行政院國家科學委員會專題研究計畫 成果報告
(子計畫二)長期追蹤嚴重急性呼吸道症候群病患之肺部機
能後遺症
計畫類別: 整合型計畫 計畫編號: NSC92-3112-B-002-042- 執行期間: 92 年 05 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學醫學院內科 計畫主持人: 余忠仁 共同主持人: 張允中,吳惠東 報告類型: 完整報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 12 月 14 日
行政院國家科學委員會補助專題研究計畫
■ 成 果 報 告
□期中進度報告
台灣地區「嚴重性呼吸道症候群」之臨床研究-
(子計畫二)長期追蹤嚴重急性呼吸道症候群病患之肺部機能後遺症
計畫類別:□ 個別型計畫 ■整合型計畫
計畫編號:NSC92-3112-B-002-042-
執行期間: 92 年 1 月 1 日至 93 年 7 月 31 日
計畫主持人:余忠仁
共同主持人:張允中、吳惠東
計畫參與人員:
成果報告類型(依經費核定清單規定繳交):□精簡報告 ■完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:國立台灣大學醫學院內科
中 華 民 國 93 年 12 月 14 日
中文摘要
關鍵字:嚴重急呼吸道症候群、急性呼吸窘迫症候群、高解像力電腦斷層檢查、肺功能檢 查、一氧化碳瀰漫量 在 2003 年 2 月至 8 月間,全球共有 8096 位嚴重急呼吸道症候群(SARS)病患,774 位死 亡,死亡率為 9.6%。在台灣,346 位病患中,37 位死於 SARS,大多數因發生急性呼吸窘 迫症候群(ARDS)而呼吸衰竭。研究顯示,ARDS 的存活者,由於經歷了嚴重的疾病過程, 產生了長期在軀體上與神經心理上的後遺症,造成肺部與肺外之病態。本研究計畫以定期 之肺功能檢查與高解像力電腦斷層檢查 SARS 病患之肺部變化。由於吾人對於 SARS 的臨 床經驗仍在累積中,精確的評估 SARS 的長期肺部機能之變化有助於臨床醫師對於治療此 一疾病之思考。 本計畫自 92 年 4 月執行至 93 年 7 月,本院共照顧 76 位嚴重呼吸道症候群之可能病患,15 位死亡。40 位於發病後 51.8±20.2 天接受第一次高解像力電腦斷層檢查,37 位接受肺功能 檢查;發病後 140.7±26.7 天,19 位接受第二次高解像力電腦斷層檢查,22 位接受第二次肺 功能檢查。第一次高解像力電腦斷層檢查顯示多數病例肺部影像仍有明顯變化(airtrapping,92.5%;ground-glass opacity,90%;reticulation,70%;parenchymal band,55%; bronchiectasis,17.5%; consolidation,10%;honeycombing,7.5%)。 發生 ARDS 之 SARS 病患其肺部變化明顯較嚴重,尤其是 ground-glass opacity(GGO)的嚴重度。肺功能檢查 有 12 位(37%)病患有囿限性通氣病變,其餘之肺功能檢查為正常。發生 ARDS 之 SARS 病 患其肺功能明顯較差。接受第二次檢查之病患,高解像力電腦斷層檢查與肺功能檢查均呈 現明顯進步,包括電腦斷層影像之 GGO 嚴重度由 8.68±6.96 分降至 4.42±5.14, (p < 0.0001) 與纖維化嚴重度由 5.79±6.13 降至 3.05±5.80,(p < 0.0001),肺功能檢查 FVC (%預測值) 由 71.3 ± 23.4 上升至 98.1 ± 19.6%,(p = 0.004),而 FEV1 (%預測值) 由 73.9 ± 21.0 上升至 96.5 ± 17.9%,(p = 0.005)。即使是發生 ARDS 之 SARS 病患其第二次電腦斷層檢查與肺功 能檢查也都有明顯進步,但仍有 50%仍有囿限性通氣病變。肺部之一氧化碳瀰漫量(DLco) 變化與電腦斷層檢查之纖維化嚴重度成明顯負向相關。本觀察研究顯示嚴重急性呼吸道症 候群之肺部傷害之預後可能較原先預期為良好,而肺功能檢查之 DLco 值可作為肺部纖維 化嚴重度之參考。
Abstract
Keywords: severe acute respiratory syndrome, acute respiratory distress syndrome,
high-resolution computed tomography, pulmonary function test, diffusion capacity Between Feb to August, 2003, in more than 29 countries, 8096 cases and causing more 774 deaths (fatality rate 9.6%). In Taiwan, 37 out of 346 SARS victims died, most of deaths were attributed to severe acute respiratory distress syndrome (ARDS).
