.; Yataugi, K.; Fujio, M.; Tsuno, Y. Mem. Fac. Sci.,
Kyushu Unau., Ser. C 1990,17,281. (c) Tsuji, Y.; Yataugi, K.; Fujio, M.;
Tsuno, Y. Zbid. 1991, 18, 121. (d) Fujio, M.; Truji, Y.; Oteu, T.; Tsuno, Y. Tetrahedron Lett. 1991,32, 1805. lOOH - 2 0 LOOT 0 % : E 'OH 3
-4t
0 -104 - 3 - 2 - 1 0 1 2 3 4 5 XTSFigure 1. Correlation of logarithms of rate constants for 3 ( 0 )
and 4 ( 0 ) against Y".
- l
t
LOOTI
QOA
-9
- 3 - 2 - 1 0 1 2 3 4 5
XTs
Figure 2. Correlation of logarithms of rate constants for 5 (O), 6 (01, and 7 (13) against Yms.
tyl- 1-phenylmethyl tosylate (5), 1-tert-butyl-1-( 3'-chloro- pheny1)methyl tosylate (6), and 1-(3',5/-dichlorophenyl)-
2,2,2-trifluoro-l-phenylethyl tosylate (7) were prepared from the corresponding alcohols using known procedures.'s Spectral data and elemental analyses all agreed with the assigned structures. Solvolytic rate constants for these tosylates in various solvents were measured by the con- ductimetric method, with the exception of the rates of acetolysis which were monitored titrimetrically. The re- sults are shown in Table I. The solvolysis products were all unarranged.20
In most cases, the rate constants for substrates 3-5 in most solvents are in good agreement, generally within lo%, with the literature data.1C16Jg However, larger deviation was observed in some occasions for which the ks at 25 O C were calculated from the data measured at quite high temperature, such as 90 O C and higher.
For
example, the extrapolation of the rates of ethanolysis for 4 at W 1 3 0"C
gave a k, 8.89 X lO-'O/s, and deviated sie;nificantJy fromthe reported data, 1.93 X 10-'o/s.16 For k(4) in 60% eth- anol, which wae calculated from the data at 55 and 75 O C , the literature value (1.20 X ~O-"/S)'~ was very close to 1.16 X 10-7/s as was found in the present work.
The plots of the logarithm of rate constants, k(3) and k(4), respectively, with Ymul showed that additional rate constants measured in aqueous acetone and in aqueous
(20) The detkils on the product study will be reported elsewhere.
(21) Although some "corrected" Y m values are available (Allard, B.; Caeadevall, E. Nouu. J. Chim. 1983, 7, 569), they are limited to etha- nol-water and fluorinated alcohol-ater mixtures only. In addition, they were derived from the rate data of 2-endo-norbomyl methanesulfonatm and might not be appropriate for toeylatea. In thie study, the revieed Y m
values reported in the recent review (ref 6) were employed.
Downloaded by NATIONAL TAIWAN UNIV on August 31, 2009 | http://pubs.acs.org
Correlation of Solvolytic Reactivities J. Chem., 11, 1992 0 - 1 -2 - 3 - 4
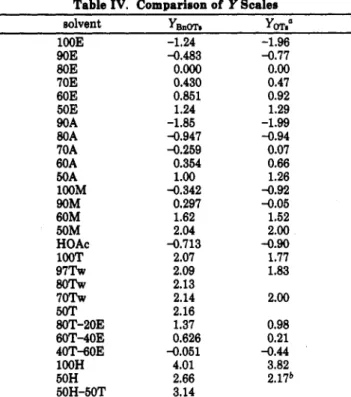
Table I. Pertinent Solvolytic Rate Constants for Tosylates k, s-l (25 "Ob
solventa 3 4 5 6 7
lOOE 4.32 X lodd 8.89 X 6.14 X lodz 1.72 x io-'&
90E 2.14 x 10-7~ 3.52 X 10" 9.06 x 1 0 - 7 ~
80E 7.02 x 10-7' 2.10 x 1o-Bp 1.07 X lo-' 6.74 x 10-7ce
60E 5.24 X lo4# 1.16 X 7.60 X lo-' 4.55 x lO-Sd
50E 1.87 X 1.18 x 10-5
90A 7.69 X 1.50 x 10-3" 5.04 X 1@**
80A 5.61 X lo4' 9.62 X 1.21 x 10-5 3.18 x 10-7"
