American Society of Mammalogists
Reproductive Biology of the Red-Giant Flying Squirrel, Petaurista petaurista, in Taiwan Author(s): Pei-Fen Lee, Yao-Sung Lin, Donald R. Progulske
Source: Journal of Mammalogy, Vol. 74, No. 4 (Nov., 1993), pp. 982-989 Published by: American Society of Mammalogists
Stable URL: http://www.jstor.org/stable/1382437 Accessed: 16/05/2009 03:37
Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at
http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use.
Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at
http://www.jstor.org/action/showPublisher?publisherCode=asm.
Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission.
JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact support@jstor.org.
American Society of Mammalogists is collaborating with JSTOR to digitize, preserve and extend access to Journal of Mammalogy.
REPRODUCTIVE BIOLOGY OF THE RED-GIANT FLYING
SQUIRREL, PETAURISTA PETAURISTA, IN TAIWAN
PEI-FEN LEE, YAO-SUNG LIN, AND DONALD R. PROGULSKE
Department of Zoology, National Taiwan University, Taipei, Taiwan, Republic of China (PFL and YSL) Department of Forestry and Wildlife Management, University of Massachusetts, Amherst, MA 01003 (DRP)
Reproductive characteristics of red-giant flying squirrels (Petauristapetaurista) were studied from December 1981 to November 1982 in Taiwan. We collected 216 adults (116 males, 100 females) and 65 immatures (23 males, 42 females). The sex ratio of adults did not deviate from 1:1 ratio (P > 0.05). Adult females tended to be heavier than adult males (1,334 and 1,260 g, respectively, P < 0.01), but the sexes showed no differences in other body characteristics. Some males maintained reproductive capacity in all months except February, and most were sexually active in March-June and October-November. Most females were sexually active in May-July and November-January. About one-half of the adult females (49%) became pregnant during each breeding season, in winter (December- February) and summer (June-August). Pregnancy rates ranged from 50 to 57% in all months except February. Litter sizes ranged from one to two, with an average of 1.04 young/litter. The estimated birth weight for P. petaurista was >56 g. This species is a seasonal breeder with low reproductive rates, which may be a result of a low predation rate combined with their high degree of folivory.
Key words: Petaurista, reproduction, flying squirrel, Taiwan
Giant flying squirrels (Petaurista) are nocturnal arboreal folivores that reside in tree cavities during the daytime (Muul and Lim, 1978; Nowak and Paradiso, 1983). They are widely distributed in eastern and southeastern Asia (Honacki et al., 1982). Five species are recognized (Nowak and Pa- radiso, 1983); white-cheeked giant flying squirrels (P. leucogenys), giant flying squir- rels (P. magnificus), spotted giant flying squirrels (P. elegans), red-giant flying squir- rels (P. petaurista), and white-faced giant flying squirrels (P. alborufus).
Little is known of the reproductive biol- ogy of Petaurista sp. (Lin et al., 1985), ex- cept for some scattered reports on litter sizes (Kuroda, 1940; Medway, 1978; Mitchell, 1979; Muul and Lim, 1978; Udagawa, 1954). P. petaurista ranges from the Hima- layas across southern China to Taiwan, southward to Sri Lanka, and eastward into
Borneo (Askins, 1977). Distributed from 300 to 2,200 m in mountainous areas in Taiwan, P. petaurista is most abundant in the lower elevations of hardwood forests and recently has adapted to secondary conifer plantations, where it has become a pest spe- cies (Lee et al., 1986; Lin and Lee, 1986).
In an effort to address some forest-man- agement issues, we initiated a series of eco- logical studies on giant flying squirrels. We found that P. petaurista was able to explore secondary conifer plantations and use this habitat as feeding and resting areas (Lee et al., 1986), and we studied bark-stripping be- havior of P. petaurista on Japanese fir (Cryptomeriajaponica- Lin and Lee, 1986). P. petaurista is most active before midnight and home ranges of adult females in conifer plantations was estimated to be 3.2 ha (Lin et al., 1988). The average density of P. pe- taurista is 2.35 and 0.56/10 ha in hardwood
November 1993 LEE ET AL. -REPRODUCTION IN PETAURISTA PETAURISTA 983 and conifer plantations, respectively (Lee et
al., 1993).
