O R I G I N A L A R T I C L E
Open Access
Genetic Diversity of Landraces and
Improved Varieties of Rice (Oryza sativa L.)
in Taiwan
Ai-ling Hour
1, Wei-hsun Hsieh
2, Su-huang Chang
2, Yong-pei Wu
3, Han-shiuan Chin
2and Yann-rong Lin
2*Abstract
Background: Rice, the most important crop in Asia, has been cultivated in Taiwan for more than 5000 years. The landraces preserved by indigenous peoples and brought by immigrants from China hundreds of years ago exhibit large variation in morphology, implying that they comprise rich genetic resources. Breeding goals according to the preferences of farmers, consumers and government policies also alter gene pools and genetic diversity of improved varieties. To unveil how genetic diversity is affected by natural, farmers’, and breeders’ selections is crucial for germplasm conservation and crop improvement.
Results: A diversity panel of 148 rice accessions, including 47 cultivars and 59 landraces from Taiwan and 42 accessions from other countries, were genotyped by using 75 molecular markers that revealed an average of 12.7 alleles per locus with mean polymorphism information content of 0.72. These accessions could be grouped into five subpopulations corresponding to wild rice, japonica landraces, indica landraces, indica cultivars, and japonica cultivars. The genetic diversity within subpopulations was: wild rices > landraces > cultivars; and indica rice > japonica rice. Despite having less variation among cultivars, japonica landraces had greater genetic variation than indica landraces because the majority of Taiwanese japonica landraces preserved by indigenous peoples were classified as tropical japonica. Two major clusters of indica landraces were formed by phylogenetic analysis, in accordance with immigration from two origins. Genetic erosion had occurred in later japonica varieties due to a narrow selection of germplasm being incorporated into breeding programs for premium grain quality. Genetic differentiation between early and late cultivars was significant in japonica (FST= 0.3751) but not in indica (FST= 0.0045), indicating effects of different breeding goals on modern germplasm. Indigenous landraces with unique intermediate and admixed genetic backgrounds were untapped, representing valuable resources for rice breeding. Conclusions: The genetic diversity of improved rice varieties has been substantially shaped by breeding goals, leading to differentiation between indica and japonica cultivars. Taiwanese landraces with different origins possess various and unique genetic backgrounds. Taiwanese rice germplasm provides diverse genetic variation for association mapping to unveil useful genes and is a precious genetic reservoir for rice improvement. Keywords: Genetic diversity, Landraces, Rice, Taiwan
© The Author(s). 2020 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. * Correspondence:ylin@ntu.edu.tw
2Department of Agronomy, National Taiwan University, Taipei 10617, Taiwan
Background
Asian cultivated rice (Oryza sativa L.), feeding more than 90% of the human population in Asia, is one of the world’s most important crops. Wild ancestors and landraces with rich genetic diversity and wide adaptation to various envi-ronments provide valuable and useful genetic resources for
crop improvement (Dwivedi et al. 2016; Kovach and
McCouch 2008; Sang and Ge 2013). Natural germplasm
preserved by in situ and/or ex situ conservation is in urgent need of systematic evaluation to unveil new genes or alleles to incorporate into breeding programs for crop improve-ment. For rice, the most well-known germplasm conserva-tion center is the Internaconserva-tional Rice Genebank Collecconserva-tion at the International Rice Research Institute (IRRI). Single nu-cleotide polymorphisms (SNPs) and structural variants re-vealed by resequencing accelerate research on genetic diversity, evolution, association studies of genotypes and phenotypes and allele mining (Huang et al.2010; Li et al.
2014; Wang et al.2018; Zhao et al.2018).
Oryza sativa was domesticated from wild rice, O.
nivara or O. rufipogon (Chang 1976; Khush 1997), and
two distinct varietal groups, ssp. indica and ssp. japon-ica, are well recognized and dated as Hsien (秈) and Keng (稉), respectively, in the Hang dynasty about 2000
years ago (Chou 1948; Wang et al. 2018). Distinct
morphology and post-reproductive barriers between ssp. indica and ssp. japonica were first thought to have re-sulted from independent domestication by different an-cient populations or a single domestication with multiple origins recently (Londo et al. 2006; Choi et al.
2017; Choi and Purugganan 2018). The genetic diversity of O. sativa was dramatically reduced by bottleneck ef-fects of selective sweeps in early domestication (Caicedo
et al. 2007; Kovach and McCouch 2008). Landraces
which are morphologically recognizable and have histor-ical origins exhibit lower genetic diversity than wild rela-tives but higher than modern cultivars because of adaptation to local environments and diversity of
farmers’ preferences (Pusadee et al. 2009; Thomson
et al.2007). The allele richness of landraces was, in gen-eral, about 30% higher than that of cultivars (Kovach
and McCouch 2008; Zhang et al. 2009) and landraces
possess a wealth of abiotic tolerances, biotic resistances and other superior characters. Taken together, investiga-tion of morphological, physiological, and genetic diver-sity of landraces will provide valuable information and resources for modern rice breeding.
In Taiwan, archaeological evidence shows that rice has been cultivated by indigenous people for more than
5000 years (Hu 1993). Excavated grains exhibit various
sizes and shapes and resemble tropical japonica and indica rice (Hsieh et al. 2011). In the early seventeenth century, immigrants from two provinces of southeast China, Fujian and Guangdong, brought indica landraces
to Taiwan. In the late nineteenth century, approximately 1197 collections of temperate japonica rice were introduced from Japan (Iso1964). Sixty Taiwanese landraces have been widely used in rice research and breeding, revealed by 16 domestication-syndrome genes (Hsieh et al.2011). Taiwan-ese landraces have contributed significantly to modern indicaand temperate japonica rice breeding in Asian coun-tries. The most renowned example is IR8, the miracle rice with high yield that mitigated a food crisis in the 1970s and evoked a Green Revolution in Asia, which inherited the semi-dwarf allele (sd1) from Taiwanese landrace
Dee-Geo-Woo-Gen (DGWG) (Evenson and Gollin 2003). Indeed,
the DGWG allele has been extensively applied to improve grain yield of both indica and japonica varieties in the past 50 years (Sasaki et al. 2002; Asano et al.2011; Zhao et al.
2010). Taichung 65 (TC65), an old japonica cultivar, inher-ited null alleles of two photoperiodic genes, Ehd1 and Hd1, from landraces and has been extensively applied in modern rice breeding and in studying flowering in response to day length (Doi et al. 2004; Hsieh et al.2011; Lin 1991; Wei et al.2016; Yano et al.2000).
Many modern cultivars integrate temperate japonica and indica rice toward meeting the major demands of daily dining and traditional food processing in Taiwan. A lot of genetic variation is found in Taiwanese rice germplasm because of natural selection for adaptation to various environments, noting that Taiwan encompasses tropical and subtropical zones in a broad range of alti-tudes (0–3952 m). The genetic diversity of Taiwanese rice germplasm, originating from different geographic areas and admixed by humans in different epochs, is ex-pected to be high (Chin et al.2016).
To unravel admixing of rice germplasm imposed by natural and artificial selection is important for basic sci-entific research and breeding, each relying on informa-tion about genetic diversity and populainforma-tion structure. In this study, a diversity panel of 148 accessions, including 53 modern varieties, 83 landraces, and 12 wild rice ori-ginating from Taiwan, Japan, China and countries of southeast Asia and south Asia, was genotyped with 75 markers to assess genetic diversity and population struc-ture, conducting principal coordinate analysis (PCoA) and producing a phylogenetic tree. In addition, Taiwan-ese landraces are scrutinized, gaining insight into their significant roles in genetic and breeding research. Results
Genetic Diversity of Polymorphic Markers
A total of 953 alleles were detected from 75 DNA markers, including 49 simple sequence repeat (SSR), 6 sequence-tagged site (STS) and 20 indel markers, across the diversity panel of 148 rice accessions, including 12 wild rices, 83 landraces, 24 indica cultivars, and 29 ja-ponica cultivars (Additional file 1: Table S1). The allele
number per locus ranged from 3 to 37 with an average of 12.7, and the majority of markers revealed 6–15 al-leles. (Additional file 2: Figure S1a, Additional file 1: Table S2). Eight markers, RM472, RM2334, RM4108, CH0509, P17G10–24, RM1761, RM4154 and RM5708, were highly polymorphic with more than 20 alleles de-tected (Additional file 1: Table S2). Polymorphism infor-mation content (PIC) values ranged from 0.18 to 0.95 with an average of 0.72, and 66 (88%), 8 (10.7%) and 1 (1.3%) markers were highly, moderately and slightly informative, with PIC ≥0.5, 0.5 > PIC ≥0.25, and < 0.25, respectively (Additional file2: Figure S1b, Additional file1: Table S2). Overall, these 75 markers provided plentiful allele infor-mation to assess genetic diversity, population structure, and genetic distances of this rice diversity panel.