Pulmonary sequelae is especially anticipated in patients developing severe pulmonary infection or acute lung injury. Survivors of the acute respiratory distress syndrome have persistent
functional disability one year after discharge from the intensive care unit. As the clinical
experience of dealing with SARS is accumulating, studies prospectively evaluating physiological, functional, and morphologicalmeasures during the year after diagnosis of SARS will provide valuable information for clinicians to handle patients with this new disease.
From April, 2003 till now, 76 patients with documented SARS were admitted to our hospital,15 died of the disease. Forty of the survivors received first HRCT examination at 51.8±20.2 days after symptom onset, 37 received pulmonary function examination;140.7±26.7 after symptom onset,19 received a second HRCT examination,22 had a second pulmonary function
examinations。HRCT of lung parenchymal change revealed air trapping (92.5%), ground-glass opacity (90%), reticulation (70%), parenchymal band (55%), bronchiectasis (17.5%),
consolidation (10%), and honeycombing (7.5%) in the first follow-up study. SARS patients who experienced ARDS (n=16) had significantly higher scores than those without ARDS (n=24) in
ground-glass opacity. Twelve of the 37 patients (37%) showed variable degrees of restrictive ventilatory defects in first PFT examiantion and 11 of them had been complicated by ARDS. On the first PFT patients without ARDS had better test results than those complicated by ARDS. Comparison between the first and second follow-up HRCT of 19 cases revealed significant improvement in ground glass opacity (CT scores 8.68±6.96 vs. 4.42±5.14, p < 0.0001) and
fibrosis (CT scores 5.79±6.13 vs. 3.05±5.80, p < 0.0001). All these impairments in PFT improved 2 months later. The FVC (% predicted) values improved from 71.3 ± 23.4 to 98.1 ± 19.6% (p = 0.004) and the FEV1 (% predicted) increased from 73.9 ± 21.0 to 96.5 ± 17.9% (p = 0.005). Most HRCT and PFT parameters in patients with SARS-ARDS significantly improved on the second examinations, but a restrictive defect was still present in 5 of the 10 patients (50%), probably because of residual pulmonary fibrosis. The DLco (% predicted) was inversely correlated with the
total fibrotic scores on the high-resolution computed tomography (HRCT) of the chest. Our observation study revealed that lung damage in SARS patients usually resolve over time. The DLco may be a useful marker to follow-up fibrosis sequelae.
Background information
Severe acute respiratory syndrome (SARS) is a new infectious disease identified since late February, 2003. The disease was first reported among people in Guangdong Province of China, Hanoi of Vietnam, and Hong Kong. It has since then spread worldwide, including North America, Europe, and other Asian countries [1]. The disease was horrible by its high infectivity, rapid progression to respiratory failure and potentially lethal in severe cases. As of April 16, 3293 cases of SARS had been reported in the world, and 159 died of the disease. In Taiwan, 27 probable cases are reported; most of them are imported from affected area, such as Hong Kong or Mainland China, or close contacts (like health care workers or family) of a SARS patient [2]. SARS does not respond to empirical antimicrobial agent for acute community- acquired typical or atypical pneumonia. Bacteriological and virological pathogens known to cause pneumonia were not identified. A new virus belonging to the family Coronaviridae was recently isolated from the body fluids and tissues of SARS victims [3-5]. More evidence supports this novel coronavirus as the causative pathogen of SARS. Yet, so far, laboratory diagnostic tests used to test clinical specimens for evidence of this novel coronavirus are still in development and are not available outside a research setting. Serologic testing for coronavirus antibody consists of indirect fluorescent antibody testing and enzyme-linked immunosorbent assays that are specific for
antibody produced after infection [5]. Although some patients have detectable coronavirus antibody within 14 days of illness onset, definitive interpretation of negative coronavirus antibody tests is possible only for specimens obtained >21 days after onset of fever. A reverse transcriptase-polymerase chain reaction (RT-PCR) test specific for RNA from the novel
coronavirus has been positive within the first 10 days after fever onset in specimens from some SARS patients, but the duration of detectable viremia or viral shedding is unknown, and RT-PCR tests on samples collected during convalescence might be negative. Viral culture followed by RT-PCR also has been used to detect the novel coronavirus in some specimens [3,4].