70A 2.87 X lO-'j 4.65 X 5.90 x 10-5 1.32 X l@"
60A 1.21 x 104" 1.99 x 1o-Bu 2.48 X lo-' 1.48 X l@Ie
50A 1.08 x 10-3 6.42 X lo4
l00M 2.07 x 10-7' 4.87 X
1.84 x lO+j
70E 1.95 X lo*# 5.00 x 10-89 2.88 x 10-4 7.21 X lo4
90M 9.46 x lo-'" 3.22 X lo-"" 2.12 x 10-4 1.28 X l0"ff 1.04 x 10-5 60M 4.41 x 10-3 2.72 x 10-5 50M 1.17 X 7.62 x 10-5 100T 5.38 X loa 1.36 X 1.25 X 5.57 x lo" 9 7 w 1.30 X 7.81 X lo-' 80w 1.44 x 10-2 7 0 W 1.48 x 10-2 5oT 1.55 X 7.76 x 10-5 80T-20E 2.21 x 10-73 2.48 x 10-3 7.93 x 10-5 40T-60E 9.52 x 10-5 5.23 x io-'@
HOAc 5.76 X lo4" 8.61 X lO-'OY 2.07 x 10-5
lOOH 2.98 x 10-3 5.66 X 1.09bb 5.54 x 10-3
60T-40E 4.52 X lo-'
50H 8.17 X lo4 4.84 x 10-2bb 2.62 X lo4
50H-50T 2.35 x 10-5 0.147bb 7.78 X lo-'
OFor abbreviation of solvents: A, acetone; E, ethanol; M, methanol; H, hexafluoro-2-propanol; T, 2,2,2-trifluoroethanol. The numbers denote the volume percent of the specific solvent in the solvent mixture, unless otherwise mentioned. The amount of water is not recorded. bObtained in this work unleas otherwise mentioned. 'Weight percent. dReference 6. -fCalculated from data at other temperature, k, s-l
( O C ) : (e) 9.73 x 10-6 (75.0), 1.88 X 10" (60.0); (f) 3.47 X lo-' (75.0), 5.82 X (60.0); 2.05 X 10" (50.0). #Reference 15. h-"Calculated from data at other temperature, k, s-l ("C): (h) 7.79 X lo-' (125.0), 7.79 X (100.0); (i) 1.54 X lo-' (90.0), 4.24 X 10" (75.0), 9.86 X lo4 (65.0);
(j) 1.58 X lo-' (75.0), 2.90 X lW6; (k) 3.64 X lo-' (70.0), 1.01 X lo4 (60.0), 3.58 X 10" (50.0); (1) 1.29 X lo-' (75.0), 2.29 X 10" (60.0); (m) 4.19
X lo4 (75.0), 8.16 X (130), 2.28 X 10" (113.6), 1.05 X
104 (105.0), 2.40 x la" (90.0). PReference 16. 9-MCalculated from data at other temperature, k, 8-l ("C): (q) 1.08 X lo-' (105.0), 2.20 X 1O-a (90.0), 4.30 X lo4 (75.0); (r) 5.93 X 10" (75.0), 6.15 X l@ (55.0); (8) 6.44 X 10" (115.0), 2.52 X 10" (105.0), 5.12 X lo4 (90.0); (t) 1.08 X lo-' (105.0), 2.20 x 10" (90.0), 4.30 X lo4 (75.0); (u) 5.37 X lo-' (llO.O), 1.91 X lo-' (lOO.O), 4.40 X lob (85.0); (v) 1.49 X lo-' (lOO.O), 3.41 X lo4 (85.0), 1.37 X 10-6 (75.0); (w) 1.64 X lo-' (75.0), 4.52 X lW5 (60.0); (x) 1.09 X lo4 (85.0), 4.48 X loa (75.0), 1.75 X lp (65.0); (y) 1.99 X lo-' (130.0); 1.94 x 10-5 (105.0); ( 2 ) 9.43 X lo4 (65.0), 4.71 X lod (40.0); (aa) 6.00 X lo-' (75.0), 3.78 X lo4 (50.0). bbExtrapolated from linear plota with log k (6). 'emcalculated from data at other temperature, k, 8-l ("C): (cc) 3.35 X lo-' (75.0), 1.91 X 10" (50.0); (dd) 6.67 X lo-' (65.0), 6.37 X 10" (40.0); (ee) 5.58 X lo4 (75.0), 3.61 X 10" (50.0); (ff) 5.78 X lo-' (75.0), 1.93 X lo4 (65.0), 6.27 X 10" (55.0); (gg) 1.94 X lo-' (75.0), 2.26 X (75.0), 4.77 X 10" (50.0); (ii) 3.92 X lo-' (75.0), 7.49 X 10" (60.0), 2.40 X 10" (50.0); Cij) 2.48 X lo-' (60.0), 6.80 x 10-5 (50.0); (kk) 2.48 X lo-' (100.0), 6.80 X 10" (75.0); (11) 1.72 X lo-' (75.0), 3.18 X 10" (60.0); (mm) 6.46 X lo-' (60.0), 5.92 X
(60.0); (n) 5.18 X lo4 (lOO.O), 1.63 X lo-' (90.0), 3.83 X (75.0); ( 0 ) 8.78 X
(50.0); (hh) 8.20 X -- -- -- -- -- (40.0).