Genetic Structure and Diversity of Subpopulations These 148 accessions could be divided into two subpop-ulations according to inferred population structures, withΔK values found to be highest at K = 2 by STRUCT URE analysis (Additional file2: Figure S2). The japonica group constituted 62 accessions, including one wild rice, O. rufipogon, 29 Taiwanese japonica cultivars and 32 landraces from Taiwan, Japan, and China. All 29 Tai-wanese japonica cultivars, except Kaohsiung 145, shared 99.9% ancestry, indicating a consistent genetic back-ground. The indica group contained 86 accessions, in-cluding 24 cultivars, 51 landraces, and 11 wild rices.
Admixture, simulated single genetic background less than 80%, was frequently observed in wild rice, except nivara-2 and rufipogon-21, but occasionally in cultivars (Taichung Sen 17 and Basmati 385) and landraces
(Tan-gengenrankatsu and Chin-Men-Tou-Men-Hung-Mi)
(Additional file2: Figure S3a).
To further subdivide this germplasm, five subpopula-tions, denoted PopI to PopV, were obtained as K = 5 was the optimum number of subpopulations identified by STRUCTURE analysis (Table 1, Additional file2: Figure S3b). The japonica group was subdivided into two sub-populations, PopII and PopIII. Most japonica landraces were grouped into PopII, including 23 and 2 accessions originating from Taiwan and China, respectively. PopIII contained 36 japonica accessions, including 29 cultivars sharing 89–99% uniform subpopulation background; and 6 landraces of which (Burieuraozu, Paotsupagaiahon, Tangengenrankatsu, Nabohai, Unknown 1 and Sin-ceyauo), were admixtures with 44–83% of subpopulation PopIII genetic background. The Taiwanese upland
ja-ponica landrace, Tangengenrankatsu, containing some
indicagenetic background, was particularly interesting. The indica group was subdivided into three subpopu-lations, PopI, PopIV and PopV (Table1, Additional file2: Figure S3b). Twenty accessions, including 17 Taiwanese cultivars, Basmati 370, Kasalasth, and IR64, were grouped in PopI. Four accessions (Tainung Sen 22, Kao-hsiung Sen 7, Taichung Sen Glutinous 2, and Basmati Table 1 The accessions of five subpopulations grouped by STRUCTURE analysis
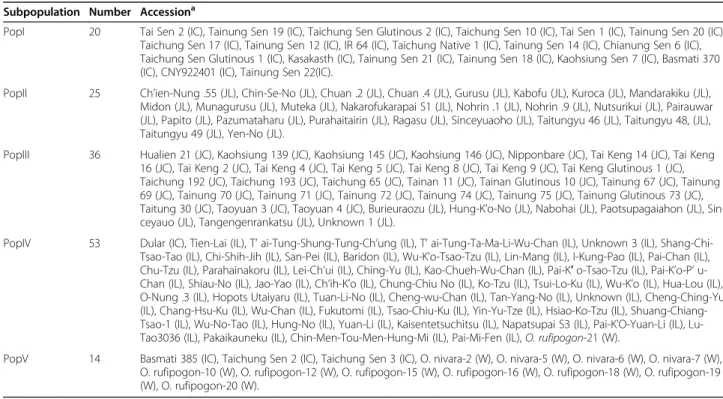
Subpopulation Number Accessiona
PopI 20 Tai Sen 2 (IC), Tainung Sen 19 (IC), Taichung Sen Glutinous 2 (IC), Taichung Sen 10 (IC), Tai Sen 1 (IC), Tainung Sen 20 (IC), Taichung Sen 17 (IC), Tainung Sen 12 (IC), IR 64 (IC), Taichung Native 1 (IC), Tainung Sen 14 (IC), Chianung Sen 6 (IC), Taichung Sen Glutinous 1 (IC), Kasakasth (IC), Tainung Sen 21 (IC), Tainung Sen 18 (IC), Kaohsiung Sen 7 (IC), Basmati 370 (IC), CNY922401 (IC), Tainung Sen 22(IC).
PopII 25 Ch’ien-Nung .55 (JL), Chin-Se-No (JL), Chuan .2 (JL), Chuan .4 (JL), Gurusu (JL), Kabofu (JL), Kuroca (JL), Mandarakiku (JL), Midon (JL), Munagurusu (JL), Muteka (JL), Nakarofukarapai S1 (JL), Nohrin .1 (JL), Nohrin .9 (JL), Nutsurikui (JL), Pairauwar (JL), Papito (JL), Pazumataharu (JL), Purahaitairin (JL), Ragasu (JL), Sinceyuaoho (JL), Taitungyu 46 (JL), Taitungyu 48, (JL), Taitungyu 49 (JL), Yen-No (JL).
PopIII 36 Hualien 21 (JC), Kaohsiung 139 (JC), Kaohsiung 145 (JC), Kaohsiung 146 (JC), Nipponbare (JC), Tai Keng 14 (JC), Tai Keng 16 (JC), Tai Keng 2 (JC), Tai Keng 4 (JC), Tai Keng 5 (JC), Tai Keng 8 (JC), Tai Keng 9 (JC), Tai Keng Glutinous 1 (JC), Taichung 192 (JC), Taichung 193 (JC), Taichung 65 (JC), Tainan 11 (JC), Tainan Glutinous 10 (JC), Tainung 67 (JC), Tainung 69 (JC), Tainung 70 (JC), Tainung 71 (JC), Tainung 72 (JC), Tainung 74 (JC), Tainung 75 (JC), Tainung Glutinous 73 (JC), Taitung 30 (JC), Taoyuan 3 (JC), Taoyuan 4 (JC), Burieuraozu (JL), Hung-K’o-No (JL), Nabohai (JL), Paotsupagaiahon (JL), Sin-ceyauo (JL), Tangengenrankatsu (JL), Unknown 1 (JL).
PopIV 53 Dular (IC), Tien-Lai (IL), T’ ai-Tung-Shung-Tung-Ch’ung (IL), T’ ai-Tung-Ta-Ma-Li-Wu-Chan (IL), Unknown 3 (IL), Shang-Chi-Tsao-Tao (IL), Chi-Shih-Jih (IL), San-Pei (IL), Baridon (IL), Wu-K’o-Tsao-Tzu (IL), Lin-Mang (IL), I-Kung-Pao (IL), Pai-Chan (IL), Chu-Tzu (IL), Parahainakoru (IL), Lei-Ch’ui (IL), Ching-Yu (IL), Kao-Chueh-WChan (IL), Pai-K′ o-Tsao-Tzu (IL), Pai-K’o-P’ u-Chan (IL), Shiau-No (IL), Jao-Yao (IL), Ch’ih-K’o (IL), Chung-Chiu No (IL), Ko-Tzu (IL), Tsui-Lo-Ku (IL), Wu-K’o (IL), Hua-Lou (IL), O-Nung .3 (IL), Hopots Utaiyaru (IL), Tuan-Li-No (IL), Cheng-wu-Chan (IL), Tan-Yang-No (IL), Unknown (IL), Cheng-Ching-Yu (IL), Chang-Hsu-Ku (IL), Wu-Chan (IL), Fukutomi (IL), Tsao-Chiu-Ku (IL), Yin-Yu-Tze (IL), Hsiao-Ko-Tzu (IL), Shuang-Chiang-Tsao-1 (IL), Wu-No-Tao (IL), Hung-No (IL), Yuan-Li (IL), Kaisentetsuchitsu (IL), Napatsupai S3 (IL), Pai-K’O-Yuan-Li (IL), Lu-Tao3036 (IL), Pakaikauneku (IL), Chin-Men-Tou-Men-Hung-Mi (IL), Pai-Mi-Fen (IL), O. rufipogon-21 (W).
