行政院國家科學委員會補助專題研究計畫成果報告
計劃名稱: 發展肝細胞專一性的蛋白微脂體複合物作為肝臟基因治療的載體
計畫類別:þ個別型計畫 □整合型計畫
計劃編號: NSC 90-2314-B-002-142
執行期間: 自 89 年 8 月 1 日至 90 年 7 月 31 日
計畫主持人:倪衍玄 共同主持人:張美惠
協同主持人:黃玲惠、張富雄 助理:韓曉芬
計畫執行機構:台大醫學院小兒科
ABSTRACTBackground/Aim
: Asiaologlycoprotein (AsG) receptors uniquely exist in hepatocytes.Protamine is a polyarginine and able to condense DNA. Liposome is widely used as a gene transfer vector. In this study, we designed a new complex containing AsG-protamine conjugate and mixed with liposome, which uses AsG as a liver specific ligand, protamine to condense DNA, and both protamine and liposome to carry foreign DNA to form an efficient hepatic gene transfer vector.
Methods
: Conjugation reaction of AsG and protamine and liposome was monitored by capillary electrophoresis. The product then carried green fluorescent protein (GFP) gene to transfect human hepatoma cell line andnon-hepatocyte cell line to demonstrate its efficacy and specificity
in vitro
. For thein vivo
study, this gene transfer complex carried GFP gene and injected into the mice either through tail veins or portal veins. The expression of GFP was assayed 48 hours later by the directfluorescent microscopic examinations and the flow cytometry.
Results
: AsG-protamine conjugate and liposome mixture had a 2 and 10 more folds increase of GFP expression than liposome or conjugate alone in hepatoma cell line respectively.However, the
in vivo
studies showed the gene expression level in the liver was minimal either through tail veins or portal veins.Conclusion
: AsG-protamine conjugate and liposome mixture is a new liver-specific gene transfer vector. This vector may work well inex
vivo
gene therapy and needs further modification to apply toin vivo
hepatic gene therapy.Key Wor ds: asialoglycopr otein, pr otamine, liposome, gene ther apy
INTRODUCTION
An optimal liver gene therapy vector should be liver-specific, able to transfer genes into quiescent hepatocytes, express the transgene on a long-term basis, not be immunogenic, not require any manipulation of the recipient, and not cause any morbidity.(1) Currently, there is no perfect vector. Therefore, an efficient vector for liver-directed gene therapy, either
in vivo
orin
vitro
, is highly desired.Viral vectors are well known to be efficient in terms of transfection, but not without risks when applied in human. (2) Nonviral vectors based on receptor-mediated endocytosis, like the asialoglycoprotein
(AsG)-polylysine-DNA complex, can direct the foreign genes to hepatocytes. (3, 4) AsG receptor is well characterized and specifically exists in hepatocytes. Polylysine is a positive-charged
protein that can carry negative-charged DNA by electrostatic force. An AsG-polylysine conjugate is able to carry foreign DNA and target to the liver. (5,6) However, this method is less efficient than the viral vector in terms of gene expression. Besides, polylysine is toxic to cells.
To overcome the above difficulties, we designed a new compound that is an
AsG-protamine conjugate instead of AsG-polylysine. Protamines are small basic proteins (MW=4000-4250 kD) with a high arginine content. This protein was naturally found in the salmon or herring sperms. Salmon sperm protamine had been sequenced and shown to contain 32 amino acids, out of which 21 of them are arginines. (7) It is also a positively charged protein and able to bind the negatively charged DNA. Furthermore, protamine is potent in folding the DNA. Although the exact
mechanism of DNA-protamine complex formation remains unknown, protamine can condense DNA into a toroid form. (8,9) In this compact structure, DNA is well protected from enzymatic hydrolysis and may reach the nucleus in an intact form.(10) Protamine had been proved to increase transfection efficiency in the liposome-mediated gene transfer system in many kinds of cells, including hepatocytes. (11,12) The other advantage of protamine is that it is an FDA-approved, antidote for heparin. The adverse drug effects and pharmacology were well known. Once we adapt protamine for
in
vivo
human gene therapy, it is clinically applicable.Asialofetuin (Af) is one of the AsG proteins and commercialized in a
pharmacological grade. Af had been used as a liver-specific ligand to carry drugs for an
hepatocytes liposome Asialoglycoprotein (AsG) Folded DNA protamine AsG receptor
Fig. 1 The conceptual design of the complex. The asialoglycoprotein
and protamine are conjugated by a chemical bond while the gene of interest and liposome are mixed with the conjugate.