Table 11. Correlation Analyses of Rate Data against YoT. 1 ,
substrate na m R SD 3 14 0.911 0.979 0.054 4 13 0.976 0.964 0.081 5 21 0.964 0.976 0.049 6 12 1.OOO 0.981 0.062 7 10 0.992 0.953 0.112 .,Number of solvents.
Table 111. Correlation Analyres of Rate Data against Ym. and N(OTs) substrate no m 1 R 3 10 0.863 -0.247 0.983 4 8 0.672 -0.217 0.990 5 12 0.837 -0.130 0.989 6 6 0.963 -0.081 0.996 7 5 0.616 -0.482 0.997
a Number of solventa whose N(0Ts) values are available.
methanol deviated from the original b e a r relationships1c16 (Figure 1). Similar deviations were also observed in the solvolysis of secondary tosylates 5 and 6 and the tertiary h y l a t e 7 as well (Figure 2). The results of correlation analyses using eqs 1 and 2 are summarized in Tables I1
and 111, respectively.
- 5 4
- 7 -6 - 5 - 4 -3 -2 - 1
Log k (6)
Figure 3. Correlation of logarithms of rate constants for 5 ( 0 )
against that for 6.
However, an excellent linear relationship between log k(5) and log k(6), m = 1.019 and
R
= 0.998, was observed. Therefore, some very fast rate constants for 5 could be calculated by extrapolation (Figure 3, open circle). Moreover, excellent linear correlations were also found if log ks for 5 were plotted against log ks for 3, 4, and 7,respectively. A new
Ym
scale of solvent ionizing powersbased on the logarithms of the ks for 5 can thus be de-
Downloaded by NATIONAL TAIWAN UNIV on August 31, 2009 | http://pubs.acs.org
in acetic acid was realized, no significant deviation was observed for those in hexafluoro-2-propanol or trifluoro- ethanol (Figures 1 and 2). The nonlinear relationships could be improved from
R
= 0.95-0.98 by using eq 1 (Table11) to
R
= 0.98-0.99 (Table 111) if the multiparameter eq2 was employed. However, this improvement cannot be interpretated by an intervention of solvent nucleophilicity because in the cases of 3 and 7 the overcrowded tertiary reaction center in the solvolytic transition state excludes the possibility of solvent participation. Moreover, the limiting s N 1 mechanism has been demonstrated for the solvolysis of secondary substrates 4,16*u*26 5, and 6.19
Therefore, it is likely that the Yml scale might not be a proper one for benzylic tosylates.