PopV 14 Basmati 385 (IC), Taichung Sen 2 (IC), Taichung Sen 3 (IC), O. nivara-2 (W), O. nivara-5 (W), O. nivara-6 (W), O. nivara-7 (W), O. rufipogon-10 (W), O. rufipogon-12 (W), O. rufipogon-15 (W), O. rufipogon-16 (W), O. rufipogon-18 (W), O. rufipogon-19 (W), O. rufipogon-20 (W).
a
The abbreviations in brackets () next to accessions are JL for japonica landrace, JC for japonica cultivar, IL for indica landrace, IC for indica cultivar, and W for wild rice
370) admixed with PopIV were noted. Most of the 53 ac-cessions of PopIV, except Dular and O. rufipogon-21, were
indicalandraces. Two Taiwanese landraces (Pakaikauneku
and Pai-Mi-Fen) and a Chinese landrace (Chin-Men-Tou-Men-Hung-Mi) were admixed with PopII. PopV con-tained 11 wild rice accessions and 3 indica cultivars (Tai-chung Sen 2, Tai(Tai-chung Sen 3, and Basmati 385).
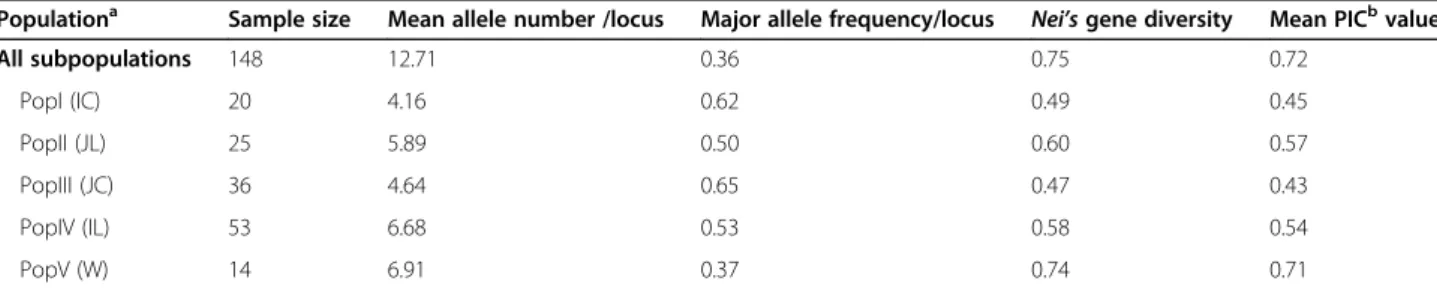
The genetic diversity of each subpopulation was evalu-ated by using 4 parameters, mean allele number per locus, major allele frequency per locus, gene diversity and PIC value (Table2). PopV, consisting of 11 wild rice and 3 indica cultivars, displayed the most diverse genetic background, revealed by the highest mean allele number (6.91), Nei’s gene diversity (0.74) and mean PIC value (0.71) but the lowest major allele frequency per locus (0.37) (Table2). On the other hand, PopIII, consisting of 29 japonica cultivars and 7 japonica landraces, ex-hibited the lowest genetic diversity. The genetic diver-sities of these five subpopulations were PopV, wild rices > PopII, japonica landraces > PopIV, indica landraces > PopI, indica cultivars > PopIV, japonica cultivars. While it was anticipated that landraces would be generally more diverse than cultivars, it was noteworthy that the genetic diversity of indica culti-vars was higher than that of japonica culticulti-vars, while the genetic diversity of indica landraces was slightly lower than that of japonica landraces albeit more indica accessions were assessed (Table 2).
Genetic Divergence in Asian Cultivated O. sativa
The genetic diversity of the 136 O. sativa accessions evaluated was relatively high as revealed by mean allele number (11.01), mean gene diversity (0.74) and mean
PIC (0.70) (Table 3). The highly diverse 83 landraces
contributed the majority of genetic variation in this panel. Genetic diversity, in general, was higher in the
indicathan the japonica group; however, japonica
land-races exhibited higher variation than indica landland-races. The genetic diversity of cultivars was relatively narrow as compared to landraces, and japonica cultivars had the least variation (Table3).
Relatively high FST values of 0.3084 and 0.3040 was
observed between indica and japonica in all O. sativa accessions and in landraces, respectively (Table 3). In modern breeding under intensified directional selection,
indica and japonica cultivars are even more diversified
from each other as revealed by the highest FST value
(0.4200). On the other hand, there was less divergence between cultivars and landraces both in indica and japonica.
Divergence of Taiwanese Rice Germplasm
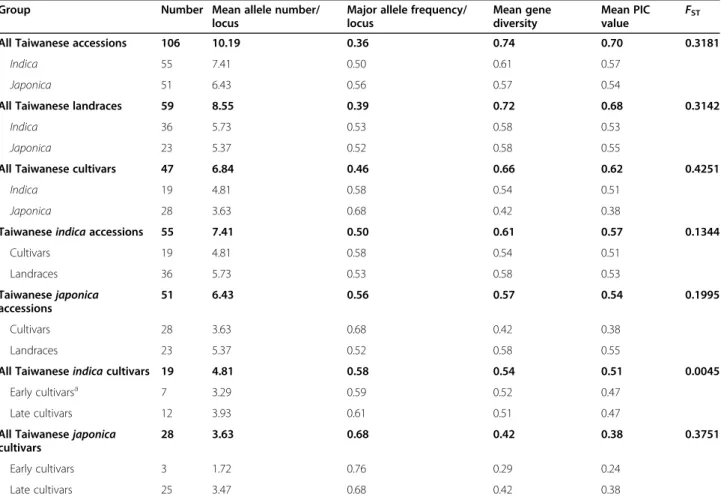
In the collection of 106 Taiwanese accessions, the gen-etic diversity of indica accessions was higher than that of japonica ones, and the genetic diversity of landraces was also higher than cultivars (Table 4). The genetic di-versity of indica landraces was not obviously different from japonica landraces; however, indica cultivars ex-hibited greater diversification than japonica cultivars. Taiwanese cultivars were divided into‘early’ cultivars or ‘late’ cultivars consistent with the government policy of rice breeding goals changing from yield (early) to pre-mium grain quality (late) in 1981. The 25 late japonica cultivars exhibited larger genetic diversity than 3 early japonica cultivars; nevertheless, the difference was not statistically significant difference between early and late indicacultivars (Table4).
For the Taiwanese accessions, great differentiation be-tween indica and japonica types was indicated by high
FST (0.3181), with Taiwanese landraces similar to this
overall trend (FST= 0.3142) but higher differentiation
be-tween indica and japonica cultivars (FST= 0.4251)
(Table4). Less differentiation between Taiwanese japon-icacultivars and landraces (FST= 0.1995) and indica
cul-tivars and landraces (FST= 0.1344) were observed. The
late indica cultivars were not differentiated from the early indica cultivars (FST= 0.0045); however, the late
ja-ponica cultivars were significantly differentiated from
the early japonica cultivars (FST= 0.3751). Relatedness Based on Genetic Distances
The 148 accessions could be separated into two groups corresponding to indica and japonica by 2-dimensional Table 2 Genetic diversity parameters of five subpopulations
Populationa Sample size Mean allele number /locus Major allele frequency/locus
Nei’s gene diversity Mean PICbvalue
All subpopulations 148 12.71 0.36 0.75 0.72 PopI (IC) 20 4.16 0.62 0.49 0.45 PopII (JL) 25 5.89 0.50 0.60 0.57 PopIII (JC) 36 4.64 0.65 0.47 0.43 PopIV (IL) 53 6.68 0.53 0.58 0.54 PopV (W) 14 6.91 0.37 0.74 0.71 a
The majority of accessions in PopI, PopII, PopIII, PopIV and PopV are indica cultivar, japonica landrace, japonica cultivar, indica landrace, and wild rice, respectively b
Table 3 Genetic diversity and divergence in O. sativa