organ-targeting therapy. (13,14)
We used liposome as the supplement vector to increase gene transfer efficiency of the conjugate. Liposomes are clinically applicable non-viral vectors for gene therapy (15). The advantages of this vector are nonimmunogenic, no limitation of gene size, can be administered repeatedly, commercialized and ready to use.(16,17) Its disadvantages are the lack of tissue targeting ability in standard
preparation.(13) It was shown liposome could be targeted to hepatocytes once labelled with AsG.(18) We expected the mixing of the liposome and AsG-protamine conjugate may increase the transfection efficiency (Fig. 1). The internal diameter of a liposome is about 0.025 to 0.1 μm, which is much less than the longest dimension of plasmid DNA (about 2 μm) (19). This means that the expressed genes need to be compacted inside the liposome. Again, protamine should be important as the
components of the complex to condense DNA.
RESULTS
Fig. 2 Capillary zone electrophoresis to monitor the conjugation of
protamine and asialofetuin (Af). The upper panel was done at zero time of the conjugation reaction. The Af and protamine had different peaks (see arrows). The lower panel was recorded 12 hours later and the conjugate had already formed a single sharp peak (the arrow).
electrophoresis (CE) Kinetics:
According to the curve, we determined 12 hours at roomtemperature was the optimal condition for the making of conjugate (Fig. 2).
Simplified gel retardation assay
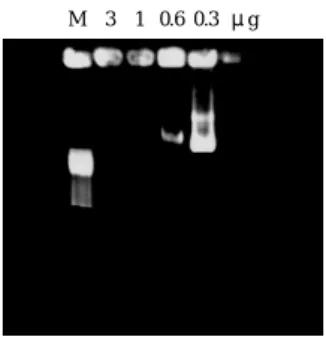
: The endpoint was the lowest amount of conjugate that can bind 5ìg of DNA completely, such that no DNA ran into the gel. This was used as the starting point for the transfection experiments (Fig. 3).Optimize the amount of conjugate and
liposome mixture:
The GFP plasmid amount was fixed at 5 ìg. The conjugate and the liposome amount varied accordingly as shown in Fig. 4. After the GFP gene expression assay, we then determined the mixture of conjugate 1ìg plus lipofectamine 3 ìl to carry 5 ìg of GFP plasmid could set up the optimal gene expressionM 3 1 0.6 0.3 μg
Fig. 3 Simplified gel retardation assay to determine the optimal
molecular ratio of the conjugate to 5ìg of plasmid (pGL-1). The corresponding amount of conjugate was 3, 1, 0.6, 0.3 ìg as labeled in the upper row of each lane. When the conjugate was 1 ìg, all plasmids were retarded in the well. When the conjugate was 0.6 ìg, some plasmids were not bound by the conjugate and released into the gel. Thus, we determined the optimal condition was 1 ìg. M: marker, ëHind III.
condition.