A proposed management strategy was to shoot the squirrels in conifer plantations (W. E. Howard, pers. comm.). Although this practice may prove useful, it was not clear how hunting affects squirrel populations be- cause no detailed reproductive information is available. In this paper, we document the reproductive characteristics ofP. petaurista, particularly the seasonal breeding patterns of males and females.
MATERIALS AND METHODS
We collected squirrels in hardwood forests near Sunmin Village of southern Taiwan (23013'N,
120041'E) between December 1981 and Novem- ber 1982. At an elevation of 500 m, Sunmin has a yearly mean temperature of 20.80C (X summer = 250C; winter = 150C). Precipitation, ca. 3,000 mm annually, occurs mostly between March and August, while other months are dry. Most hard- wood trees are members of the families Laura- ceae and Fagaceae. These trees provide abundant nuts and other food for squirrels.
Each squirrel was weighed on a custom-built spring scale accurate to 0.1 g. The lengths of body, tail, ear, hind foot, and styliform cartilage were measured to the nearest 1 mm. Paired testes and epididymides were weighed to the nearest 10 mg. Testes were preserved in Bouin solution, later transferred to 70% alcohol, and serially sec- tioned at 6-8 Am. Sections taken from the middle of testes were used to measure diameters of sem- iniferous tubules. Two diameters from each tu- bule were measured by an ocular micrometer. An average of 10 measurements for each animal was taken as the diameter of the seminiferous tubule of the squirrel, as recommended by Pud- ney (1976). The presence of enlarged nipples was recorded for females. Both ovaries and uteri were removed from the body and weighed to the near- est 10 mg. The number of embryos and placental scars were recorded. Crown-rump lengths of em- bryos were measured to the nearest 0.1 mm with a vernier caliper, and embryos were cleaned and weighed to the nearest 10 mg. Ovaries from in- dividuals that bore embryos were fixed in 70% alcohol and serially sectioned at 6-8 tm, stained with haematoxylin and eosin, and examined for corpora lutea (Nixon and McClain, 1975).
The sexual maturity of males was classified
according to the histological criteria suggested by Kirkpatrick (1955) and Hoffmann and Kirkpat- rick (1956) for gray squirrels (Sciurus carolinen- sis) and fox squirrels (S. niger). Five stages were identified; infantile (small seminiferous tubules with small or no lumena), prepubertal (most tu- bules containing primary spermatocytes in the interiors), functional (most tubules with full sperm production), degenerating (early stage-loose primary and secondary spermatocytes, and other cellular debris cluttering the tubules; middle stage-more cellular debris cluttering the tu- bules, and shrinking tubules; late stage-ad- vanced degeneration with shrinking tubules and disappearing lumina), and redeveloping (form- ing lumina and layers of developing spermato- cytes in tubules). The first two stages were re- garded as immature and the rest as adult. Females were classified as adult if any of the following were present: enlarged nipples, placental scars, embryos, or the presence of type seven follicles in the ovary (Pederson and Peters, 1968). Adult females were further classified into three groups based on counts of placental scars; those with no placental scar, with one placental scar, and with two or more placental scars.
Analysis of variance and Student's t-tests were performed using SAS (SAS Institute Inc., 1989). Statistical assumptions of these tests were checked and no significant departures were found.
RESULTS
Sex ratio and body characteristics. -We collected 216 adults (116 males, 100 fe- males) and 65 immatures (23 males, 42 fe- males). With the exception of September, when only two P. petaurista were collected, monthly samples ranged from 15 to 36. Sex ratio of adult squirrels did not deviate from 1:1 ratio (P > 0.05). For immature squir- rels, our sample was too small to detect the actual sex ratio.