The deviation from linear Grunwald-Winstein plota against
Yx
(eq 1) observed in the present study is very similar to thathaa
been shown in our previous work.11-13 For instance, individual linear relationships could be found for the data in aqueous acetone, aqueous ethanol, aqueous methanol, and ethanol-trifluoroethanol, respectively. Thedata points for those measured in aqueous acetone always showed depression from the line defined by those mea- sured in aqueous ethanol or methanol. Thus, the scattered plots did not result from random errors. Moreover, the order of reactivities such as k(100M)
>
k(90E)>
k(80A) could not be accomodated by Swain's parameterB,%
which denotes the solvation ability of localized cations. Another possible explanation by the intervention of ion pair return would be unlikely, since no dependence of deviation on reactivities (Table I)n or on the amount of returnlgC could be observed. Consequently, it is likely that the Ym scale is not appropriate to apply to systems with delocalized cationic transition states as that for bromides, chlorides, and p-nitrobenzoates, which have been found in the pre- vious ~ t u d i e s . ~ ~ - ~ ~Since the tertiary h y l a t e Id would be very unstable and very reactive, other reference substrates should be choeen. Evidence for the absence of nucleophilic solvent assistance or methyl participation in the solvolysis of l-aryl-l-tert- butyhethyl tosylates
haa
already been shown.19 The lack of solvent intervention in the solvolysis of the sterically hindered tosylate 51e can be further proved by additional evidence. The relative rate ratios of 141-256 for tosy- late/bromide in aqueous ethanol% are in line with the value of 231 observed for the 2-adamantyl system.29In
addition, the a-deuterium kinetic isotope effect, 1.19-1.21 in aqueous ethano1,malso
agreea with that predicted for s N 1 prye~a.3~ A new Y scale based on the rates of solvolysis of 5 u thus a reasonable choice. Accordingly, a YBnm scale could be developed on the basis of eq 1 for correlating the solvolytic reactivities of benzylic h y l a t m by using 5 as the reference standard and 6 for extrapolation.The comparison of YBam with Y m 6 (Table
IV)
reveals that the latter is always more negative except in ace- tone-water and in ethanol-water. In those solvents the YoTe may be more negative than the new YBnms at lower M 9 ci -2 - 4 - 6 -8 - 1 0 4 J I - 3 - 2 - 1 0 1 2 3 4 5 YBnOTsFigure 4. Correlation of logarithms of rate constants for 3 ( O ) ,
4 (01, and7 (a) against YBnm,.
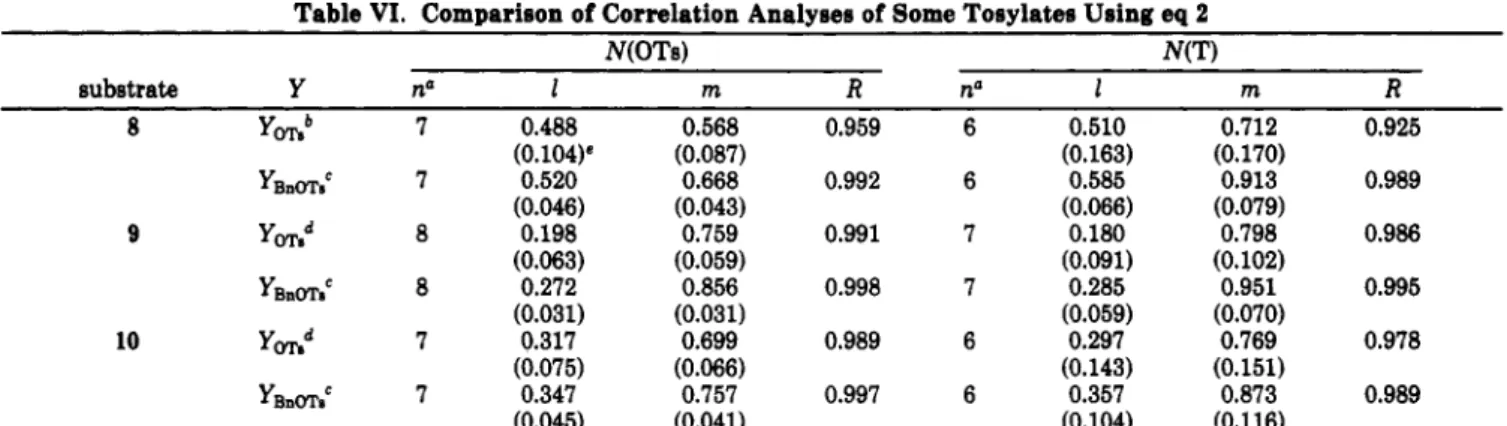
Table IV. Comparison of Y Scales
solvent YFI., y0T.a
l00E -1.24 -1.96 90E -0.483 -0.77 8OE O.OO0 0.00 70E 0.430 0.47 60E 0.851 0.92 50E 1.24 1.29 90A -1.85 -1.99 80A -0.947 -0.94 70A -0.259 0.07 60A 0.354 0.66 5OA 1.00 1.26 l00M -0.342 -0.92 90M 0.297 -0.05 60M 1.62 1.52 50M 2.04 2.00 HOAc -0.713 -0.90 100T 2.07 1.77 97% 2.09 1.83 80% 2.13 7oTw 2.14 2.00 5oT 2.16 8OT-20E 1.37 0.98 60T-40E 0.626 0.21 40T*OE -0.051 -0.44 l00H 4.01 3.82 50H 2.66 2.17b 50H-SOT 3.14
"Reference 2b. bAllard, B.; Casadevall, E. Nouv. J. Chem. 1983,
7, 569.