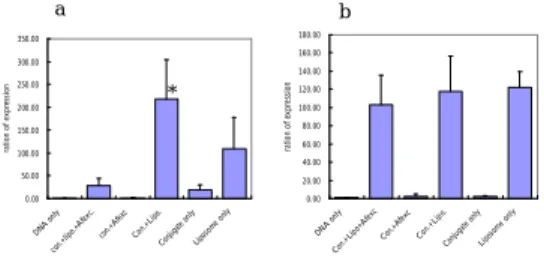
Transfection of the conjugate to Huh7
: The results showed the transfection caused by conjugate could be competed away by the excess amount of Af. The conjugate and the liposome mixture indeed increased the transfection efficiency in the in vitro system (Fig. 5a). The transfection study done in lung cancer cell line also demonstrated the conjugate worked well for hepatoma cell line and not for the lung cancer cell line. This is an evidence to support its tissue specificity (Fig. 5b).Intravenous and intraportal injection of the
conjugate into the mice
: Thein vivo
GFP expression after either tail vein or portal vein injection was generally of very low level irrespective the conjugate alone or plus the liposome mixture. In addition to liver, we checked the gene expression in heart, lung, kidney and spleen. There was no expression in0 10 20 30 0.2 0.4 0.8 1.0 0 0.5 1. 02.0 3 .04. 0 pGL-1+ conj. (μg) LIPOFECTAMINE ul(μl) G F P e x p re ss io n in d ex
Fig. 4 The determination of optimal ratio of plasmid (pGL-1), the
conjugate and liposomes. The pGL-1 can express GFP and its expression index was used to evaluate the optimal condition. The mixture of conjugate 1ìg plus lipofectamine 3 ìl to carry 5 ìg of GFP plasmid was the optimal gene expression condition.
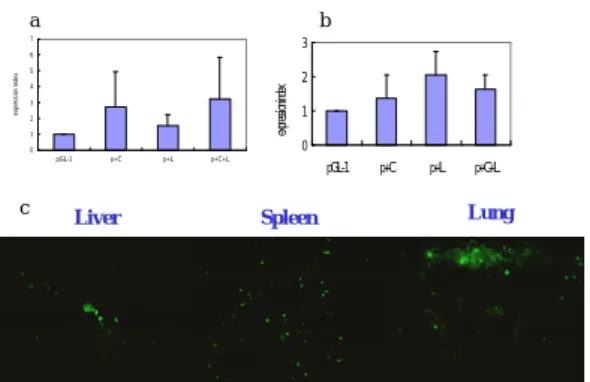
heart and kidney when the conjugates (with and without liposome mixture) were injected either intravenously or intraportally. For the tail vein injection, there was some minimal expression in the liver, spleen and lung, however, it was only 1-3 folds more than the background signal (Fig. 6a and 6c). The intraportal injection also showed a 1-2 fold increase of expression as compared with the background signal. (Fig 6b)
DISCUSSION
Previous study done by Li et al showed an appropriate formulated liposome- protamine complex could successfully gain a high gene expression through intravenous administration route, particularly in the lung.(20) Thus, they had already defined the optimal liposome/DNA ratio and pointed out the target organ this complex work toward. Our design was to add asialoglycoprotein, a liver-specific ligand in the liposome-protamine complex. The complex did work well
in vitro
that it showed a higher EI than liposome or conjugate only.0.00 50.00 100.00 150.00 200.00 250.00 300.00 350.00 DNA only con.+ lipo. +Afe xc. con.+Af exc Con. +Lipo . Conju gate only Lipos ome only ra ti o n o f ex p re ss io n * a 0.00 20.00 40.00 60.00 80.00 100.00 120.00 140.00 160.00 180.00 DNA only Con .+Lipo+ Afexc Con.+ Afexc Con.+ Lipo. Con juga te on ly Liposome onl y ra tio n o f ex pr es si on b
Fig. 5 Transfection of the conjugate plus liposome mixture into (a)
Huh7 cell line and (b) NCI-H292 lung cancer cell line. There were six experimental groups in the transfection (1) DNA only as the negative control. (2) conjugate plus liposome competed by non-conjugate Af (3) conjugate competed by non-conjugate Af (4) conjugate and lipofectamine (5) conjugate only and (6) liposome only as the positive control. The detail amounts of each components were described in the “materials and methods” section.