Group Number Mean allele number/locus Major allele frequency/locus Mean gene diversity Mean PICavalue FST b All O. sativa 136 11.01 0.36 0.74 0.70 0.3084 Indica 75 8.31 0.50 0.61 0.58 Japonica 61 7.05 0.54 0.59 0.55 All landraces 83 9.57 0.39 0.72 0.69 0.3040 Indica 51 6.52 0.53 0.58 0.54 Japonica 32 6.25 0.50 0.60 0.57 All cultivars 53 7.28 0.44 0.68 0.64 0.4200 Indica 24 5.43 0.57 0.56 0.52 Japonica 29 3.65 0.68 0.43 0.39 All indica 75 8.31 0.50 0.61 0.58 0.1166 Cultivars 24 5.43 0.57 0.56 0.52 Landraces 51 6.52 0.53 0.58 0.54 All japonica 61 7.05 0.54 0.59 0.55 0.1913 Cultivars 29 3.65 0.68 0.43 0.39 Landraces 32 6.25 0.50 0.60 0.57 a
PIC is the abbreviation for polymorphism information content b
Fixation index (FST) indicates genetic differentiation between two subpopulations by the reduction of heterozygosity due to genetic drift and / or selection
Table 4 Genetic diversity and divergence in Taiwanese germplasm
Group Number Mean allele number/
locus
Major allele frequency/ locus Mean gene diversity Mean PIC value F ST
All Taiwanese accessions 106 10.19 0.36 0.74 0.70 0.3181
Indica 55 7.41 0.50 0.61 0.57
Japonica 51 6.43 0.56 0.57 0.54
All Taiwanese landraces 59 8.55 0.39 0.72 0.68 0.3142
Indica 36 5.73 0.53 0.58 0.53
Japonica 23 5.37 0.52 0.58 0.55
All Taiwanese cultivars 47 6.84 0.46 0.66 0.62 0.4251
Indica 19 4.81 0.58 0.54 0.51
Japonica 28 3.63 0.68 0.42 0.38
Taiwanese indica accessions 55 7.41 0.50 0.61 0.57 0.1344
Cultivars 19 4.81 0.58 0.54 0.51 Landraces 36 5.73 0.53 0.58 0.53 Taiwanese japonica accessions 51 6.43 0.56 0.57 0.54 0.1995 Cultivars 28 3.63 0.68 0.42 0.38 Landraces 23 5.37 0.52 0.58 0.55
All Taiwanese indica cultivars 19 4.81 0.58 0.54 0.51 0.0045
Early cultivarsa 7 3.29 0.59 0.52 0.47
Late cultivars 12 3.93 0.61 0.51 0.47
All Taiwanese japonica cultivars
28 3.63 0.68 0.42 0.38 0.3751
Early cultivars 3 1.72 0.76 0.29 0.24
Late cultivars 25 3.47 0.68 0.42 0.38
a
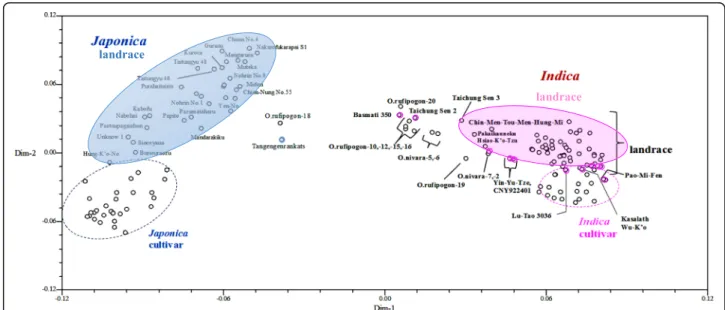
PCoA analysis, in which the first and the second dimen-sion explained 18.1% and 7.7% of variation, respectively (Fig. 1). Japonica accessions were distinct from indica accessions, and japonica accessions were distributed more sparsely than indica accessions. The indica cultivars could be distinguished from indica landraces by the third di-mension, accounting for 3.12% of variation (Additional file2: Figure S4). The cultivars were more closely aggregated than landraces for indica and japonica, indicating more similar genetic background. Three landraces (Tangengenrankatsu, Pakaikauneku and Hsiao-K’o-Tzu) and 2 cultivars (Taichung Sen 2 and Taichung Sen 3) were close to wild rice.
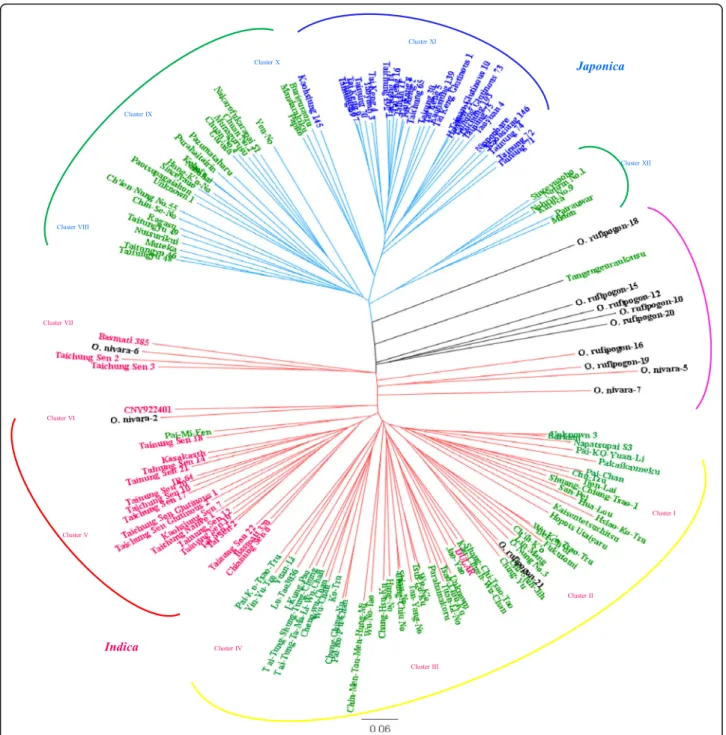
The unrooted phylogenetic tree according to Nei’s genetic distances revealed two distinct major clusters, indica and ja-ponica, indicated by red and blue branches, respectively (Fig.2). The indica cluster could be further subdivided into 7 clades, Clade I to VII, and the japonica cluster could be subdi-vided into 5 clades, Clade VIII to XII. The indica cultivars were grouped in Clade V and distinguished from the indica landraces, which formed one group with 4 distinct clades, Clade I– IV. Clades VIII, IX, X and XII were primarily com-prised of japonica landraces. On the other hand, japonica cul-tivars entirely constituted Clade XI, with the exception that Kaohsiung 145 was closely-related to three japonica landraces, Burieuraozu, Mandarakiku and Papito. These results show that the genetic background of modern indica and japonica cultivars have deviated from those of traditional landraces under intensive selection for breeding goals of high yield and premium grain quality. Finally, wild rice, indicated with a black branch, could not form a distinct group and fell into inter-mediate locations in the phylogenetic tree. One japonica land-race, Tangengenrankatsu, and four indica cultivars (Basmati
385, Taichung Sen 2, Taichung Sen 3, and CNY922401) also fell into intermediate locations allied with wild rice.
Discussion
Genetic Diversity Revealed by Assessment of Molecular Markers
Genetic diversity evaluated by molecular markers provides useful and fundamental information for crop improvement. Among an assortment of markers, SSRs exhibiting relatively high polymorphism level per locus provide rich allelic infor-mation for genetic diversity analysis. A currently-preferred phylogenetic relationship of rice germplasm was established by using SSRs that discerned 5 major groups– specifically, aus, indica, aromatic, temperate japonica and tropical japon-ica(Garris et al.2005). Phylogenetic trees of rice germplasm using genome-wide SNPs or structural variants were in ac-cordance with these 5 groups (Wang et al.2018; McNally et al.2009; Fuentes et al.2019). In the present study, a total of 953 alleles were detected by 75 polymorphic markers, varying from 3 to 37 alleles per locus with an average of 12.7 (Additional file 2: Figure S1, Additional file 1: Table S2), which was higher than other studies (Chakhonkaen et al.
2012; Jin et al.2010; Nachimuthu et al. 2015). PIC values, which are good indicators of marker polymorphism levels, were in the range of 0.18 to 0.95, with a mean of 0.72, higher than those reported in European (0.49), Chinese (0.42) and Indonesian (0.66) rice germplasm, respectively (Courtois et al.2013; Jin et al.2010; Thomson et al.2007). Moreover, 66 of 75 markers (88%) were considered highly informative with PIC values > 0.5 (Additional file 2: Figure S1). Thus, these 75 markers provided rich allelic information for genetic diversity analysis.