SARS is defined by clinical and radiographic categories. Probable SARS includes fever (>38°C), newly developed respiratory symptoms (such as cough, shortness of breath, chest pain), and radiographic evidence of infiltrates consistent with pneumonia or respiratory distress syndrome (RDS) on chest radiograph [1, 6-8]. All SARS patients eventually develop pulmonary
complications during the course of the disease. About 25%-40% were admitted to the ICU due to respiratory failure, after a certain period of fever, shortness of breath and hypoxemia. About 4% of all SARS patients eventually die of the disease. The typical finding on chest X-ray and thoracic CT scan is ill-defined, ground-glass opacification in the periphery of the affected lung parenchyma, usually in subpleural location. The characteristic peripheral alveolar opacities are very similar to those found in bronchiolitis obliterans organizing pneumonia. Histologic examination of the lung reveals gross consolidation of the lungs. Both early phase( pulmonary edema with hyaline membrane formation) and organizing phase (cellular fibromyxoid
inflammatory infiltrate in interstitium, vacuolated and multinucleated pneumocytes were identified, the latter finding suggested viral infection, such as measles, parainfluenzavirus, respiratory syncytial virus and Nipahvirus infection, but not include ordinary human coronavirus [6,8].
Usually, after viral pneumonia, sequelae of respiratory system may persist for certain duration [9]. The pathology of pulmonary sequelae may include bronchiectasis, obliterative bronchiolitis, bronchiolitis obliterans with organizing pneumonia. Pulmonary function testing performed after the convalescence of infection may show either obstructive or restrictive disorder[10,11]. Bronchial hyperreactivity is especially common after RSV infection. Pulmonary sequelae is especially anticipated in patients developing severe pulmonary infection or acute lung injury. Patients who survive the acute respiratory distress syndrome are at risk for physical and
neuropsychological complicationsof the lung injury itself, associated multiorgan dysfunction,and their long stay in the intensive care unit (ICU). Herridge et al had evaluated 109 survivors of the acute respiratory distress syndrome 3, 6, and 12 months after discharge from the intensive care unit. Although lung volume and spirometric measurements were normal by 6 months, carbon monoxide diffusion capacity remained low throughout the 12-month follow-up. Six percent of patients had arterial oxygen saturation values below 88 percent during exercise. The median score for the physical role domain of the Medical Outcomes Study 36-item Short-Form General Health Survey (a health-related quality-of-life measure) increased from 0 at 3 months to 25 at 12 months (score in the normal population, 84). The distance walked in six minutes increased from a median of 281 m at 3 months to 422 m at 12 months; all values were lower than predicted. Survivors of the acute respiratory distress syndrome have persistent functional disability one year after discharge from the intensive care unit. Muscle weakness and fatigue were the reasons for their functional limitation [12].
According to our experience, muscle weakness and functional impairment in SARS patients persists two weeks after weaning from ventilator. Although the general condition improved gradually, pulmonary functional test and thoracic image (chest X-ray and CT scan) performed one month after SARS revealed abnormalities. As the clinical experience of dealing with SARS is accumulating, studies prospectively evaluating physiological, functional, and morphological measures during the year after diagnosis of SARS will provide valuable information for clinicians to handle patients with this new disease. Therefore, thegoal of this study was to characterize long-term pulmonary andfunction in a prospectively identified cohortof patients who survived SARS, especially with ARDS.
