Molecular Cloning and Expression of a Phase I Flagellin Gene
from Salmonella enterica serovar Choleraesuis
Gan-Nan Chang
(1), Chen-Shan Chen
(2)and Kuo-Chieh Ho
(2,3)(Manuscript received 10 December, 2006; accepted 16 March, 2007)
ABSTRACT: P56, a protein with a molecular weight of 56 kDa, was isolated from the cell-free
supernatant of Salmonella enterica serovar Choleraesuis CH12440 (Salmonella Choleraesuis CH12440) culture. Comparison of the N-terminal amino acid sequence of P56 with the sequences in GenBank indicated that it was flagellin, the product of fliC gene and the subunit of flagellar filament. The gene encoding P56 was cloned and expressed. Although the gene displayed a 99.2 % nucleotide sequence homology and a 98.6 % amino acid sequence homology with other S. Choleraesuis phase-1-c flagellin genes, there was an insert of 36 nucleotides absent in other phase-1-c flagellin genes. The expressed P56 protein was toxic to macrophage cell Raw264.7, and caused the programmed death of the cells in vitro.
KEY WORDS: Cytotoxin, Flagellin, Salmonella enterica serovar Choleraesuis, Programmed death.
INTRODUCTION
Salmonella enterica serovar Typhi (S. Typhi), Salmonella enterica serovar Typhimurium (S.
Typhimurium) and Salmonella enterica serovar Choleraesuis (S. Choleraesuis) are three major pathogenic Salmonella species. They are etiological agents of typhoid fever, diarrhea and abdominal pain, and septicemia, enteritis and pneumonia, respectively (Nnalue, 1990; Salyers and Whitt, 1994; Chang and Tsai, 1996). S. Choleraesuis normally is a swine pathogen and occasionally causes systemic infection in human (Chang and Tsai, 1996). The endotoxin is considered a major virulence factor of Salmonella spp. and is the cause of death in people with systemic infection. Enterotoxin has also been reported for a large number of Salmonella serotypes and, at least in part, responsible for the loss of electrolytes and fluid from small intestine (Giannella et al., 1975; Caprioli et al., 1982; Singh et al., 1985).
In the past decade, the cytotoxins produced by enteric pathogens have been increasingly investigated. These toxins have been referred to as verotoxins because they are cytotoxic to Vero cells (Konowalchuk et al., 1977). Some cytotoxins are lethal to mammalian cells, such as Shiga toxin __________________________________________ 1.Department of Biotechnology, Tajen University, 20, Wei-Shin
Rd., Shin-II Tsun, Yan-Pu Hsiang, Pingtung 907, Taiwan. 2. Department of Life Science and Graduate Institute of Plant
Biology, National Taiwan University, 1, Sec. 4, Roosevelt Rd., Taipei 106, Taiwan.
3. Corresponding author. Tel: 886-2-33662508; Email: kch@ ntu.edu.tw
produced mainly by Shigella dysenteriae serotype 1 (Bartlett et al., 1986) and the closely related Shiga-like toxin (SLT) produced by Escherichia coli (Cleary et al., 1985; Marques et al., 1986). A similar cytotoxin is produced by Campylobacter jejuni (Guerrant et al., 1987). Although evidence has evolved regarding the role of cytotoxins produced by toxigenic organisms in the pathogenesis of particular diseases, very limited data are available concerning the cytotoxin produced by Salmonella spp. We previously isolated a cytotoxin from the cell-free supernatant of S. Choleraesuis culture, named P56. It caused degeneration and necrosis of mouse macrophage cell line P388-D1 in vitro, and hepatomegaly, splenomegaly and pneumonia in ICR mice following intravenous injection (Chang and Tsai, 1996). In this study, we demonstrated that cytotoxin P56 was flagellin, the subunit of flagellar filament.
Flagella are thin, hair-like and rigid appendages of bacteria, and are bacterial locomotive structures. A flagellum has three basic parts: The outmost and longest part is a filament which consists of around 20,000 protein subunits of a single protein called flagellin (FliC) with a molecular weight of 50 to 60 kDa. The flagellin is made within the cell and then passed along the hollow core of the helical filament to be added to the distal end. The filament is attached to a slightly wider and shorter hook which arises from the basal body. The basal body anchors the flagellum to the cell wall and plasma membrane, and is the motor of the flagellum (Kondoh and Hotani, 1974; Macnab, 1996).
Flagella have been recognized as one of the potential virulence factors of microorganisms. However, most suspicion has been focused on motility necessary for colonization and the aid of virulence factor secretions (Penn and Luke, 1992; Josenhans and Suerbaum, 2002; Hirano et al., 2003). In this communication, we reported the cloning and characterization of the gene encoding P56. The expressed P56 was directly involved in the pathogenesis of bacteria.