Fig. 1 Principle coordinate analysis (PCoA) of 148 rice accessions. After 2-dimension analysis of PCoA, the first and second dimension explained 18.1% and 7.7%, respectively. Each accession is indicated by a circle. Japonica and indica rices are distinctly separated, with japonica and indica landraces indicated by blue and magenta solid ellipses, and japonica and indica cultivars indicated by blue and magenta dashed ellipses, respectively
The Genetic Diversity and Differentiation in the Collected Rice Germplasm
The diversity panel of 148 accessions could be separated into two subpopulations according to STRUCTURE ana-lysis, clearly corresponding to indica and japonica groups (Additional file 2: Figure S3a). Further division into five subpopulations, indica cultivars, indica land-races, japonica cultivars, japonica landraces and wild
rice, were supported by K = 5 (Table1, Additional file2: Figure S3b). Most accessions were classified into the ex-pected groups according to the records of the National Plant Genetic Resource Center (NPGRC) Taiwan that were classified by plant and seed morphology, however some were incongruent due to admixed genetic back-ground. For example, landraces, Sinceyauo from Japan, Hung-K’o-No from China and Burieuraozu, Nabohai,
Fig. 2 Unrooted neighbor-joining tree of 148 rice accessions. Genetic distance was calculated according to Nei (1983) with the genotypes of 75 markers and cluster analysis by the neighbor-joining method. Japonica cultivars, indica cultivars, landrace and wild rices are indicated by blue, red, green and black, respectively. Cluster I-VII belong to indica sub-groups and Cluster VIII-XII belong to japonica sub-groups. Bar represents genetic distance
Paotsupagaiahon, and Tangengenrankatsu from Taiwan, were grouped with japonica cultivars (Pop III); an aus cultivar (Dular) and O. rufipogon-21 were assigned to the indica landrace group (Popn IV); and three indica cultivars, Basmati 385, Taichung Sen 2 and Taichung Sen 3, were placed in the wild rice group (PopV) (Table1). These accessions might still share identical by descent segments since derivation from common ances-tors. One possible factor contributing to such incongru-ous findings that cannot be neglected is introgression owing to gene flow among wild species, landraces, and cultivars (Ishikawa et al.2006; Wang et al.2018). For ex-ample, a mega variety TC65 inherited photoperiod-insensitive alleles of Heading date 1 (Hd1) and Early
heading date 1 (Ehd1) from two landraces, Muteke and
Nakabo, by spontaneous introgression of natural gene
flow during modern breeding (Wei et al. 2016). Indeed,
landraces have been commonly used in breeding pro-grams especially in the early purification breeding stage e.g. two old Taiwanese indica cultivars, Taichung Sen 2 and Taichung Sen 3, derived from landraces based on breeding records. Thus, admixed accessions are not ne-cessarily rare outcomes of natural introgression, but de-rive from intentional cross hybridization in at least some cases.
Morphology and genetic background are quite differ-ent between indica and japonica rice through
independ-ent origins, long-term adaptation to diverse
environments and selection for various human prefer-ences. The extent of genetic differentiation between
these two subspecies was revealed by FST analysis
(Ike-hashi 2009; Zhang et al. 2007). High genetic
differenti-ation (FST= 0.3084) was observed between indica and
japonica groups in our rice diversity panel (Table 3), in
agreement with several studies (Thomson et al. 2007;
Lin et al. 2012). The level of differentiation between
indica and japonica landraces (FST= 0.3040) was lower
than that between indica and japonica cultivars (FST=
0.4200). Landraces were selected by farmers for adapta-tion to local environments and various preferences; while modern cultivars result from intense directional selection for specific traits. Less differentiation in land-races than in cultivars was associated with different se-lection intensity.
The gene diversity of indica accessions was higher than that of japonica accessions since the bottleneck ef-fect was more severe in japonica rice during early
do-mestication (Kovach and McCouch 2008; Wang et al.
2018; Zhu et al. 2007). In the present study, genetic di-versity was much lower in japonica than indica popula-tions as well (Fig. 1, Table 3), the same tendency as in previous studies using Taiwan breeding germplasm and a collection from Borneo Island (Lin et al. 2012; Thom-son et al.2009). Nevertheless, in our collection the level
of diversity of japonica landraces was higher than that of
indica landraces (Table 3) because the former included
both upland and lowland accessions. Unveiling Taiwanese Rice Germplasm
Today, indigenous peoples still cultivate their own land-races with unique traits, such as large grain and aroma, on upland fields in Taiwan. The cultivation of rice, ac-companied by foxtail millet, can be dated back to 5000 years ago by unearthed grain remains from some arch-aeological sites in Tainan Science Park, southern Taiwan
(Tsang 2012). Approximately 98% and 83% of the
exca-vated carbonized rice grains from the Tapenkeng Cul-ture period (4800–4200 B.P.) and Niuchoutzu CulCul-ture period (3800–3300 B.P), respectively, were classified as
japonica rice according to grain morphology (Tsang
2012; Wang2007).
In the present study, 17 landraces labeled with ‘#’ in the Additional file1: Table S1, were grouped in Clusters VIII, IX, X and XII which belong to the japonica clade (Fig.2). These indigenous landraces were genetically dis-tinct from modern temperate japonica cultivars, Cluster XI (Fig.2), and presumed to belong to tropical japonica rice (javanica). The upland landrace, Tangengenran-katsu, has admixed genetic background and is genetically close to O. rufipogon-18. Only few indigenous landraces were clustered in indica clades, albeit some were classi-fied as japonica rice by morphology according to NPGR C records, such as Pakaikauneku, Kaisentetsuchitsu, Napatsupa S3, and Baridon (Additional file 1: Table S1, Fig. 2). Tropical japonica, diverged from temperate ja-ponica, is thought to have originated in the upper Thai-Malay Peninsula and might have moved from the Thai-Malay
Archipelago northward through Indonesia, the
Philippines, Taiwan, Ryukyus, and Japan (Chang 1976;
Gutaker et al. 2020). Thus, Taiwan was on the dispersal route of tropical japonica and 2/3 of carbonized rice grains unearthed from remains of Niaosung Culture (1400–1000 B.P.) had grain length larger than 4 mm
which resembled tropical japonica (Wang 2007). In
ac-cordance with archaeobotanical evidence, phylogenetic analysis of SSR genotypes classified indigenous upland landraces as tropical japonica (Fig.2).
In the indica clusters, only 6 accessions were recorded with indigenous language pronunciations, including 5 (Baridon, Napatsupai S3, Pakaikameku, Kaisentetsu-chitsu, Hopot Utatyaru) in cluster I and Parahainakoru in cluster III (Fig. 2). These 6 indica landraces might have been preserved and cultivated by indigenous people for thousands of years, however archaeobotanical evi-dence is lacking. We cannot rule out that these indica landraces were adopted by indigenous people only hun-dreds of years ago, after Chinese introduced much
Taiwan and China and showed no significant isolation-by-distance (Fig. 2). However, the indica landraces were divided into two large clades, Cluster I & II and Cluster III & IV, which might reflect two origins, Guangdong and Fu-jian. The genetic diversity of indica landraces in Taiwan is relatively high (Tables 3, 4) which might result from in-trinsic high variation in indica rice and multiple origins as well. Taiwanese indica cultivars, closer to IR64 than Dular, an Aus cultivar in India (Fig. 2), might be resulted from modern breeding that 14 of 17 Taiwanese indica cultivars can be traced back to IRRI accessions or DGWG as their breeding parents (Lu and Lu2010).
Landraces, intermediate between wild relatives and cul-tivars, are important genetic reservoir for crop improve-ment to cope with climate changes and increase sustainability. In Taiwan, 16 officially acknowledged indi-genous peoples have their own cultures and diet prefer-ences, including diversified crop germplasm. Taiwanese rice landraces compromised of tropical japonica and indica rice revealed diverse genetic variation in plant
architecture and seeds (Hsieh et al. 2011) and herein
showed much SSR diversity (Tables3,4). This high gen-etic variation indicates that Taiwanese landraces are a res-ervoir of genetic diversity and beneficial genes/alleles for rice breeding and improvement. Taiwanese landraces have had great impact on modern rice breeding not only in Taiwan but also elsewhere in the world. According to the database of rice breeding pedigrees (Taiwan Rice Informa-tion System, TRIS), Taiwanese landraces were commonly used to introgress useful genes for rice improvement, es-pecially in the early breeding programs a half-century ago. The most prominent varieties, japonica TC65 with photoperiod insensitivity and indica variety Taichung Native 1 (TCN1) with semi-dwarf stature, have had great impact on rice breeding and research. Because photoperiod insensitivity was a highly desired trait, TC65 had been extensively applied in modern rice breeding programs, leading to all current Taiwanese
temperate japonica cultivars inheriting the ehd1 and
hd1 alleles. Taiwanese temperate japonica cultivars can
be cultivated in two crop seasons under tropical and subtropical environments, making Taiwan the south-ernmost region of temperate japonica cultivation. The
indicavariety TCN1 inherited null function of sd1 with
a 383-bp deletion from the landrace DGWG (Sasaki et al.2002), and, this DGWG allele has been widely ap-plied to improve grain yield of both indica and japonica varieties in the past 50 years (Asano et al. 2011; Zhao et al. 2010). Yet, there are still numerous useful genes/ alleles existing in the genetic reservoir of Taiwanese landraces, for example conferring large grain size, aroma, and biotic and abiotic resistance. Untapped beneficial genes from landraces can help to breed new varieties for resilient and sustainable agriculture.