Subjects and Methods
Study Population
Seventy-six patients were diagnosed as probable cases in our institution. Fifteen of the 76 cases died in the period of admission. Sixty of the remaining sixty-one cases were discharged after clinical improvement. All patients fulfilled the clinical criteria of SARS according to the definition of the World Health Organization (WHO), with fever of more than 38°C, cough or breathing difficulty, history of exposure within 10 days prior to onset of symptoms, and an
abnormal chest radiograph (CXR). All cases should have positive RT-PCR (real-time polymerase chain reaction assay) for SARS-coronavirus (SARS-CoV) in clinical specimens, or positive seroconversion in 28-day convalescent sera.
Testing Procedure
Upon entry into the study, all SARS subjects and control subjects will undergo complete
pulmonary function testing and HRCT imaging at 3, 6, 9 and 12months after the initial diagnosis of disease.
Baseline Pulmonary Function Testing
Pulmonary function studies were performed on a Keystone model pulmonary function analyzer (S&M Instrument Co. Inc., Doylestown,PA) or a Sensormedics Series V6200 Autobox
Reproducibility between the two machines was documentedby testing a number of individuals on both systems. Lung volumeswere determined using the helium dilution technique (Keystone)or nitrogen washout (Sensormedics); diffusing capacities weredetermined using the single-breath method and adjusted for hematocrit. The most appropriate reference equations for our laboratory and testing conditions were chosen by applying a number of referenceequations to pulmonary function results of normal volunteers.
Diffusion capacity measurement
Subjects performed a total of six diffusingcapacity maneuvers, 2 each at low, medium, and high fraction ofinspired oxygen (FIO2). Before performance of the DLCO measurementsa sample of gas
was taken and measured for CO to estimate baselineCO in equilibrium with blood carboxyhemoglobin.
HRCT Technique
Computed tomographic (CT) scanning was performed on all subjects with helical CT scanner (PQ6000, Marconi, USA; High-Speed, General Electric Medical System, Milwaukee, Wis., USA) with 1mm collimationat 10-mm intervals through the chest during suspended inspirationat TLC with the patient in the supine position. Images are reconstructedusing the high spatial frequency algorithm and photographed atlung (window width 1,500 Hounsfield units [HU], level 700 HU) and mediastinal (window width 400 HU, level 0 HU) windows. Nointravenous contrast will be administered.
HRCT Evaluation
All scans were interpreted by an experienced chest radiologist blinded to SARS/ control status and physiologic data. The HRCT findings were described according to the recommendations of the Nomenclature Committee of the Fleischner Society. The images were viewed at a
window/level setting of 1000/600 in standard DICOM (Digital Imaging and Communication in Medicine) viewer. HRCT findings including ground-glass opacity, reticulation, honeycombing, parenchymal band, consolidation, air trapping and bronchiectasis were recorded. Table 1 outlines a modified scoring system used for evaluating HRCT findings. The system has been used to describe idiopathic pulmonary fibrosis and correlated well with the degree of fibrosis manifested by pathologic specimen.(13) As paired inspiration and expiration HRCT were performed in this study, we modified the system to evaluate the extent of hypoattenuation on expiratory HRCT as air trapping (Table 1). Forthe purpose of analysis, each lobe was scoredseparately and the sum of all lobes was incorporated into a total score for groundglass attenuation (CT-alv), fibrosis or reticulation (CT-fib), air trapping (CT-air trap) for each study. There was a scaleof 0-5 for each lobe and the total score obtained from HRCT ranged from 0 to 25. The scores were obtained with consensus between two chest radiologists.
Results
HRCT findings and score
Forty patients received first HRCT examinations, with about 51.83±20.23 days after onset of symptoms, 16 of them experiencing ARDS. Lung parenchymal change on first HRCT (43.4±9.5 days after the onset of symptoms) including air trapping (92.5%), ground-glass opacity (90%), reticulation (70%), honeycombing (7.5%), parenchymal band (55%), bronchiectasis (17.5%), and consolidation (10%) (Figures 1). Nine patients presented with ground-glass opacity and air trapping, 3 patients presented with only air trapping and one case had only ground glass opacity. The remaining 28 patients had more than two HRCT findings. Among the 19 patients who followed up second HRCT, 4 (24%) presented with ground glass opacity and air trapping, each one case had only ground glass opacity (6%), air trapping (6%), parenchymal band (6%) and bronchiectasis (6%). The remaining 9 (53%) had more than two HRCT findings. CT evidence of small airway change including ground glass opacity, air trapping or both is noted in 13 patients (32%) in the first HRCT study and 5 patients (30%) in the second HRCT study. None of the 40 patients had pleural effusion, cavitation or lymphadenopathy in either the first or second HRCT
study. None of the cases in the first and second HRCT studies was without abnormality. The average HRCT scores in the first evaluation of all these patients were: CT-alv 7.0±6.8, CT-fib 4.93±6.39, CT-air trap 4.68±3.68. The average scores of the second HRCT in 19 patients were: CT-alv 4.42±5.14. CT-fib 3.05±5.80, CT-air trap 5.05±5.17.