*: The expression index (EI) of conjugate plus liposome was about twice of that of lipofectamine only, but not statistically significant (p=0.056, Mann-Whitney test). Conjugate plus liposome group significantly had a higher EI than the groups of conjugate only (p=0.008, Mann-Whitney test). The EI was of no statistical difference among the groups of conjugate plus liposome competed by non-conjugate Af, conjugate and lipofectamine, and liposome only in the lung cancer cell line study. The experiments were done in triplicate.
This complex appeared to be liver-specific since the lung cancer cell culture was only transfected by the liposome-mediated pathway, but not the conjugate. Although we did not apply this conjugate to other kinds of tissues, the negative result of transfection in lung cancer cell line may be representative enough to illustrate the tissue specificity of this conjugate. The conjugate likely entered the hepatocytes through the AsG receptors because the excess amount of unconjugated Af could compete away the conjugate. In terms of gene expression, the
Liver c 0 1 2 3 4 5 6 7 pGL-1 p+C p+L p+C+L ex pr es si on i nd ex a 0 1 2 3 pGL-1 p+C p+L p+C+L ex pre ssi on ind ex Spleen Lung b
Fig. 6 The in vivo transfection studies; (a) the tail vein injection (b)
intraportal injection (c) The section of liver, spleen and lung 48 hours after tail vein injection of pGL-1, conjugate plus liposome mixture under fluorescent microscope (400X). That the cells were emitting green light denotes the successful transfections. EI of each group showed no statistical significance either by tail vein injection or intraportal injection.
conjugate plus liposome had a three-fold increase when compared with liposome only; while it was about four- fold increase when compared with the conjugate only. The
conjugate plus liposome mixture seemed to have a synergistic effect
in vitro
.The
in vivo
intravenous and intraportal injection of the conjugate into the mice led to a minimal gene expression in this experiments. For the tail vein injection, the conjugate plus liposome mixture must carry the GFP DNA to travel a long distance from the injection site to the liver. It was shown that the cationic liposome: DNA complex can activate a strong inflammatory response. (21) We thought theconjugate:liposome: DNA complex in this experiment was inactivated by the macrophages or the reticuloendothelial system or other serum factors, for example, the complements in the circulation.
The intraportal route avoids the first pass of the
complex to the pulmonary circulation. and is supposed to avoid the above difficulties, but still in vain when we applied this conjugate. Though it is relatively a short distance from the portal vein to the hepatocytes, the gene transfer vector still exposes to the host immune system and may be cleared in the portal system. Intraperitoneal route may be one alternative. It gives the complex a chance to avoid the serum factors in the circulation. It had been well demonstrated the application of adenovector-liposome complex through the intraperitoneal route in the mice.(22) Anyway, the route of administration was still the critical factor in gene
expression.(23)
For the future refinement of this vector, the addition of a molecule to combat these serum factors may be helpful. A decay-accelerating factor (DAF) had ever been incorporated into the envelope of virus vector to render it
complement-resistant.(24) DAF is also possible to be incorporated into our design. The potential demerit to incorporate DAF is the size of the complex perhaps will be quite large and hard to escape the trap of the reticuloendothelial system. In summary, we had designed a very powerful tool for gene transfer and gene therapy
ex vivo
. The components of this complex consist of AsG, a liver-specific ligand, protamine, helpful to fold DNA and liposome. All three are likely to contribute the gene transfer efficiency of this complex. It can be uptaken through the AsG receptor and/or liposome endocytosis pathway. Some modifications are mandatory to help shaping this complex to be effectivein vivo
.REFERENCES
cell transplantation and liver-directed gene therapy. Semin Liver Dis 1999;19:1-6. 2. Kay MA, Glorioso JC, Naldini L. Viral
vectors for gene therapy: the art of turning infectious agents into vehicles of
therapeutics. Nat Med 2001; 7: 33-40. 3. Wu GY, Wu CH. Receptor-mediated gene
delivery and expression
in vivo
. J Biol Chem 1988;263:14621-4.4. Wu GY, Wu CH. Receptor-mediated
in
vitro
gene transformation by a soluble DNA carrier system. J Biol Chem 1987;262:4429-32.5. Wu GY, Wilson JM, Shalaby F, Grossman M, Shafritz DA, and Wu CH.