Modern cultivars are a result of intensive directional selection for specific traits which are frequently deter-mined by government policy and demands of markets. In Taiwan, the major dining staple was indica rice before War World II but changed to temperate japonica rice because of government policy during Japanese occupa-tion. Now, japonica rice is for dining; while indica rice is used for various food processing needs, such rice noo-dles, pudding, and cakes. Thus, japonica and indica im-provement have different breeding goals. For indica rice, high yield with resistances to biotic and abiotic stresses are breeding goals; thus, diverse germplasm from land-races or introduced from other countries are commonly utilized as donor parents (Lin et al. 2012). Therefore, there was no obvious difference in genetic diversity and differentiation between early and late indica cultivars (FST= 0.0045, Table 4). On the other hand, the breeding goal of japonica rice was changed from high yield to pre-mium grain quality that the germplasm used for improv-ing different traits seemed to be associated with high differentiation between early and late japonica cultivars, FST= 0.3751 (Table4). In order to improve grain quality, a few Japanese elite temperate japonica cultivars were intro-duced and used extensively in recurrent breeding crosses (Lin et al. 2012). This led to modern Taiwanese japonica cultivars being grouped at the same clade, Cluster XI with the Japanese elite cultivar, Nipponbare (Fig.2), as japonica varieties from Taiwan and Japan did not differ significantly in the pattern of genetic diversity (Lin et al. 2012). The genetic distances between any two Taiwanese japonica cultivars were in the range of 0.43–0.58 (Fig. 2); conse-quently, the gene pool of japonica cultivars is relative nar-row as compared to either japonica landraces or modern indica cultivars (Tables3, 4), resulting in genetic vulner-ability in rice cultivation and management.
To overcome severe genetic vulnerability of temperate ja-ponica cultivars, wild relatives and indica rice were intro-duced to breeding programs. For example, japonica Tainung 67 was the descent of a cross of japonica Tainung 61 and O. rufipogon, and japonica Taichung 192 was an indica/japon-ica-crossed variety (Lu and Lu 2010). Recently, numerous advanced breeding lines introduced from IRRI and wild rela-tives have been used in breeding programs to improve biotic and abiotic stresses for sustainable agriculture, e. g. IRBB66 pyramided with 5 bacterial blight resistant genes (Yap et al.
2016). Thus, current rice breeding goals in Taiwan
emphasize grain quality first, followed by other traits such as resistances and multi-dimensional utilizations (forage and landscape). To achieve various goals, germplasm for breeding are not limited to the domestics but also exotics.
Conclusions
A diversity panel of 148 rice accessions, including 47 cultivars and 59 landraces from Taiwan and 42
accessions from other countries, could be grouped into five major subpopulations: wild rices, japonica landraces, indicalandraces, indica cultivars, and japonica cultivars. The genetic diversities, without exception, were wild rices > landraces > cultivars, and indica rice > japonica rice. The majority of Taiwanese japonica landraces pre-served by indigenous peoples were classified as tropical ja-ponicaby morphology and phylogenetic analysis, consistent with archaeobotanical evidence. Thus, japonica landraces had greater genetic variation than indica landraces. The Taiwanese indica landraces could be separated into two clusters on phylogenetic trees, reflecting two sets of intro-ductions from China. The genetic variation and divergence of modern cultivars are largely influenced by government policies and market demands, exemplified by premium grain quality for japonica rice, and yield and resistances for indica rice. Large genetic diversification was unveiled in Taiwanese landraces, as well as intermediate and admixed genetic background, providing a precious and valuable gen-etic reservoir for rice breeding in the future.
Materials and Methods Plant Materials
A diversity panel of 148 rice accessions, including 136 O. sativa, 4 O. nivara, and 8 O. rufipogon, were analyzed in this study. The germplasm originated from Taiwan, Japan, and China or was introduced from the Inter-national Rice Research Institute (IRRI), and was obtained from the National Plant Genetic Resource Center (NPGRC), Taiwan. These germplasms were propagated and used for rice improvement by rice breeders, Dr. Chih-Shen Sheu in Taichung District Agricultural Re-search and Extension Station and Dr. Yong-pei Wu in Chiayi Agricultural Experiment Branch, Taiwan Agricul-tural Research Institute. Each accession denoted indica or japonicaand cultivars or landraces was in accordance with the record in NPGRC based on the classification according to morphology and collection sites. For cultivars, there were 29 japonica varieties including 28 Taiwanese and 1 Japa-nese cultivar and 24 indica varieties including 19 Taiwan-ese, 1 Pakistani and 3 Indian cultivars, and IR64. For landraces, there were 59, 18, and 6 accessions from Taiwan, China and Japan, respectively. For the 59 Taiwanese land-races, 23 accessions were recorded with pronunciations of indigenous languages, 36 with Chinese characters, and 3 la-beled as Unknown, Unknown 1 and Unknown 3, respect-ively. The 12 wild rices were collected from China, Bangladesh and Laos (Additional file1: Table S1).
DNA Extraction and Genotyping Assay
Genomic DNA was extracted from leaf tissues of rice seedlings at the three-leaf stage as described previously (Lin et al.2012). A total of 75 markers including 49
pub-lished SSRs (McCouch et al. 2002), 6 STSs (Wu et al.
2010), and 20 newly-designed SSRs and indels
(Add-itional file1: Table 2) distributed across the rice genome were applied for genotyping assay.
Among the 75 markers, 56 were analyzed with a QIAxcl System -GT12™ Genetic Analyzer (Qiagen, USA). The PCR reaction was in a total volume of 15μL containing 30 ng genomic DNA, 0.3 nmol/μL forward and reverse primer
each, and 8μL Taq DNA Polymerase Master Mix
(Ampli-qon, Denmark). Amplification was performed on a thermo-cycler (Model T1, Biometra, Germany) with the following thermal profile: 94 °C for 3 min for 1 cycle; 94 °C for 40 s, 55 °C for 40 s, 72 °C for 40 s, for 35 cycles; 72 °C for 3 min for 1 cycle. Amplicons were resolved by QIAxcel DNA High Resolution Kit (1200) with QX size marker 25–450 and QX alignment marker 15 bp/500 bp (Qiagen, USA). The other 19 markers were assessed on an ABI 3730 DNA Analyzer (Applied BioSystems, USA). PCR reactions were set in a
total volume of 20μL containing 20 ng of genomic DNA,
10 pmol/μL of primer labeled with a fluorescent dye, 2 μL
of 10× PCR buffer, 2μL of 2.5 nmol/μL dNTPs, 1.5 μL of
5 U/μL Amplitaq Gold® DNA polymerase (Applied
Biosys-tems, USA), and 2μL of 1 mol/L betaine. Amplifications
were performed with the following thermal profile: 94 °C for 5 min for 1 cycle; 95 °C for 30 s, 55 °C for 55 s, 72 °C for 35 s, for 35 cycles; and 72 °C for 1 min for 1 cycle. DNA fragment analysis of amplified products were carried out by using an ABI 3730 DNA Analyzer with ABI GeneS-can™ -600 LIZ™ Size Standard following the manufac-turer’s instructions (Applied BioSystems, USA).
Data Analysis
To evaluate genetic relatedness of these 148 accessions, geno-types of 75 markers were subjected to genetic diversity, popu-lation structure simupopu-lation, principle coordinate analysis (PCoA), and phylogenetic analysis. Five genetic diversity pa-rameters including mean allele number per locus, major allele frequency per locus, Nei’s gene diversity, mean polymorphic information content (PIC), and fixation index (FST) were assessed by using PowerMarker V3.25 (Liu and Muse2005).
Population structures of 148 accessions were analyzed by STRUCTURE V 2.3.3, a Bayesian model-based ap-proach (Pritchard et al. 2000). Simulation was performed under the admixture model with 100,000 burn-in itera-tions of Markov Chain Monte Carlo (MCMC) for K values set from 1 to 11, andΔK (an ad hoc quantity) was used to determine subpopulation number (Evanno et al.2005).