Comparison of first vs. second follow-up HRCT
Nineteen patients had two serial HRCT studies, at 140.68±26.68 days after symptom onset, 8 were convalescing from ARDS, while the other 11 were non-ARDS. For all the 19 cases
receiving second follow-up HRCT, there were significant improvement in CT-alv from 8.68±6.96 to 4.42±5.14 (p<0.0001) and in CT-fib from 5.79± 6.13 to 3.05±5.79 (p< 0.0001). No difference was found in CT-air trapping (5.37±4.41 vs. 5.05±5.17, p = 0.45). (Fig 2) The finding remained significant in subgroup analysis. For ARDS group, CT-alv decreased from 13.12±7.53 to 6.50± 6.65 (p=0.0078) and CT-fib decreased from 8.37±7.67 to 4.87± 8.25 (p=0.0156). No significant change was noted in the CT-air trap (6.62±1.99 vs. 6.12±4.32, p = 0.5781). For non-ARDS group, CT-alv score decreased from 5.45±4.50 to 2.91±3.24 (p = 0.002) and CT-fib decreased from 3.91±4.16 to 1.73±2.87 (p = 0.0039). No significant change was noted in CT-air trap (4.46±5.48 vs. 4.27±5.78, p = 0.6875). (Figure 2).
Pulmonary function tests
Thirty-seven patients received pulmonary function tests at the same time of CT examinations. Twelve of the 37 patients (37%) showed variable degrees of restrictive ventilatory defects, 11 of them had been complicated by ARDS. All these impairments in PFT improved 2 months later except for the DLco (Table 2). The FVC (% predicted) values improved from 71.3 ± 23.4 to 98.1 ±
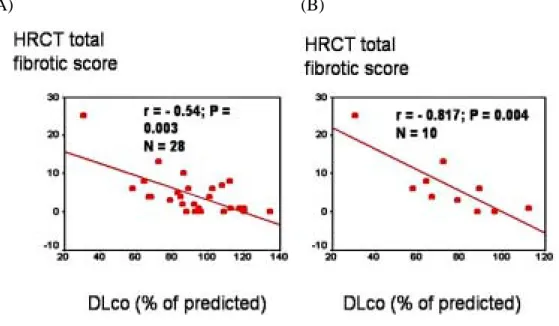
19.6% (p = 0.004) and the FEV1 (% predicted) increased from 73.9 ± 21.0 to 96.5 ± 17.9% (p = 0.005). On the first PFT patients without ARDS had better test results than those complicated by ARDS, but these differences did not reach statistical significance 3 months later (Table 3). Most PFT parameters in patients with SARS-ARDS significantly improved on the second PFT, but a restrictive defect was still present in 5 of the 10 patients (50%), probably because of residual pulmonary fibrosis. The DLco (% predicted) was inversely correlated with the total fibrotic scores
on the high-resolution computed tomography (HRCT) of the chest (Figure 3A and 3B). The DLco
(% predicted) was also associated with the duration of mechanical ventilation in the 7 patients with ventilatory support (r = - 0.86, p = 0.026).