Table V. Correlation Analyses of Rate Data against Yeam.
substrata no m R SD 3 14 0.964 0.996 0.024 4 14 0.999 0.992 0.038 6 14 0.979 0.999 0.010 7 10 1.06 0.994 0.043 a Number of solventa.
veloped. The values are shown in Table
ZV,
and the reaults of correlation analyses by using these newYs
are listed in TableV.
Discussion
It
hae
been shown that solvent nucleophilicity plays an important role in the solvolysis of primary benzyl tosy- lateen and secondary deactivated 1-arylethyl t o s ~ l a t e s . ~ ~For
tosylatea 3-7, although a small deceleration of the rates(22) Kevill, D. N.; Ren, H. J. Org. Chem. 1989,54,5654.
(23) Wen, A. D.; Knuagaaabathy, V. M.; Tidwell, T. T. J. Am. Chem.
Soc. 1986,107,4613.
(24) Richard, J. P. J . Am. Chem. SOC. 1989,111, 1455.
(25) Murata, A.; Sakagachi, S.; Fujiyama, R.; Muhima, M.; Fujio, M.; Tsuno. Y . Bull. Cheni. SOC. J m . 1990. 63.1138.
(26j Swain, C. G.; Swain, M: S.; Powell, A. L.; Alumni, S . J. Am. Chem.
Soc. 1985.106.502.
(27) S i m i k r&ta were also found in the solvolyeis of substituted naphthylmethyl toaylates. See: Liu, K.-T.; Hsu, H.-Y.; Yang, J . 4 .
Tetrahedron Lett., in press.
(28) Yang, J . 8 . M.S. Thesis, National Taiwan University, June 1990. (29) Bingham, R. C.; Schleyer, P. v. R. J. Am. Chem. SOC. 1971,93,
3189.
(30) Sheu, H.-C. Ph.D. Thesis, National Taiwan Univemity, July 1991. (31) Melander, L.; Saunders, W. H., Jr. Reaction Rates of Isotopic Molecules; Wiley-Interscience: New York, 1980; Chapter 6.
Downloaded by NATIONAL TAIWAN UNIV on August 31, 2009 | http://pubs.acs.org
Table VI. Comparison of Correlation Analyses of Some Tosylates Using eq 2 N(0Ts) substrate Y nn 1 m R no 1 m R 8 Yonb 7 0.488 (0.104)e YBBOTle 7 0.520 (0.046) 9 ym: 8 0.198 (0.063) YBnOTt 8 0.272 (0.031) 10 Yond 7 0.317 (0.075) YBBOTt 7 0.347 (0.045) 0.568 0.959 6 (0.087) 0.668 0.992 6 (0.043) 0.759 0.991 7 (0.059) 0.856 0.998 7 (0.031) 0.699 0.989 6 (0.066) 0.757 0.997 6 (0.041) 0.510 (0.163) 0.585 (0.066) 0.180 (0.091) 0.285 (0.059) 0.297 (0.143) 0.357 (0.104) 0.712 0.925 (0.170) 0.913 0.989 (0.079) 0.798 0.986 (0.102) 0.951 0.995 (0.070) 0.769 0.978 (0.151) 0.873 0.989 (0.116)
Number of solvents whose N values are available. *Recalculation of the literature data (ref 34) using YOT8 (ref 6). Recalculation of the
literature data (ref 34) using Y B , , ~ ~ . dRecalculation of the literature data (ref 23) using YOT. (ref 6). e Standard deviation.