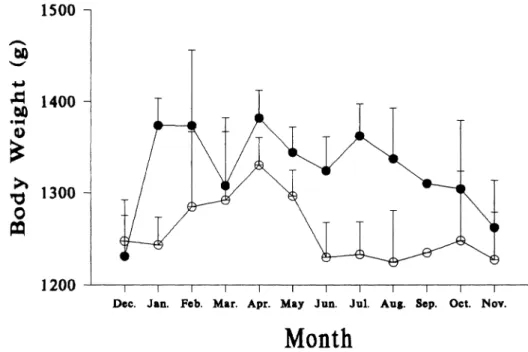
Average body mass of adult females was significantly greater than that of adult males (1,334 and 1,260 g, P < 0.001). Except for the uncertainty in September samples, monthly average body mass of adult fe- males were greater than those of males in every month but December (Fig. 1). The differences were greater in January-Febru- ary and June-August, with significant dif-
984 JOURNAL OF MAMMALOGY Vol. 74, No. 4 1500 '• 1400 0 '• 1300-
1200
Dec. Jan. Feb. Mar. Apr. May Jun. Jul. Aug. Sep. Oct. Nov.
Month
FIG. 1.-Monthly changes (mean ? SE) of adult male (open circles) and female (closed circles) body weights of Petaurista petaurista in Sunmin, Taiwan, December 1981-November 1982. ferences in January and July (P < 0.05) after
excluding pregnant females from the anal- ysis. Adult males weighed 989-1,569 g, whereas females varied from 1,005 to 1,597 g. Other morphological characters, i.e., lengths of body (X = 378 mm), tail (X = 459 mm), ear (1 = 73 mm), hind foot (X = 47 mm), and styliform cartilage (I = 88 mm), were not significantly different be- tween sexes (P > 0.05). There was consid- erable overlap between immature and adult squirrels in these measurements.
Reproductive cycle of males. -The repro- ductive development of males showed a seasonal trend (Table 1). Of the 116 adult males, 65 were classified as functional, 48 degenerating, and 3 redeveloping. Func- tional individuals were found throughout the year, except February (Table 1). Peak abundances of sperm in testes occurred in March-June and October-November, when males were most sexually active. Active spermatogenesis was observed and sperm filled the seminiferous tubules. Immediate- ly following the functional period, some squirrels entered sexual quiescence. Squir-
rels with degenerating testes were found from December to February and from June to August. Three developmental stages can be distinguished. Early degenerating testes were found in these months. The middle-degen- erating stage followed and occurred in Jan- uary, February, July, and August. The late- degenerating stage occurred in February, March, and August. The sexual-redevel- oping period was short for males as only three individuals were found in this con- dition in February and August, following the degenerating periods. Testes underwent involution in February and August and re- covered from this stage rapidly.
Development of male reproductive or- gans supported the previous observations. Mean weights of testes and epididymides and diameter of seminiferous tubules showed two major peaks during the year (Fig. 2). Reproductive activity was maxi- mum during March-June and October-No- vember.
Reproductive pattern offemales. - Of 100 adult females, 78 showed previous breeding experience. About 52% of adult females had
November 1993 LEE ET AL.--REPRODUCTION IN PETAURISTA PETAURISTA 985 TABLE 1l.-Monthly distribution of reproductive status of adult male Petaurista petaurista in Sunmin, Taiwan, December 1981-November 1982. Reproductive status was determined according to the stage of spermatogenesis proposed by Kirkpatrick (1955).
Degenerating
Year and month Functional Early Middle Late Redeveloping Total
1981 December 2 7 9 1982 January 2 2 9 13 February 2 2 4 2 10 March 4 1 5 April 14 14 May 9 9 June 9 4 13 July 2 1 5 8 August 5 3 3 5 1 17 September 1 1 October 8 8 November 9 9 Total 65 19 19 10 3 116
only one placental scar, while 22 and 26% had no placental scars and two or more pla- cental scars, respectively. These groups in- dicate female age structure in the popula- tion. The recruitment rate for the population of females was ca. 22%.
