行政院國家科學委員會專題研究計畫 成果報告
子計劃一:奈米發光膠粒合成與粒間作用研究(2/2)
計畫類別: 整合型計畫 計畫編號: NSC91-2120-E-002-009- 執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日 執行單位: 國立臺灣大學材料科學與工程學研究所 計畫主持人: 韋文誠 計畫參與人員: 林建村教授,虞邦英, 林頌恩 報告類型: 完整報告 處理方式: 本計畫可公開查詢中 華 民 國 92 年 11 月 17 日
奈米光粒子合成能隙晶體結構製作與應用
子計劃一:奈米發光膠粒合成與粒間作用研究
計畫編號:NSC 91-2210-E-002-009
執行期限:91 年 8 月 1 日至 92 年 7 月 31 日
主持人:韋文誠 臺灣大學材料系
協同主持人:林建村博士 中研院化學所
計畫參與人員:虞邦英、林頌恩 臺灣大學材料系
I. 中文摘要 本研究第二年主要工作是合成具有核 殼結構單徑二氧化矽/二氧化鈦及銦酸鍶螢 光粒子,並探討顆粒之間作用行為,及分 析顆粒對於光閘晶體之光學性質的影響。 實驗使用粒徑均一之二氧化矽顆粒,在強電 性斥力下自組成光閘晶體。再利用反應控 制,以溶膠凝膠法生成的奈米二氧化鈦顆粒 覆蓋於二氧化矽顆粒表面,創造出核殼結 構的光閘晶體。銦酸鍶則由硝酸前導溶液 反應析出而得。顆粒及組合物之微結構主 要利用各種電子顯微鏡進行分析觀察。並 以反射光譜分析儀量測其光學性質的變 化 。 組 合 測 試 中 加 入 高 分 子 界 面 劑 (PMAA-NH4, PDAAE or PVP),藉由表面電 性的量測,了解高分子電解質之功能。此 外,藉由反射光譜分析儀的量測可知由二氧 化矽/二氧化鈦核殼結構所組成的光閘晶體 能有效濾除500nm 反射峰,只保留 600nm 處的反射峰,使反射峰的範圍窄化。 關鍵詞:溶膠凝膠法、二氧化矽、二氧化 鈦、銦酸鍶、自我組成、單徑、光閘晶體、 AbstractThe objectives of this study are to synthesize SiO2 core/TiO2 shell and luminescence
SrIn2O4 spherical particles, to understand the
interparticle forces, and to assemble photonic crystals so to reveal the band gap properties. Nano-sized and homogeneous TiO2 was
prepared from the reaction of Ti alkoxide (TBOT) with the water provided by the esterification of butanol and acetic acid. SrIn2O4 was synthesis from nitrate solution.
The configurations of the SiO2/TiO2 particles
and the microstructure of assembly were characterized by SEM, AFM, and TEM. In
addition, the PBG crystals were measured by reflective spectrometer and the particles by ζ-meter, respectively. With the addition of three polymers PMAA-NH4, DAAE, and
PVP dispersive role was investigated. The silica/titania core-shell structure could filter the visible light near 500 nm, and sharper the 600 nm spectrum comparing to that of the PBG crystal assembled by mono-silica particles.
Keywords: sol-gel method, SiO2, TiO2,
SrIn2O4 self-assembly, mono-size, PBG
crystal,
II. Motivation and Objective
The synthesis of submicron mono- dispersive oxide particles starting from TEOS (tetraethyl orthosilicate) has been studied in the past project. Particle ripening and coalescence were the major growth mechanism after the nucleation of silica species after hydrolysis and condensation of TEOS. Essentially, the method reported by Stöber et al1 is the most essential work on the synthesis of submicron mono-dispersive silica particles in diameter from 0.05 to 2.0 µm. Various processing parameters were used by Bowen et al.2 and the others
demonstrating the possibility of size changing by the bath temperatures (-20℃~80 ℃).
In recent years there has been renewal of interest in silica spherical particles because of their self-assembly property like natural opal to form photonic bandgap crystals (PBG crystals).3 One of the main aims of PBG technology is the fabrication of three- dimensional (3-D) PBG in the visible and infrared (IR) region of the
electromagnetic spectrum. Colloidal process was utilized to carry the synthesized particles, then organized or self- assembled to an ordered 3-D structure.
TiO2 particles could be synthesized by
sol-gel method as well.4 The material shows interesting optical (refractive index is 2.52-2.71), dielectric constant (DC 31-114), and colloidal properties (iso-electric point, iep = pH 4-6), which are different from silica powders (refractive index is 1.44-1.46; DC is 4.3-8.5; iep is pH 3-4).5,6 In addition to those, the bandgap energy of TiO2 is about 3.2 eV.