Table 1. severe acute respiratory syndrome (SARS): HRCT scoring system
Alveolar score (CT-alv) 0 No alveolar disease
1 Ground glass opacity <5% of the lobe (minimal but not normal) 2 Ground glass opacity involving up to 25% of the lobe
3 Ground glass opacity involving 25-49% of the lobe 4 Ground glass opacity involving 50-75% of the lobe 5 Ground glass opacity involving >75% of the lobe Fibrotic score (CT-fib)
0 No interstitial disease
1 Interlobular septal thickening; no discrete honeycombing
2 Honeycombing (+/- septal thickening) involving up to 25% of the lobe 3 Honeycombing (+/- septal thickening) involving 25-49% of the lobe 4 Honeycombing (+/- septal thickening) involving 50-75% of the lobe 5 Honeycombing (+/- septal thickening) involving >75% of the lobe Air trapping score (CT-air trap)
0 No bronchiolar disease (no air trapping on expiration)
1 Air trapping involving <5% of the lobe (minimal but not normal) 2 Air trapping involving up to 25% of the lobe
3 Air trapping involving 25-49% of the lobe 4 Air trapping involving 50-75% of the lobe 5 Air trapping involving >75% of the lobe
Table 2. Followed-up PFT in patients with SARS Timing First PFT (n = 37) Second PFT (n = 22) p FVC (%) 71.3 ± 23.4 98.1 ± 19.6 0.004 FEV1 (%) 73.9 ± 21.0 96.5 ± 17.9 0.005 FEV1/FVC (%) 91.0 ± 6.8 85.8 ± 8.0 0.16 FRC (%) 95.6 ± 30.7 117.5 ± 30.2 0.04 TLC (%) 86.4 ± 20.3 104.0 ± 14.1 0.03 DLco (%) 88.8 ± 25.7 102.6 ± 16.8 0.19 MVV (%) 80.7 ± 18.9 95.9 ± 14.7 0.03
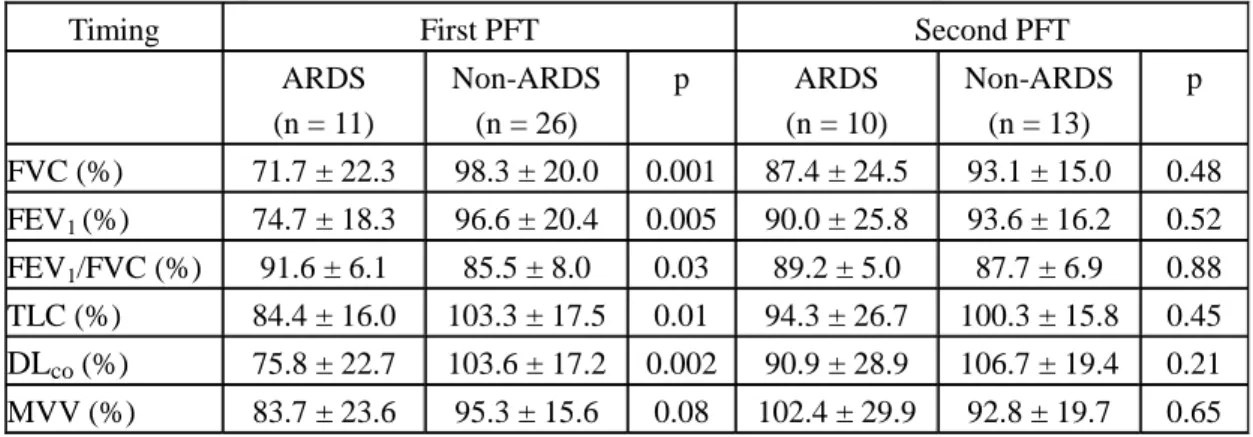
Table 3. PFT of patients with SARS: ARDS vs. non-ARDS groups
Timing First PFT Second PFT
ARDS (n = 11) Non-ARDS (n = 26) p ARDS (n = 10) Non-ARDS (n = 13) p FVC (%) 71.7 ± 22.3 98.3 ± 20.0 0.001 87.4 ± 24.5 93.1 ± 15.0 0.48 FEV1 (%) 74.7 ± 18.3 96.6 ± 20.4 0.005 90.0 ± 25.8 93.6 ± 16.2 0.52 FEV1/FVC (%) 91.6 ± 6.1 85.5 ± 8.0 0.03 89.2 ± 5.0 87.7 ± 6.9 0.88 TLC (%) 84.4 ± 16.0 103.3 ± 17.5 0.01 94.3 ± 26.7 100.3 ± 15.8 0.45 DLco (%) 75.8 ± 22.7 103.6 ± 17.2 0.002 90.9 ± 28.9 106.7 ± 19.4 0.21 MVV (%) 83.7 ± 23.6 95.3 ± 15.6 0.08 102.4 ± 29.9 92.8 ± 19.7 0.65
Fig 1A
Fig 1B
Fig 1C
Fig 1D
Fig. 1 HRCT of case 1 (49 days after symptom onset, 26 days after removal of the endotracheal tube) in suspended inspiration (A) demonstrates fibrotic bands, bronchiectasis, ground glass opacities. The expiratory phase (B) demonstrates mosaic attenuation suggesting air trapping. The lung
parenchymal change and air trapping at corresponding location on inspiration (C) and expiration status (D)
disappeared in the second follow-up CT study (114 days after symptom onset).