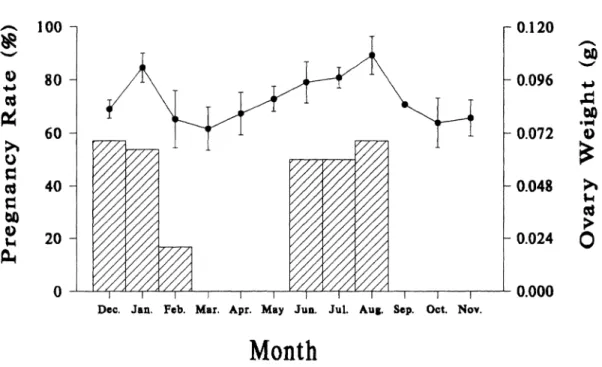
Like males, females exhibited two dis- tinct breeding seasons per year. Paired weights of ovaries showed a bimodal pat- tern with peaks occurring in January and August (Fig. 3). During the breeding sea- sons, only 49% of adult females became pregnant and were found only in winter (De- cember-January) and summer (June-Au- gust). Except in February, when the rate was 16.7%, pregnancy rates were 50-57% for the other months. Based on the occurrence of pregnancy and the development of ovaries, November-January and May-July are the two most sexually active periods for fe- males. The winter breeding season began in late November-early December, and ended in late February, whereas the summer breeding season ran from late May-early June to the end of August-early September. Pregnant females found in December and June had small embryos, whereas those found in later breeding seasons had larger embryos. Based on sizes of embryos, most young were born in January-February and
8- 4 1 3 2 2 3 00- S50-
a
100 MonthFIG. 2.-Trends of mean (?SE) paired testes
weight (a), epididymides weight (b), and diam- eter of seminiferous tubules (c) for adult male Petaurista petaurista in Sunmin, Taiwan, De- cember 1981-November 1982.
986 JOURNAL OF MAMMALOGY Vol. 74, No. 4 100 - - 0.120 80 - - 0.096 60 - - 0.072 O e
40
-0.048
,
20 -
-0.024
0 / X0.000 iDec. Jan. Feb. Mar. Apr. May Jun. Jul. Aug. Sep. Oct. Nov.
Month
FIG. 3.--Monthly changes of paired ovarian weight (line, mean + SE) and pregnancy rate (bars) for adult female Petaurista petaurista in Sunmin, Taiwan, December 1981-November 1982.
July-August. Young also were born in Sep- tember because of the high pregnancy rate and early development of embryos in preg- nant females found in August.
Pregnant females did not show greater body dimensions (i.e., body mass, and length of body, tail, hind foot, ear, and styliform cartilage) than the non-breeders (P > 0.05). No significant differences in body measure- ments between breeding females in winter and summer were found (P > 0.05).
Only one squirrel had two embryos whereas the others had one, resulting in an average of 1.04 young/litter. Litter sizes were not significantly different between seasons and between first-time and experienced breeders (P > 0.05).
The number of corpora lutea varied from one to two, with a mean (?+SD) of 1.13 ? 0.34. Of the 24 pregnant females, only two of 27 ova were unsuccessfully implanted. Mortality of embryos after implantation was low; no dead fetuses were observed. Thus, the total prenatal mortality was ca. 7.4%.
Based on pregnancy period and when young were observed in this study and other nearby sites (Lee et al., 1986), we estimated
the gestation period for Petaurista to be at least 45 days. The birth weight for P. petau- rista was >56.3 g, the largest embryo ob- tained in this study.
DIscussIoN
The breeding patterns of Petauristinae vary among species and geographic loca- tion. In subtropical Taiwan, P. petaurista is a seasonal breeder with two breeding sea- sons (winter and summer) per year. In tem- perate areas such as Japan, P. leucogenys breeds from spring to autumn (Udagawa, 1954). In North America, the southern fly- ing squirrel (Glaucomys volans) breeds only in spring and autumn (Dolan and Carter,
1977; Geortz et al., 1975; Muul, 1968, 1969; Sollberger, 1943; Sonenshine et al., 1979). In tropical rainforests, such as those in Ma- laysia, Muul and Lim (1974) found that no particular temporal patterns occurred in Hylopetes lepidus and Pteromyscus pulver- ulentus.