MATERIALS AND METHODS
Bacterial strain
Salmonella Choleraesuis CH12440 was isolated
from lungs, hilus lymph node of lungs, spleen, liver, and gallbladder of pigs naturally infected with systemic septicemic salmonellosis (Chang and Tsai, 1996).
Cell line
Murine macrophage cell line RAW264.7 was purchased from American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 medium supplemented with 10 % fetal calf serum.
Amino acid determination
The purified P56 protein showed a single band on a 9% SDS-polyacrylamide gel (SDS-PAGE) was used for amino acid sequence determination. The N-terminal amino acid sequence of protein was determined by Edman degradation using an ABI Procise model 491 protein sequencer (Applied Biosystem, USA) according to the method described by Tam et al. (1998).
Bacterial genomic and plasmid DNA isolation
Genomic DNA was isolated using the procedure described by Roussel and Chabbert (1978) with some modifications. A 300 mL overnight culture in LB broth (Luria-Bertani broth: 10g bacto-tryptone, 5g bacto-yeast extract, 10 g NaCl per liter) was harvested at 4,000xg. Pellet was suspended in 4 mL of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0), then 100 μL lysozyme (10 mg/mL in TE) and 100 μL RNAase A (10 mg/mL in water) were added. The solution was incubated at 37℃ for 30 min. Incubation was continued for 1 hr after addition of 1.5 mL proteinase k/sarkosine (5 mg proteinase k and 0.1 g sarkosine/mL TE). After centrifugation at 25,000xg, 20 min, 4 ℃ , the supernatant was transferred to a new tube and extracted with phenol/chloroform/isoamlyalcohol (25:24:1) twice, and then chloroform/isoamylalcohol (24:1) two
times. The aqueous layer was transferred to a new tube and mixed with 0.04 volumes of 5 M NaCl and 2.5 volume of 100% alcohol. The DNA was precipitated at 12,000xg, 4℃, 20 min after chilling at –20℃ for 1 hr. The pellet was rinsed with 75% alcohol, air dried and then resuspended in 1.5 mL TE.
Plasmid DNA was prepared by alkaline extraction procedure (Sambrook et al., 1989). For sequencing, plasmid DNA was isolated by QIAprep Spin Miniprep Kit (QIAGEN, Germany).
DNA probe preparation
After P56 was identified by partial protein sequencing as flagellin, two oligonucleotides (SC1: 5’-TGACCCAGAATAACCTG-3’ and SC2: 5’-GAGTCGAGGTCAGACTG-3’) were made according to the conserved regions of phase-1 and phase-2 flagellin genes of several S. Choleraesuis and S. Typhimurium strains (Joys, 1985; Vanegas and Joys, 1995; Wei and Joys, 1985). A 295-bp DNA fragment, named fragment FL295 was amplified on the S. Choleraesuis CH12440 genomic DNA using this pair of oligonucleotides in a PCR reaction. The PCR reaction was carried out as described previously (Ho et al., 1992). The fragment was cloned into pUC18 at SmaI site and sequenced to confirm the existence of the fliC sequence.
Genomic library construction and screening
Library construction and screening were done as described previously (Ho and Chang, 2000; Ho et al., 2001). The S. Choleraesuis CH12440 DNA was partially digested with EcoRI and cloned into λzap (ZAP Express System, Stratagene). The library contained 1.1 × 105 plaque-forming unit (pfu) with
greater than 95% recombinants determined in the presence of isopropyl-β-D-thiogalactopyranoside (IPTG) and 5-bromo-4-chioro-3-indolyl-β-D- galactoside (Xgal). The library was screened for the fliC gene by plaque hybridization using 32P-labeled
fragment FL295 as probe. The positive plaque areas were selected and re-screened until a single, isolated plaque could be picked up. A clone named λSC170 was obtained. The recombinant λ DNA was then converted into the phagemid pSC170.
Construction of flagellin gene in expression vector
In order to express P56 protein, a PCR DNA fragment containing the coding region of fliC was amplified on pSC170 DNA using oligonucleotides of Sal-B and Sal-MS (Sal-B: 5’-GCGGGATCCATGGCA CAAGTAATCAACAC-3’ and Sal-SM: 5’-TCC CCCGGGTTAACGCAGTAAAGAGAGGAC-3’. The underlined sequences were the recognition site for
BamHI and SmaI, respectively.), and cloned into
expression vector pGEX-6P-1 (Amersham Biosciences Ltd) to generate a recombinant plasmid pSC15. The cloned DNA of pSC15 was subjected to sequencing to insure its entirety. The recombinant plasmid was used to transform E. coli DH5α.