water content (lOOE, 9OE, 9OA, and 8OA), or more positive at higher water content (7OE-50E and 70A-50A). It seems
plausible that the delocalized benzylic cationic transition state will be relatively less favored than the localized 1- adamantyl cation in solvents of higher solvation energy,
as has been realized in previous ~ ~ r k . ~ ~ - ~ ~ i ~ ~ Furthermore, both the electrophilicity and the nucleophilicity of the
solvents33 are likely to be important as in the cases of bromidesI2 and chlorides.llb
The advantages of employing this new Y scale can be illustrated by the excellent correlation for 3, 4, 6, and 7 ion Table V ( R
>
0.99) as compared with the less satis- factory results in Table I1 (R = 0.95-0.98). The scattered log k-
Yml plots in the cases of 3 ( R = 0.979) and 7(R
= 0.953) (Figure 1) are likely the outcome of using of an improper Y d e . Since both tosylatea are highly hindered and are not susceptible to nucleophilic solvent participa- tion, the observed good linear correlation for these sub- strates (Table 111) with the multiparameter eq 2 (vide supra) could therefore be considered as artifacts. In the solvolytic study of benzyl tosylate (81, Kevill and co- workersM suggested the use of
Ym
and N(Et3O+)= in eq2. Other N scales, N(T) and N'(OTs), were also developed recently.% Recalculation using the new YBnms values, as compared Ym, indicated the combination of YBnmS and N(OTS)~' (R = 0.992) or N'(0Ts)
(R
= 0.997) would be better than the use of other values(R
= 0.925-0.989) in the correlation analyses. In other words, N(0Ts) or N'- (OTs) seems to be the choice for neutral substrates. In the cases of 1-(3'-bromophenyl)ethyl (9) and 1-[3'-(tri-fluoromethyl)phenyl]ethyl tosylates (lo), Tidwell and co- workems have found linear relationships by using Ym and N(0Ts) in eq 2 for correlation analyses. Owing to the fact that only 4 6 N'(0Ts) or N(EhO+) values are available for s u h a t e a 9 and 10, the results of regression analyses using these values are not given in Table VI for comparison. Better linear relationships for 9 ( R = 0.998) and 10
(R
= 0.997) could be obtained if YBnms and NOTs) were used for calculati~n.~ Thestandard
deviations for 1 and m were(32) Bentley, T. W.; Koo, 1. S.; Norman, S. J. J . Org. Chem. 1991,56,
1604.
(33) Abraham, M. H.; Doherty, R. M.; Kalmet, M. J.; Harris, J. M.;
T&, R. W. J. Chem. Sac., Perkrn Tram. 2 1987,913.
(34) Kevill, D. N.; Rissmann, T. J. J . Chem. Res., Synop. 1986, 252. (35) Kevill, D. N.; Anderson, S. W.; Fujimoto, E. K. In Nucleophilic-
ity; Harris, J. M., McManus, S. P., Ed.; Advances in Chemistry 215; American Chemical Society: Washington DC, 1987; pp 269-283.
(36) Kevill, D. N.; Anderson, S. W. J. Org. Chem. 1991, 56, 1845. (37) Schadt, F. L.; Bentley, T. W.; Schleyer, P. v. R. J. Am. Chem. Soc. 1976,98,7667.
also
smaller, 80 the superiority of using Y B m was obvious.The importance of solvent nucleophilicity in the solvolysis of 9 and loz3 could thus be confirmed.
Consequently, the Ym, scale will not be as good as the new YBnOTs scale in correlating reactivities and in exam- ining the nucleophilic solvent effect in the solvolysis of benzylic tosylates, in which delocalized cationic transition states were formed. The importance of the solvation on the cationic moiety in the transition state can also be demonstrated in the solvolysis of substituted naphthyl- methyl tosylate~.~' More work on the solvolysis of other tosylates is in progress.
Experimental Section
General Remarks. Capillary melting points were uncorrected. NMR spectra were determined on a Varian EM390 instrument using tetramethylsilane as internal standard. IR spectra were measured on a Perkin-Elmer Model 983G spectrometer. Mass spectral analyses were obtained with a Finigan TSQ-16C in- strument. Elemental analyses were done in the Microanalytical Laboratory of this department.
Materials. Solvents for the kinetic studies were spectral grade or reagent grade and purified according to standard ~ r o c e d u r e s . ~ Dry solvents were freshly distilled before use.