The average litter size (1.04) found in this study is similar to that previously reported for P. petaurista in Taiwan (Kuroda, 1940) and in Malaysia (Medway, 1978; Muul and
November 1993 LEE ET AL.--REPRODUCTION IN PETA URISTA PETA URISTA 987
Lim, 1978). This small litter size also is commonly found in other species of Petau- rista; P. magnificus in Nepal (Mitchell, 1979), P. elegans in the Malaysian Penin- sula (Muul and Lim, 1978), P. leucogenys in Japan (Udagawa, 1954), and P. alborufus in Taiwan (P. F. Lee and Y. S. Lin, in litt.). The litter size for Petaurista sp. does not seem to be affected by latitude and geo- graphic location and does not follow Lord's (1960) observation of gradual increase of litter size in North American tree squirrels (Sciurus sp.) at higher latitudes.
Muul and Lim (1978) suspected that the small litter size of Petaurista sp. resulted from a low predation rate and was corre- lated with their high degree of folivory. In Taiwan, predation pressure on P. petaurista is relatively low because their natural pred- ators have been eliminated or significantly reduced due to habitat destruction and il- legal hunting. The composition of the diet of P. petaurista was at least 55% leaves (Lee et al., 1986). With high leaf contents in the overall diet, mammalian folivores tend to have relatively low metabolic rates (Mc- Nab, 1978). The low metabolic rate is cor- related with low reproductive rate (McNab,
1980).
Apparently the breeding pattern of P. pet- aurista was controlled by females. Some male squirrels were capable of breeding each month, except February, as indicated by males with functional testes. However, pregnant females were only found in De- cember-February and June-August. Only about one-half (49%) of the adult females became pregnant during the breeding sea- sons. This low pregnancy rate among fe- males in each breeding season probably is due to few females breeding twice in one year and some females that do not breed at all in a given year. Although our data does not indicate that female P. petaurista have only one litter per year, field observations indicate that this is possible. In the field, young were observed foraging with an adult female at ca. 3-4 months of age, when they were ca. 50% of adult weight (Lee et al.,
1992); this bonding may last for several months (Lee et al., 1986; Muul and Lim, 1978). Lin et al. (1988) reported an unusual case of two female squirrels (mother and offspring) living together in conifer planta- tions for 16 months and apparently did not breed for the entire period although both showed signs of estrus.
The exact factors behind the timing of breeding season remain unknown. Factors, such as temperature, precipitation, photo- period, and plant chemicals, have been pro- posed to account for the onset of breeding seasons in mammals (Herbert, 1977; Jame- son, 1988; Reddi and Prasad, 1968). Except for photoperiod and food, factors such as precipitation and temperature cannot ex- plain the breeding pattern in P. petaurista, because breeding occurred in both the coldest and warmest seasons as well as in wet and dry seasons. It is reasonable to as- sume that the reversals in change of day- length may be the most predictable factor that contributes to the observed seasonal- breeding patterns in P. petaurista. Many mammalian species show reproductive re- sponses that change with daylength (Bron- son, 1989; Jameson, 1988). Muul (1968) found that change in photoperiod was the major contributor for triggering the breed- ing of G. volans. Food is another possible factor in controlling the timing of repro- duction in P. petaurista, because it relies heavily on vegetation that contains many secondary plant compounds (Jameson,
1988). Further studies on these aspects are necessary.
The increasing differences in body weights between adult male and female squirrels during periods of pregnancy probably are a result of seasonal breeding conditions. So- nenshine et al. (1979) reported that body weights of female G. volans increased at the time of the breeding season and male body weights declined during periods of sexual activity. Our results on P. petaurista from Taiwan agree with findings for adult sciurids in temperate climates showing a seasonal cycle of body mass; however, for P. petau-
988 JOURNAL OF MAMMALOGY Vol. 74, No. 4
rista the minimum and maximum weights occur in winter and spring, respectively, in contrast to late winter and autumn as seen for fox squirrels (S. niger-Nixon et al., 1991).