The genetic distance of similarity matrix was calcu-lated using modified Rogers’ distance (Goodman and
Stuber 1983). The genetic distances were consequently
subjected to two-dimension principle coordinate analysis
with Decnter and Eigene modules (Rohlf1987) and used
in construction of an unrooted phylogenetic tree by neighbor-joining in PowerMarker V3.25 (Liu and Muse
Additional Files
Additional file 1: Table S1. Name, type, subspecies/species and origin of 148 rice accessions used in this study. Table S2. Chromosomal position, locus name and PIC value of 75 SSR marker used for this study. Additional file 2: Figure S1. The frequency distribution of allele number and polymorphic information content (PIC) with 75 molecular markers. (A) Allele number per locus ranges from 3 to 37 with an average of 12.7. (B) PIC ranges from 0.18 to 0.95 with an average of 0.72. Figure S2. (a) Structure simulation analysis to determine best K. (A) LnP(D), the log likelihood for each K, was calculated by 100,000 permutations and mean LnP(D) value was taken from 10 replications.ΔK, an ad hoc quantity, is transferred by mean LnP(D) value andΔK of 148 accessions. (B) LnP(D) value andΔK of 86 indica accessions. (C) LnP(D) value and ΔK of 86 japonica accessions. Figure S3. Population structure analysis of 148 accessions. Each individual is indicated by a vertical bar. (A) For K = 2, pop2–1 and pop2–2, indicated by red and green, are composed of japonica and indica rice, respectively. (B) For K = 5, pop5–1, pop5–2, pop5–3, pop5–4 and pop5–5, indicated by red, green, blue, yellow and magenta, are composed of indica cultivar, japonica landrace, japonica cultivar, indica landraces and wild rices, respectively. The numbers of accessions in each subpopulation are indicated in brackets (). Figure S4. Three-dimensional plot from principle coordinate analysis of 148 rice ac-cessions. Japonica and indica are separated on opposite sides. Japonica and indica cultivars are marked with circles.
Abbreviations
IRRI:International Rice Research Institute; SNP: Single nucleotide polymorphism; DGWG: Dee-Geo-Woo-Gen; PCoA: Principal coordinate analysis; SSR: Simple sequence repeat; STS: Sequence-tagged site; indel: Insertion/deletion; PIC: Polymorphic information content; Pop: Population; NPGRC: National Plant Genetic Resource Center; TC65: Taichung 65; TCN1: Taichung Native 1
Acknowledgements
The authors are thankful to Dr. Chih-Shen Sheu for providing some germ-plasm. We also appreciate the services of Professor Hawkeye, LLC for critical review and English editing.
Authors’ Contributions
AH and YL designed and supervised the research; YW prepared genetic materials; WH and SC conducted genotype assay and analyzed data; AH, WH, and YL wrote the manuscript. AH, HC, and YL edited and revised the manuscript. All authors read and approved the final manuscript. Funding
This work was supported by the National Science Council (NSC 98 –2324-B-002-001) of Taiwan.
Availability of Data and Materials
Dataset and figures supporting the results are included as additional files. Ethics Approval and Consent to Participate
Not applicable. Consent for Publication
All authors have provided consent for publication. Competing Interests
The authors declare that they have no competing interests. Author details
1Department of Life Science, Fu-Jen Catholic University, New Taipei City
242062, Taiwan.2Department of Agronomy, National Taiwan University, Taipei 10617, Taiwan.3Department of Agronomy, Chiayi Agricultural
Experiment Branch, Taiwan Agricultural Research Institute, Chiayi 600015, Taiwan.
Received: 17 July 2020 Accepted: 6 December 2020
References
Asano K, Yamasaki M, Takuno S, Miura K, Katagiri S, Ito T, Doi K, Wu J, Ebana K, Matsumoto T, Innan H, Kitano H, Ashikari M, Matsuoka M (2011) Artificial selection for a green revolution gene during japonica rice domestication. Proc Natl Acad Sci U.S.A 108(27):11034–11039.https://doi.org/10.1073/pnas. 1019490108
Caicedo AL, Williamson SH, Hernandez RD, Boyko A, Fledel-Alon A, York TL, Polato NR, Olsen KM, Nielsen R, McCouch SR, Bustamante CD, Purugganan MD (2007) Genome-wide patterns of nucleotide polymorphism in domesticated rice. PLoS Genet 3(9):1745–1756.https://doi.org/10.1371/ journal.pgen.0030163
Chakhonkaen S, Pitnjam K, Saisuk W, Ukoskit K, Muangprom A (2012) Genetic structure of Thai rice and rice accessions obtained from the international rice research institute. Rice 5(1):19.https://doi.org/10.1186/1939-8433-5-19
Chang TT (1976) The origin, evolution, cultivation, dissemnination, and diversificaiton of Asian and Africa rice. Euphytica 25:425–441 Chin HS, Wu YP, Hour AL, Hong CY, Lin YR (2016) Genetic and evolutionary
analysis of purple leaf sheath in rice. Rice 9(1):8.https://doi.org/10.1186/ s12284-016-0080-y
Choi JY, Platts AE, Fuller DQ, Hsing YL, Wing RA, Purugganan MD (2017) The rice paradox: multiple origins but single domestication in Asian rice. Mol Biol Evol 34(4):969–979.https://doi.org/10.1093/molbev/msx049
Choi JY, Purugganan MD (2018) Multiple origin but single domestication led to Oryza sativa. G3 (Bethesda) 8(3):797–803.https://doi.org/10.1534/g3.117. 300334
Chou SL (1948) China is the place of origin of rice. J Rice Soc China 7:53–54 (in Chinese)
Courtois B, Audebert A, Dardou A, Roques S, Ghneim-Herrera T, Droc G, Frouin J, Rouan L, Gozé E, Kilian A, Ahmadi N, Dingkuhn M (2013) Genome-wide association mapping of root traits in a japonica rice panel. PLoS One 8(11): e78037.https://doi.org/10.1371/journal.pone.0078037
Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18(8):926–936.https://doi.org/10.1101/gad.1189604
Dwivedi SL, Ceccarelli S, Blair MW, Upadhyaya HD, Are AK, Ortiz R (2016) Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci 21(1):31–42.https://doi.org/10.1016/j.tplants.2015.10.012
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14(8): 2611–2620.https://doi.org/10.1111/j.1365-294X.2005.02553.x
Evenson RE, Gollin D (2003) Assessing the impact of the green revolution, 1960 to 2000. Science 300(5620):758–762.https://doi.org/10.1126/science.1078710
Fuentes RR, Chebotarov D, Duitama J, Smith S, De la Hoz JF, Mohiyuddin M, Wing RA, McNally KL, Tatarinova T, Grigoriev A, Mauleon R, Alexandrov N (2019) Structural variants in 3000 rice genomes. Genome Res 29(5):870–880.
https://doi.org/10.1101/gr.241240.118
Garris AJ, Tai TH, Coburn J, Kresovich S, McCouch S (2005) Genetic structure and diversity in Oryza sativa L. Genetics 169(3):1631–1638.https://doi.org/10.1534/ genetics.104.035642
Goodman MM, Stuber CW (1983) Races of maize: VI. Isozyme variation among races of maize in Bolivia Maydica 28:169–187
Gutaker RM, Groen SC, Bellis ES, Choi JY, Pires IS, Bocinsky RK, Slayton ER, Wilkins O, Castillo CC, Negrao S, Oliveira MM, Fuller DQ, Guedes JAD, Lasky JR, Purugganan MD (2020) Genomic history and ecology of the geographic spread of rice. Nat Plants 6(5):492–502. https://doi.org/10.1038/s41477-020-0659-6
Hsieh JS, Hsing YI, Hsu TF, Li JK, Li KT, Tsang CH (2011) Studies on ancient rice—where botanists, agronomists, archeologists, linguists, and ethnologists meet. Rice 4(3–4):178–183.https://doi.org/10.1007/s12284-011-9075-x
Hu CW (1993) Historical review of semidwarf Rices and breeding of a new plant type for sustainable agriculture. Res Bull Taichung Dist Agric Improv Stn 38: 45–63
Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z, Li M, Fan D, Guo Y, Wang A, Wang L, Deng L, Li W, Lu Y, Weng Q, Liu K, Huang T, Zhou T, Jing Y, Li W, Lin Z, Buckler ES, Qian Q, Zhang QF, Li J, Han B (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42(11):961–967.https://doi.org/10.1038/ng.695
Ikehashi H (2009) Why are there indica type and japonica type in rice?— history of the studies and a view for origin of two types. Rice Sci 16:1–13.https:// doi.org/10.1016/S1672-6308(08)60050-5
Ishikawa R, Yamanaka S, Fukuta Y, Chitrakon S, Bounphanousay C, Kanyavong K, Tang LH, Nakamura I, Sato T, Sato YI (2006) Genetic erosion from modern varieties into traditional upland rice cultivars (Oryza sativa L.) in northern Thailand. Genet Resour Crop Evol 53:245–252.https://doi.org/10.1007/ s10722-004-6132-y
Iso E (1964) Talks on Horai rice. Amayomikai, Yamakuchi, p 89 in Japanese Jin L, Lu Y, Xiao P, Sun M, Corke H, Bao J (2010) Genetic diversity and population
structure of a diverse set of rice germplasm for association mapping. Theor Appl Genet 121(3):475–487.https://doi.org/10.1007/s00122-010-1324-7
Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35(1–2):25–34
Kovach MJ, McCouch SR (2008) Leveraging natural diversity: back through the bottleneck. Curr Opin Plant Biol 11(2):193–200.https://doi.org/10.1016/j.pbi. 2007.12.006
Li JY, Wang J, Zeigler RS (2014) The 3,000 rice genomes project: new
opportunities and challenges for future rice research. Gigascience 3:8.https:// doi.org/10.1186/2047-217X-3-8
Lin HY, Wu YP, Hour AL, Ho SW, Wei FJ, Hsing YC, Lin YR (2012) Genetic diversity of rice germplasm used in Taiwan breeding programs. Bot Stud 53:363–376 Lin MS (1991) Field uniformity of the japonica rice region of Taiwan as estimated
by relative genetic contribution. Theor Appl Genet 83:115–118 Liu K, Muse SV (2005) PowerMarker: an integrated analysis environment for
genetic marker analysis. Bioinformatics 21(9):2128–2129.https://doi.org/10. 1093/bioinformatics/bti282
Londo JP, Chiang YC, Hung KH, Chiang TY, Schaal BA (2006) Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc Natl Acad Sci U.S.A 103(25):9578–9583.