Fig. 2 Changes of HRCT scores in 19 SARS patients receiving two HRCT examinations. See text for description and results of statistical analysis between HRCT examinations. 1st: first HRCT study; 2nd: second HRCT study; Alveolar= CT-alv, Fibrosis= CT-fib, Air-trap= CT-air trap.
Alveolar Fibrosis Air-trap 0.0 2.5 5.0 7.5 10.0 12.5 1st 2nd Category of HRCT score Sc o re
Figure 3. DLco(%) vs. HRCT fibrotic scores. (A) Total study population; (B) SARS-ARDS patients.
(A) (B)
Conclusion
HRCT evidence of ground-glass opacity and fibrosis in lung parenchyma of convalescent SARS patients usually resolve over time. However, air trapping persists. Subclinical airway damage should be considered as a potential complication in patients recovering from SARS, whatever the severity of the clinical disease. All patients with ARDS complicating SARS had restrictive ventilatory defects within 1 month after discharge, and these impairment still persisted in 50% of them 2 months later. The DLco correlated inversely with the fibrotic scores on HRCT in these patients, suggesting that it may be a useful marker to follow-up pulmonary fibrosis.
References
1. World Health Organization. Severe acute respiratory syndrome (SARS). Wkly Epidemiol Rec 2003;78:81-3.
2. World Health Organization. Cumulative Number of Reported Probable Cases of Severe Acute Respiratory Syndrome (SARS) from 1 Nov 2002 to 16 Apr 2003. (Accessed April 16, 2003, at http://www.who.int/csr/sarscountry/2003_04_16/).
3. Ksiazek TG, Erdman D, Goldsmith C, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Nghiem KH, Dowell S, Ling AE, Humphrey C, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes J, LeDuc JW, Bellini WJ, Anderson LJ. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N Engl J Med 2003 Apr 10; [epub ahead of print].
4. Drosten C, Gunther S, Preiser W, Van Der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome. N Engl J Med 2003 Apr 10; [epub ahead of print]
5. Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, Nicholls J, Yee WKS, Yan WW, Cheung MT, Cheng VCC, Chan KH, Tsang DNC, Yung RWH, Ng TK, Yuen KY, and
members of the SARS study group. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003; Apr 8; [published online].
6. Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN. A Cluster of Cases of Severe Acute Respiratory Syndrome in Hong Kong. N Engl J Med 2003 Apr 11; [epub ahead of print]
7. Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ. Identification of Severe Acute Respiratory Syndrome in Canada. N Engl J Med 2003 Apr 10; [epub ahead of print]
8. Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ. A Major Outbreak of Severe Acute Respiratory
Syndrome in Hong Kong. N Engl J Med. 2003 Apr 14 [epub ahead of print]
9. Smyth A. Pneumonia due to viral and atypical organisms and their sequelae. British Med Bull 2002;61:247-62.
10. Wennergren G, Kristjansson S. Relationship between respiratory syncytial virus bronchiolitis and future obstructive airway diseases. Eur Respir J. 2001;18:1044- 58.
11. Fischer GB, Teper A, Colom AJ.Acute viral bronchiolitis and its sequelae in developing countries. Paediatr Respir Rev. 2002;3:298-302.
12. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348: 683-93.
13. Flaherty KR, Colby TV, Travis WD, Toews GB, et al. Fibroblastic foci in usual interstitial pneumonia. Idiopathic versus collagen vascular disease. Am J Respir Crit Care Med 2003;167:1410-1415.