Exposure on the UV light, TiO2, one of
photocatalysts, forms two carriers, electrons and holes, which introduce either reduction or oxidation reactions, respectively.
On the use of colloidal process to coat the other nano-materials on the surface of silica spheres changes the stability of the particles dispersed in the suspension. The mixture of two oxides may show a combinative colloidal properties.7 but also changes the behavior of the PBG crystals. The purpose of this paper is to synthesize the SiO2 core/TiO2 shell particles and observe
the microstructure of those particles and how core-shell structure affects the optic behavior of PBG crystal.
III. Result and Discussion
3-1 Synthesis of Nano-sized TiO2
In order to reduce the reaction rate of TBOT, the synthesis of nano-sized TiO2 used
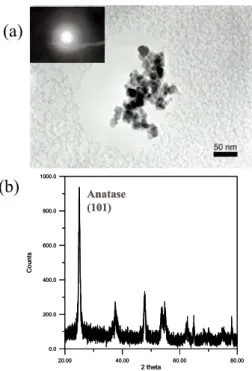
sol-gel method via the reaction of water which provided by the esterification of butanol and acetic acid. After drying and calcinations, the anatase phase is formed. Figs. 1(a) and 1(b) show the TEM macrograph and XRD result of TiO2,
respectively
3-2 Silica/titania Core-Shell Structure
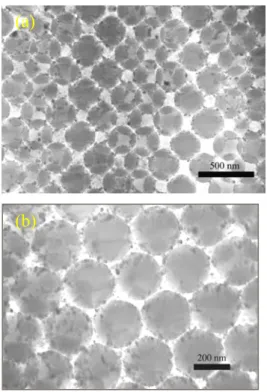
Figs. 2 (a) and 2(b) are the SEM and the TEM of silica sediment. The packing structure of the sediment surface is in hexagonal closed packing (hcp). TEM result shows that more complicate packing structure of the silica sediment was observed and the more detail discussion of the packing structure was seen in our previous report,8 which reported many kinds of defects on the surface of the SiO2 package, such as the
vacancies, faults, domain boundary, stacking faults, and the others. The main reasons for the formation of these defects are the force turbulence inducing different arrangement of SiO2 particles during the sedimentation or
packing. Those defects are related to the interparticle forces between the SiO2
particles and the packed plane during the sedimentation. Other reasons, such as the thermal vibration of particles and the stress (capillary forces) induced during the drying, or/and sintering, should be well controlled.
The synthesis method for core-shell (a) 20.00 40.00 60.00 80.00 2 theta 0.0 200.0 400.0 600.0 800.0 1000.0 Co unt s Anatase (101) 20.00 40.00 60.00 80.00 2 theta 0.0 200.0 400.0 600.0 800.0 1000.0 Co unt s Anatase (101) (b)
Fig. 1 (a) TEM macrograph and (b) XRD of nano
TiO
Fig. 2 (a) SEM and (b) TEM of self-assembled colloidal SiO2 particles sintered at 950oC/2 h.
structure was conducted by immersed silica sediment in titania sol, then calcined amorphous titania to anatase phase. Fig. 3 is the TEM pictures of this sediment. The packing structures of Figs. 3(a) and 3(b) are cubic (fcc) and hcp, respectively. The surface of silica spheres are covered by a layer of nano-sized TiO2 particles. The thickness of
the TiO2 layer is not uniform and about
10-30 nm that depended on the crystalline size of TiO2.
3-3 Heterogeneous Aggregation
The aim of heterogeneous aggregates of two kinds of oxide particles in solution is to create the shell structure on SiO2 particles.
This method adjusts pH value of the solution, which is between the iep values of two oxides. At the pH, one oxide particle shows positive charge and the other is negative. Figs. 4 and 5 are the ζ-potential of the SiO2
and TiO2 particles versus pH of the aqueous
and ethanol suspensions. In ethanol suspension, both particles carried less charge on the surface and the iep shift to basic side. One TEM result of the heterogeneous aggregates of SiO2 and TiO2 particles at pH 3
in ethanol is shown in Fig.6. The particles show nano-TiO2 coating but not uniform in
thickness. This process needs further improvement.
Fig. 4 Zeta potential of the silica and titania particles versus pH of the aqueous suspension.
Fig. 5 Zeta potential of silica and titania particles versus pH of ethanol suspension.
Fig. 6 TEM of colloidal silica/titania spheres by heterogeneous aggregation method.