Receptor-mediated gene delivery
in vivo
. Partial correction of genetic analbuminemia in Nagase rats. J Biol Chem1991;266:14338-42.
6. Stankovics J, Crane AM, Andrews E, Wu CH, Wu GY, and Ledley FD.
Overexpression of human methylmalonyl CoA mutase in mice after
in vivo
gene transfer with asialoglycoprotein/ polylysine/DNA complexes. Hum Gene Ther 1994;5:1095-104.7. Warrant RW, Kim SH:α-Helix double helix interaction shown in the structure of a protamine-transfer RNA complex and a nucleoprotamine model. Nature 1978;271:130-5.
8. Brewer LR, Corzett M, Balhorn R. Protamine-induced condensation and decondensation of the same DNA molecule. Science 1999;286:120-3.
9. Allen MJ, Bradbury EM, Balhorn R. AFM analysis of DNA-protamine complexes bound to mica. Nucleic Acid Res
1997;25:2221-6.
10. Bianchi F, Rousseaux-Prevost R, Bailly C, Rousseaux J. Interaction of human p1 and p2 protamines with DNA. Biochem Biophysic Res Comm 1994;201:1197-204. 11. Ni YH, Hsu HY, Chen PJ, Chang MH.
Protamine enhances the efficiency of liposome-mediated gene transfer to cultured human hepatoma cell line. J Formos Med Assoc 1999; 98:562-6. 12. Sorgi FL, Bhattacharya S, Huang L:
Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther 1997;4:961-8. 13. Wu J et al. Increased liver uptake of
liposomes and improved targeting efficacy by labeling with asialofetuin in rodents. Hepatology 1998;27:772-8.
14. Kaneo Y, Tanaka T, Iguchi S: Targeting of mitomycin C to the liver by the use of asialofetuin as a carrier. Chem Pharm Bull 1991;39:999-1003.
15. Felgner PL: Improvements in cationic liposomes for
in vivo
gene transfer. Hum Gene Ther 1996;7:1791-3.16. Gao X, Huang L: Cationic
liposome-mediated gene transfer. Gene Ther 1995;2:710-22.
17. Ledley FD: Nonviral gene therapy: the promise of genes as pharmaceutical products. Hum Gene Ther 1995;6:1129-44. 18. Yu HY, Lin CY: Uptake of charged
liposomes by the rat liver. J Formos Med Assoc 1997;96:409-13.
19. San H, Yang ZY, Pompili VJ, Jaffe ML, Plautz GE, Xu L, Felgner JH, Wheeler CJ, Felgner PL, Gao X. Safety and short-term toxicity of a novel cationic lipid
Gene Ther 1993;4:781-8.
20. Li S and Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther 1997;4:891-900.
21. Tousignant JD, Gates AL, Ingram LA, Johnson CL, Nietupski JB, Cheng SH, Eastman SJ, Scheule RK. Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid:plasmid DNA complexes in mice. Hum Gene Ther 2000;11:2493-513. 22. Xing X, Liu V, Xia W, Stephens LC, Huang
L, Lopez-Berestein G, Hung MC. Safety studies of the intraperitoneal injection of E1A--liposome complex in mice. Gene Ther 1997;4:238-43
23. Liu Y, Mounkes LC, Liggitt HD, Brown CS, Solodin I, Heath TD, Debs RJ. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol 1997;15:167-73. 24. Huser A, Ruldoph M, Hofmann C.
Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol 2001;19:451-5.