ACKNOWLEDGMENTS
We thank S. Y. Tu for collecting the specimens and Y. J. Chao for laboratory assistance. A. H. T. Yu provided important references. Earlier drafts of this paper benefited from the sugges- tions by S. L. Garman and two anonymous re- viewers. This work was supported by National Science Council, Republic of China, grant num- bers NSC-70-0409-B002-28, NSC-71-04090- B002-49, and NSC-73-0201-B002-01.
LITERATURE CITED
ASKINS, R. 1977. Family Sciuridae. Pp. 337-387, in Mammals of Thailand (B. Lekagul and J. A. Mc- Neely, eds.). Association for the Conservation of Wildlife, Bangkok, Thailand, 758 pp.
BRONSON, F. H. 1989. Mammalian reproductive bi-
ology. The University of Chicago Press, Chicago, 325 pp.
DOLAN, P. G., AND D. C. CARTER. 1977. Glaucomys volans. Mammalian Species, 78:1-6.
GEORTZ, J. W., R. M. DAWSON, AND E. E. MOWBRAY.
1975. Response to nest boxes and reproduction by Glaucomys volans in northern Louisiana. Journal of Mammalogy, 56:933-939.
HERBERT, J. 1977. External factors and ovarian ac- tivity in mammals. Pp. 457-505, in The ovary phys- iology (L. Zuckerman and B. J. Weir, eds.). Academ- ic Press, New York, 2:1-555.
HOFFMANN, R. A., AND C. M. KIRKPATRICK. 1956.
An analysis of techniques for determining male squirrel reproductive development. Transactions of the North American Wildlife and Natural Resources Conference, 21:346-355.
HONACKI, J. H., K. E. KINMAN, AND J. W. KOEPPL (EDS.). 1982. Mammal species of the world: a tax- onomic and geographic reference. Allen Press, Inc., and The Association of Systematics Collections, Lawrence, Kansas, 694 pp.
JAMESON, E. W., JR. 1988. Vertebrate reproduction.
John Wiley & Sons, New York, 526 pp.
KIRKPATRICK, C. M. 1955. The testis of the fox squir- rel in relation to age and seasons. American Journal of Anatomy, 97:229-256.
KURODA, M. N. 1940. A monograph of the Japanese mammals. The Sanseido Company, Ltd., Tokyo, Ja- pan, 311 pp. (in Japanese)
LEE, P. F., D. R. PROGULSKE, AND Y. S. LIN. 1986.
Ecological studies on two sympatric Petaurista spe- cies in Taiwan. Bulletin of the Institute of Zoology, Academia Sinica, 25:113-124.
1993. Spotlight counts of giant flying squir- rels Petaurista petaurista and P. alborufus in Taiwan. Bulletin of the Institute of Zoology, Academia Sin- ica, 32:54-61.
LEE, P. F., D. R. PROGULSKE, Y. T. DAY, AND Y. S. LIN. 1992. Growth pattern of the red-giant flying squirrel Petaurista petaurista in Taiwan. Acta Zoo-
logica Taiwanica, 3:165-170.
LIN, Y. S., AND P. F. LEE. 1986. Debarking on Cryp-
tomeria trees by red-giant flying squirrel (Petaurista petaurista) in Chitou. Quarterly Journal of Chinese Forestry, 19:55-64 (in Chinese, English abstract). LIN, Y. S., L. Y. WANG, AND L. L. LEE. 1988. The
behavior and activity pattern of giant flying squirrels
(Petaurista p. grandis). Quarterly Journal Chinese
Forestry, 21:81-94 (in Chinese, English abstract).
LIN, Y. S., D. R. PROGULSKE, P. F. LEE, AND Y. T.