https://doi.org/10.1073/pnas.0603152103
Lu CT, Lu HY (2010) Establishment and application of Taiwan rice information system. J Taiwan Agric Res 59:61–69 (Chinese with English abstract) McCouch SR, Teytelman L, Xu YB, Lobos KB, Clare K, Walton M, Fu BY, Maghirang
R, Li ZK, Xing YZ, Zhang QF, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9(6): 199–207.https://doi.org/10.1093/dnares/9.6.199
McNally KL, Childs KL, Bohnert R, Davidson RM, Zhao K, Ulat VJ, Zeller G, Clark RM, Hoen DR, Bureau TE, Stokowski R, Ballinger DG, Frazer KA, Cox DR, Padhukasahasram B, Bustamante CD, Weigel D, Mackill DJ, Bruskiewich RM, Ratsch G, Buell CR, Leung H, Leach JE (2009) Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc Natl Acad Sci U.S.A 106(30):12273–12278.https://doi.org/10.1073/pnas.0900992106
Nachimuthu VV, Muthurajan R, Duraialaguraja S, Sivakami R, Pandian BA, Ponniah G, Gunasekaran K, Swaminathan M, Suji KK, Sabariappan R (2015) Analysis of population structure and genetic diversity in rice germplasm using SSR markers: an initiative towards association mapping of agronomic traits in Oryza sativa. Rice 8(1):30.https://doi.org/10.1186/s12284-015-0062-5
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Pusadee T, Jamjod S, Chiang YC, Rerkasem B, Schaal BA (2009) Genetic structure and isolation by distance in a landrace of Thai rice. Proc Natl Acad Sci U.S.A 106(33):13880–13885.https://doi.org/10.1073/pnas.0906720106
Rohlf F (1987) NTSYS-pc: microcomputer programs for numerical taxonomy and multivariate analysis. Am Stat 41:330.https://doi.org/10.2307/2684761
Sang T, Ge S (2013) Understanding rice domestication and implications for cultivar improvement. Curr Opin Plant Biol 16(2):139–146.https://doi.org/10. 1016/j.pbi.2013.03.003
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416(6882):701– 702.https://doi.org/10.1038/416701a
Thomson MJ, Polato NR, Prasetiyono J, Trijatmiko KR, Silitonga TS, McCouch SR (2009) Genetic diversity of isolated populations of indonesian landraces of rice (Oryza sativa L.) collected in East Kalimantan on the island of Borneo. Rice 2(1):80–92.https://doi.org/10.1007/s12284-009-9023-1
Thomson MJ, Septiningsih EM, Suwardjo F, Santoso TJ, Silitonga TS, McCouch SR (2007) Genetic diversity analysis of traditional and improved Indonesian rice (Oryza sativa L.) germplasm using microsatellite markers. Theor Appl Genet 114(3):559–568.https://doi.org/10.1007/s00122-006-0457-1
Tsang CH (2012) Issues relating to the ancient rice and millet grains unearthed from the archaeological sites in Tainan Science Park. J Chin Dietary Culture 8: 1–14 (Chinese with English abtract)
Wang W, Mauleon R, Hu Z, Chebotarov D, Tai S, Wu Z, Li M, Zheng T, Fuentes RR, Zhang F, Mansueto L, Copetti D, Sanciangco M, Palis KC, Xu J, Sun C, Fu B, Zhang H, Gao Y, Zhao X, Shen F, Cui X, Yu H, Li Z, Chen M, Detras J, Zhou Y, Zhang X, Zhao Y, Kudrna D, Wang C, Li R, Jia B, Lu J, He X, Dong Z, Xu J, Li Y, Wang M, Shi J, Li J, Zhang D, Lee S, Hu W, Poliakov A, Dubchak I, Ulat VJ, Borja FN, Mendoza JR, Ali J, Li J, Gao Q, Niu Y, Yue Z, Naredo MEB, Talag J, Wang X, Li J, Fang X, Yin Y, Glaszmann JC, Zhang J, Li J, Hamilton RS, Wing RA, Ruan J, Zhang G, Wei C, Alexandrov N, McNally KL, Li Z, Leung H (2018) Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557(7703):43–49.https://doi.org/10.1038/s41586-018-0063-9
Wang YH (2007) The preliminary notes on the ancient rice grains excavated in Taiwan. Master thesis. Department of Agronomy, National Taiwan University, Taipei, Taiwan. (Chinese with English abstract)
Wei FJ, Tsai YC, Wu HP, Huang LT, Chen YC, Chen YF, Wu CC, Tseng YT, Hsing YC (2016) Both Hd1 and Ehd1 are important for artificial selection of flowering time in cultivated rice. Plant Sci 242:187–194.https://doi.org/10.1016/j.plantsci.2015.09.005
Wu YP, Ko PY, Lee WC, Wei FJ, Kuo SC, Ho SW, Hour AL, Hsing YI, Lin YR (2010) Comparative analyses of linkage maps and segregation distortion of two F2
populations derived from japonica crossed with indica rice. Hereditas 147(5): 225–236.https://doi.org/10.1111/j.1601-5223.2010.02120.x
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the arabidopsis flowering time gene CONSTANS. Plant Cell 12(12):2473–2483.
https://doi.org/10.1105/tpc.12.12.2473
Yap R, Hsu YC, Wu YP, Lin YR, Kuo CW (2016) Multiplex PCR genotyping for five bacterial blight resistance genes applied to marker-assisted selection in rice (Oryza sativa). Plant Breed 135:309–317
Zhang D, Zhang H, Wang M, Sun J, Qi Y, Wang F, Wei X, Han L, Wang X, Li Z (2009) Genetic structure and differentiation of Oryza sativa L. in China revealed by microsatellites. Theor Appl Genet 119(6):1105–1117.https://doi. org/10.1007/s00122-009-1112-4
Zhang H, Sun J, Wang M, Liao D, Zeng Y, Shen S, Yu P, Mu P, Wang X, Li Z (2007) Genetic structure and phylogeography of rice landraces in Yunnan, China, revealed by SSR. Genome 50(1):72–83.https://doi.org/10.1139/g06-130
Zhao KY, Wright M, Kimball J, Eizenga G, McClung A, Kovach M, Tyagi W, Ali ML, Tung CW, Reynolds A, Bustamante CD, McCouch SR (2010) Genomic diversity and introgression in O. sativa reveal the impact of domestication and breeding on the rice genome. PLoS One 5(5):e10780
Zhao Q, Feng Q, Lu H, Li Y, Wang A, Tian Q, Zhan Q, Lu Y, Zhang L, Huang T, Wang Y, Fan D, Zhao Y, Wang Z, Zhou C, Chen J, Zhu C, Li W, Weng Q, Xu Q, Wang ZX, Wei X, Han B, Huang X (2018) Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat Genet 50(2): 278–284.https://doi.org/10.1038/s41588-018-0041-z
Zhu Q, Zheng X, Luo J, Gaut BS, Ge S (2007) Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: severe bottleneck during domestication of rice. Mol Biol Evol 24(3):875–888.https://doi.org/10.1093/molbev/msm005
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.