3-4 Interparticle Force with Polymer Addition In order to get a long-range stability, two polymer electrolytes were selected to disperse SiO2 in aqueous solution. As the
amount of dispersion increases, the ζ-potential of SiO2 decreases due to the
electrolyte concentration. (Fig. 7) PDAAE
0.0 2.0 4.0 6.0 8.0 10.0 12.0 14.0 pH -70.0 -60.0 -50.0 -40.0 -30.0 -20.0 -10.0 0.0 10.0 20.0 30.0 40.0 zeta-p o ten tial (mV ) Silica Titania 0.0 2.0 4.0 6.0 8.0 10.0 12.0 14.0 pH -20.0 -10.0 0.0 10.0 zeta-p otential ( m V) Silica Titania
Fig. 3 TEM micrographs of the self-assembly colloidal silica/titania spheres sintered at 500oC
(a)
and PMAA-NH4 seem can’t absorbed on
SiO2, but show steric effect between silica
surface to stabilize the particles in the aqueous solution. 0.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 pH -60.0 -50.0 -40.0 -30.0 -20.0 -10.0 0.0 10.0 20.0 ζ -pot ent ial PDAAE 10.0ml/1000g PDAAE 5.0ml/1000g PDAAE 2.5ml/1000g PDAAE 1.0ml/1000g PDAAE 0.5ml/1000g PDAAE 0.0ml/1000g 0.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 pH -70.0 -60.0 -50.0 -40.0 -30.0 -20.0 -10.0 0.0 10.0 20.0 ζ -pot ent ia l PMAA-NH4 10.0ml/1000g PMAA-NH4 5.0ml/1000g PMAA-NH4 2.5ml/1000g PMAA-NH4 1.0ml/1000g PMAA-NH4 0.5ml/1000g PMAA-NH4 0.0ml/1000g
Fig. 7 ζ-potential of SiO2 powder in aqueous solution with (a) PDAAE (b) PMAA-NH4 in a concentration of 5 wt% SiO2 powder.
3-5 Optical Properties of PBG crystal
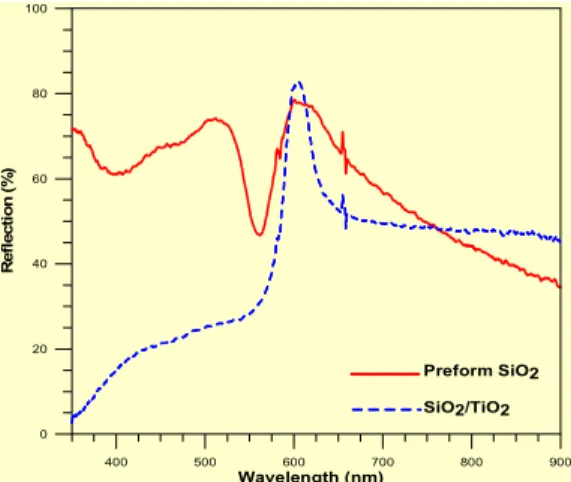
With a thin layer coating of 30 nm anatase phase, the sediment reflects different spectra from the original one. The PBG crystals of silica/ titania core-shell structure could filter the visible light and the reflective spectra is mainly at 600 nm, which is sharper than that in the PBG crystal of mono-silica particles. (Fig. 8)
3-6 Synthesis of SrIn2O4
One example is shown in Fig. 9 with elongated shape (aspect ratio is 6)
400 500 600 700 800 900 Wavelength (nm) 0 20 40 60 80 100 R e fl ec ti on ( % ) Preform SiO2 SiO2/TiO2
Fig. 8 Reflective spectra of the silica and silica/titania PBG crystals observed at reflective angle 0o with fixed angle 0o of the incident light. ……….
Fig. 9 SrIn2O4 crystals in elongated shape
IV. Self-Evaluation
Most of the planned objectives are achieved, except the measurement of interparticle force.
V. Reference
1
W. Stöber and A. Fink, J. Colloid Interface Sci., 26, 62-69 (1968)
2 C. G. Tan, B. D. Bowen, and N. Epstein, J. Colloid Interface Sci., 118 [1], 290-293 (1987)
3 H. Miguez et al., J. Lightwave Tech. Vol. 17, no. 11, Nov. (1999)
4 E. A. Barringer and H. K. Bowen, Langmuir, 1, 420-28 (1985)
5 J. S. Reed, Introduction to the Principles of Ceramic Processing, John Wiley & Sons Pte. Ltd., pp 150-171 (1986).
6 Y. M. Chiang, D. Birnie III, and W. D. Kingery, Physical Ceramics: Principles for Ceramic Science and Engineering, p.75, John Wiley & Sons, Inc., New York (1996).
7 G. Wang, and P. S. Nicholson, J. Am. Ceram. Soc.,
84 [6] 1250-56 (2001)
8. T-W. Chen and W. J. Wei, “Sythesis and characterization of mono-sized SiO2 ceramic particles in meso-structure,” Ceram. Nanomat’ls & Nanotech., Ceram. Trans., Vol 137, pp. 23-31, Am. Ceram. Soc.