The tosylates 3-7 were prepared according to literature pro- cedures.' The spectral data are in harmony with the assigned structures, and the melting points for 3-6 agree with the reported values: 3, mp 103.5 OC;' 4, 82-83 "C (lit? mp 81.5-82 "C; lit." mp 85.5-85.8 "C); 5, 67-68 "C (lit.B mp 68.2-68.9 "C); 6, 83-84
OC.9 The mp and pertinent spectral data for 7 are listed aa follow mp 117-118 OC; 'H NMR, 6 (CDClJ 2.28 ( s , 3 H, CHJ, 7.03-7.33
(m, 12 H, aromatic protons); IR vrmU (KBr) 1370,1180 (8, S=O) cm-'; MS m/z (12 eV) 303 (100, M - OTs+), 305 (65, M
+
2'+-
OTs), 474 (17, M'+), 476 (11, M
+
2 9 . Anal. Calcd for Kinetic Measurements. Conductimetric rate constants were measured, with the exception of acetolysis, wing an automatidy PC-AT or XT monitored multichannel system developed in this laboratory. The conductivity cells were placed in the thermostat with temperature variation of h0.02 OC. Solutions of 10-C106M were employed in this study. In some cases, the addition of a small amount of 2,6-lutidine to the solution was found to be necessary to prevent curvature of the rate constant plot. A ti- trametrical method was employed with a solution of 0.005-0.01
M for the study of acetolysis.a
All reactions were followed to 10 or more half-lives and showed excellent first-order behavior. Arrhenius plots of rate data ob-
tained at other temperature were used to estimate rate constants C20H1603F3C12: C, 53.06; H, 3.18. Found, C, 53.26; H, 3.18.
(38) R = 0.978-0.995 if N(EqO+) was used, and R = 0.989-0.996 if
(39) Perrin, D. D.; Armarego, W. L. F. Purification of Laboratory
(40) Fainberg, A. H.; Winstein, S. J. Am. Chem. SOC. 19S6, 78, 2770. N'(0Ts) waa used.
Chemicals, 3rd ed.; Pergamon Press: New York, 1988.
Downloaded by NATIONAL TAIWAN UNIV on August 31, 2009 | http://pubs.acs.org
at 25 O C which could not be measured directly. Each rate constant National Science Council for financial S U D D O ~ ~
_.
of this re-was determined at least in duplicate, and thedeviation was smaller than *3%. The mean values of these data are listed in Table
1 Redstry No. 3, 73572-26-6; 4, 84877-44-1; 5, 91787-10-9; 6,
search.
I.
120136-10-9; 7, 96236-00-9; 8, 1024-41-5; 9, 139760-46-6; 10,
Acknowledgment. The authors are grateful to the 139760-47-7.
Synthesis of New Amphiphilic Perfluoroalkylated Bipyridines
Nathalie Garelli and Pierre Vierling*Laboratoire de Chimie MolBculaire, Unit6 de Recherche AssociBe au C.N.R.S., Universit6 de Nice-Sophia Antipolis, 06108 Nice Ceder 2, France
Received November 5, 1991
General and versatile synthetic methods have been developed for the preparation of a large variety of 2,2’- bipyridines bearing two various perfluoroalkylated side chains with an ester or a methylene junction in the 4,4’-positions, e.g., 4,4’-bis[ [ 2”- (F-alkyl)ethenyl] alkyl] -2,2’-bipyridines, 4,4’-bis [ [ [ (F-alkyl)alkyl] oxy] carbonyl]- 2,2’-bipyridines, and 4,4’-bis[ [ [ [2”-(F-alkyl)ethenyl]alkyl]oxy]carbonyl]-2,2’-bipyridines.
Introduction
An area of growing interest lies in the use of ligands and their transition-metal complexes exhibiting amphiphilic properties capable of being inserted into interfacial films, membranes, liposomes, and other organized supramole- cular systems. A more important objective is to develop novel chemistry based on membrane-mediated processes in relatively simple systems. Such organized systems have been utilized in reactivity control, photochemical solar energy conversion and storage, transport, and drug en- capsulation and providing unique environments for sub- strates and enzymes.’
Our goal is to develop metal complexes which may be transported by vesicles (or liposomes) or by injectable fluorocarbon emulsions to be used simultaneously as drug delivery systems and as artificial oxygen carriers. In order to achieve this goal, it was necessary to synthesize new highly amphiphilic ligands bearing, in particular, per- fluoroalkylated side chains. Such derivatives may find several potential applications, namely in therapy for the transport and targeting of drugs based on transition-metal complexes and in the constitution of functionalized dis- persed systems which may operate as catalytic micro- reactors.
We describe here the preparation of a new class of am- phiphilic ligands in which the coordinating head consists of a bipyridine moiety and the hydrophobic part of two hydrocarbon chains of various lengths terminated by highly perfluoroalkylated tails.