DAY. 1985. Bibliography of Petauristinae (Roden- tia, Sciuridae). Journal of Taiwan Museum, 38:49- 57.
LORD, R. D., JR. 1960. Litter size and latitude in North American mammals. The American Midland Naturalist, 64:488-499.
McNAB, B. K. 1978. Energetics of arboreal folivores: physiological problems and ecological consequences of feeding on an ubiquitous food supply. Pp. 153-
162, in The ecology of arboreal folivores (G. G. Montgomery, ed.). Smithsonian Institution, Wash- ington, D.C., 574 pp.
. 1980. Food habits, energetics, and the pop- ulation biology of mammals. The American Natu- ralist, 116:106-124.
MEDWAY, L. 1978. The wild mammals of Malaya (Peninsular Malaysia) and Singapore. Second ed. Oxford University Press, Oxford, United Kingdom, 193 pp.
MITCHELL, R. M. 1979. The sciurid rodents (Roden- tia: Sciuridae) of Nepal. Journal of Asian Ecology, 1:21-28.
MUUL, I. 1968. Behavioral and physiological influ- ences on the distribution of the flying squirrel, Glau- comys volans. Miscellaneous Publications of the Mu- seum of Zoology, University of Michigan, 134:1-66. . 1969. Photoperiod and reproduction in flying squirrel, Glaucomys volans. Journal of Mammalogy, 50:542-549.
MUUL, I., AND B. L. LIM. 1974. Reproductive fre-
quency in Malaysian flying squirrels, Hylopetes and Pteromyscus. Journal of Mammalogy, 55:393-400.
. 1978. Comparative morphology, food habits, and ecology of some Malaysian arboreal rodents. Pp. 361-368, in The ecology of arboreal folivores (G. G. Montgomery, ed.). Smithsonian Institution, Wash- ington, D.C., 574 pp.
NIXON, C. M., AND M. W. MCCLAIN. 1975. Breeding
seasons and fecundity of female gray squirrels in Ohio. The Journal of Wildlife Management, 39:426- 438.
NIXON, C. M., L. P. HANSEN, AND S. P. HAVERA. 1991.
Growth patterns of fox squirrels in east-central Il- linois. The American Midland Naturalist, 125:168- 172.
NOWAK, R. M., AND J. L. PARADISO. 1983. Walker's
mammals of the world. Fourth ed. The Johns Hop- kins University Press, Baltimore, 1:1-568.
PEDERSON, T., AND H. PETERS. 1968. Proposal for a
classification ofoocytes and follicles in mouse ovary. Journal of Reproduction and Fertility, 17:555-557. PUDNEY, J. 1976. Seasonal changes in the testis and
November 1993 LEE ET AL. -REPRODUCTION IN PETAURISTA PETAURISTA 989
epididymis of the American grey squirrel, Sciurus carolinensis. Journal of Zoology (London), 179:107-
120.
REDDI, A. H., AND M. R. N. PRASAD. 1968. The reproductive cycle of the male Indian palm squirrel, Funambulus pennanti Wroughton. Journal of Re- production and Fertility, 17:235-245.
SAS INSTITUTE INC. 1989. SAS/STAT user's guide, version 6. Fourth edition. SAS Institute Inc., Cary, North Carolina, 1686 pp.
SOLLBERGER, D. E. 1943. Notes on the breeding hab- its of the eastern flying squirrel (Glaucomys volans). Journal of Mammalogy, 24:163-173.
SONENSHINE, D. E., D. M. LAUER, T. C. WALKER, AND B. L. ELISBERG. 1979. The ecology of Glaucomys volans (Linnaeus, 1758) in Virginia. Acta Therio- logica, 26:363-377.
UDAGAWA, T. 1954. The damage of forests by the giant flying squirrel and a new method of extermi- nation. Bulletin of Governmental Forest Experi- mental Station, Japan, 68:133-144 (in Japanese, En- glish summary).
Submitted 31 May 1991. Accepted 5 May 1993. Associate Editor was Joseph F. Merritt.