The presence of the bippidine head on these new com- pounds opens up a large field of applications. Very few organic ligands have received more attention than 22’- bipyridine and ita analogues. Bipyridine ligan& are widely used in coordination chemistry and catalysis.2 The in- terest in such ligands stems, in particular, from their ex- ceptional photoredox properties and from the peculiar photochemical and photophysical properties exhibited by several of their transition-metal c~mplexes.~ Elaborate
(1) Fendler, J. H. In Membrane Mimetic Chemistry; John Wiley &
(2) Zeissel, R. Nouveau J. Chim. 1983, 7, 613.
(3) Juris, A.; Barigelletti, F.; Campagna, S.; Balzani, V.; Belser, P.; von Sons: New York, 1982.
Zeleneky, A. Coord. Chem. Reo. 1988,84,85.
systems exploiting such properties have indeed emerged, especially in redox electro~atalysis~ and light-induced electron-transfer reactions that convert solar energy into chemical energyS6 Furthermore, several 2,2’-bipyridine- metal complexes were found to be endowed with antimi- crobial: antifungal,’ and antineoplasmic* activity.
The perfluoroalkylated tails are intended to increase the hydrophobic and fluorophilic character of these amphi- philic ligands, hence of their transition-metal complexes,
to facilitate, respectively, their incorporation into liposomes and into oxygen-delivering fluorocarbon emulsions. The encapsulation into liposomes of a drug usually improves ita therapeutic index, hence ita efficiency, by reducing ita toxicity, prolonging ita intravascular persistence, and modifying ita biodistrib~tion.~ Furthermore, the incor- poration of a drug in fluorocarbon emulsions is expected
to combine the numerous advantages of drug encapsulation with the capacity of the fluorocarbon to deliver oxygen in radio-1° and chemoresistant” tumors, thus enhancing the tumoricidal effects of radiations or of cytotoxic drugs. Such a synergistic effect has been shown in the treatment of tumors with alkylating agents, antimetabolites, and antibiotics used in conjunction with fluorocarbon emul- sions.12 In addition, the use of fluorocarbon emulsions in therapy as drug delivery systems is particularly at- tractive in view of their high intravascular persistence and
(4) Collin, J. P.; Sauvage, J. P. Coord. Chem. Reu. 1989,93,264.
( 5 ) (a) Gritzel, M.; Kalyanasundaram, K.; Kiwi, J. S t r u t . Bonding
(Berlin) 1982,49, 37. (b) Lehn, J. M. Actual. Chim. 1982, 12, 13.
(6) Craciunescu, D.; Scarcia, V.; Furlani, A,; Gbirvu, C.; Ravalico, L.;
Doadrio, A. Acta Pharm. Fennica 1987, 96, 157.
(7) Cristalli, G.; Franchetti, P.; Nasini, E.; Vittori, S.; Grifantini, M.;
Barzi, A.; Lepri, E.; Ripa, S. Eur. J. Med. Chem. 1988,23, 301.
(8) Gill, D. S. In Platinum Coordination Complexes in Cancer Che- motherapy; Hacker, M. P., Douple, D. P., Krakoff, H. I., Eds.; Martinus Nishoff: Boston, 19% p 290.
(9) (a) Lopez-Berestein, G.; Fidler, I. J. Liposomes in the Therapy of
Znfectiow Diseases and Cancer; A. R. Lias. New York, 1989. (b) Mayew,
E.; Papahadjopoulos, D. In Liposomes; Ostro, M. J., Ed.; M. Dekker: New York, 1983; p 289. (c) Gregoriadis, G. Liposomes as Drug Carriers; John Wiley & Sons: New York, 1988.
(10) (a) Rockwell, S. Int. J. Radiat. Oncol. Biol. Phys. 1985,11,97. (b) Thomas, C.; Riess, J. G.; Guichard, M. Int. J. Radiat. B i d . 1991,59,433. (11) Teicher, B. A.; Herman, T. S.; Tanaka, J.; Mer, J. P.; Holden, S.
A.; Bubley, G.; Coleman, C. N.; Frei, E., 111. Cancer Res. 1991,51,1086.
( 1 2 ) Teicher, B. A,; Mcintosh-Lowe, N. L.; Rose, C. M. In Biomut., Art.
Cells, Art. Org.; Chang, T. M. S., Geyer, R., Eds.; M. Dekker: New York,
1988; Vol. 16, p 533.
0022-3263/92/1957-3046$03.00/0 0 1992 American Chemical Society
Downloaded by NATIONAL TAIWAN UNIV on August 31, 2009 | http://pubs.acs.org