Expression of the recombinant protein
The recombinant protein was expressed as follows: 2.5 mL of an overnight-culture were added to a 47.5 mL LB broth containing 50 μg/mL of ampicillin. The bacteria grew at 37℃until the OD600
of the culture was 0.7 to 0.9, then an IPTG solution was added to a final concentration of 1 mM. The cultivation was continued for 2 hr. The bacteria were harvested at 10,000xg, 4℃, 10 min and resuspended in 1 mL cold lysis buffer (50 mM NaH2PO4/300 mM
NaCl, pH 8.0) containing 1 mg lysozyme, and then incubated on ice for 30 min. The cells were sonicated on ice for 15 sec each with cooling pause of 15 sec in between at 30 W (Ultrasonic processor, grade 5, Heat systems, NY) until the lysate became transparent and yellowish. The bacterial debris was removed at 10,000xg, 4℃, 30 min. The recombinant proteins were further purified by Glutathione Sepharose 4B column according the instruction of manufacture (Amersham Biosciences Ltd) and/or then cleaved by PreScission protease. Proteins were analyzed on a 12% SDS-PAGE. Proteins were stored at –70℃ if they were not used immediately.
Purification of cytotoxin, preparation of antibodies, Western blot analysis and DNA sequence determination and cytotoxicity test
These experiments were performed as described previously (Ho et al., 2003).
Nucleotide sequence Accession numbers
The nucleotide sequence of fliC was deposited in GenBank (accession no. AF159459).
RESULTS
Cloning the gene encoding protein P56
In order to clone the gene coding for P56, the purified protein was subjected to the amino acid analysis by Edman degradation using an ABI Procise model 491 protein sequencer. A sequence of AlaGlnValIleAsnThrAsnSerLeuSerLeuLeuThrGln Asn from the N-terminus was obtained and demonstrated to be a part of amino acid sequence of flagellin, the product of fliC gene and the protein subunit of flagellar filament by comparison with the proteins deposited in GenBank.
A DNA fragment, named fragment FL295, containing 295 nucleotides of fliC was amplified in a PCR reaction on S. Choleraesuis CH12440 genomic DNA using a pair of primers (SC1 and SC2) designed according to the conserved regions of bacterial fliC genes. The fragment FL295 was used as a probe to screen a λZAP S. Choleraesuis genomic library, and a
positive clone (λSC170) containing a 3,787-bp insert DNA was obtained.
Comparison of the nucleotide sequence of the insert DNA with sequences of the genes in GenBank showed that it had a high homology with the sequences of fliD,
fliC, fliU and fliV genes of Salmonella enterica serovar
Muenchen (Doll and Frankel, 1993; Ho and Chang, 2000).
Characteristics of fliC gene
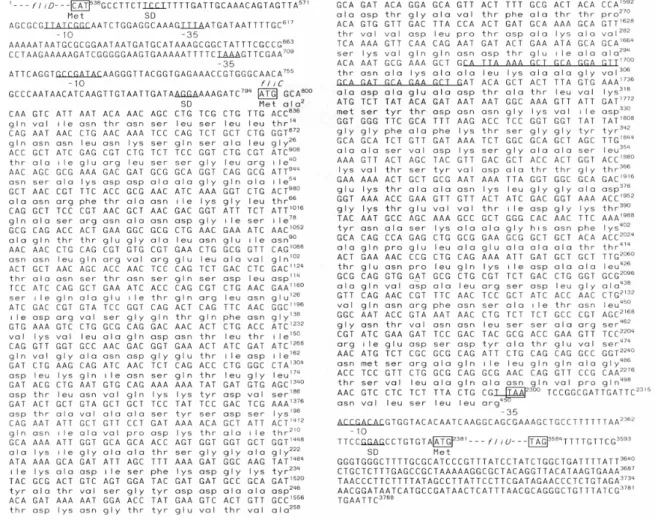
The fliC gene contained 1,506 nucleotides encoding a 501-amino acid flagellin protein (FliC) with a predicted molecular weight of 52 kDa and a pI of 4.7. The gene had an ATG as a start codon which was preceded by AGGA Shine-Dalgarno sequence, and flagellar operon-specific –10 (GCCGATAC) and –35 (TAAA) promoter consensus sequences (Kutsukake et al., 1990). Comparison with the S. Choleraesuis phase-1-c flagellin genes published by Wei and Joys (1985), the genes had a 99.2 % similarity in nucleotide sequence and 98.6 % in amino acid sequence. The high homology indicated our fliC was a phase-1 flagellin gene. However, there was a region of 36 nucleotides absent in other S. Choleraesuis phase-1-c flagellin genes (Fig. 1).
Expression of the recombinant proteins
E. coli DH5α was transformed with plasmid pSC15 to over-express the recombinant proteins in the presence of IPTG. The cell lysate was analyzed on a 12% SDS-PAGE. Figure 2 showed that a protein with a molecular weight of approximate 56 kDa (named as P56A, lane 4) was detected in the lysate of pSC15-transformed cells, but not in that of pGEX-6P-1 transformed ones. Western blot analysis shown in Fig. 3 indicated that expressed protein P56A could be stained by the antibody raised against purified P56.
Cytotoxicity assay for the P56 and P56A proteins
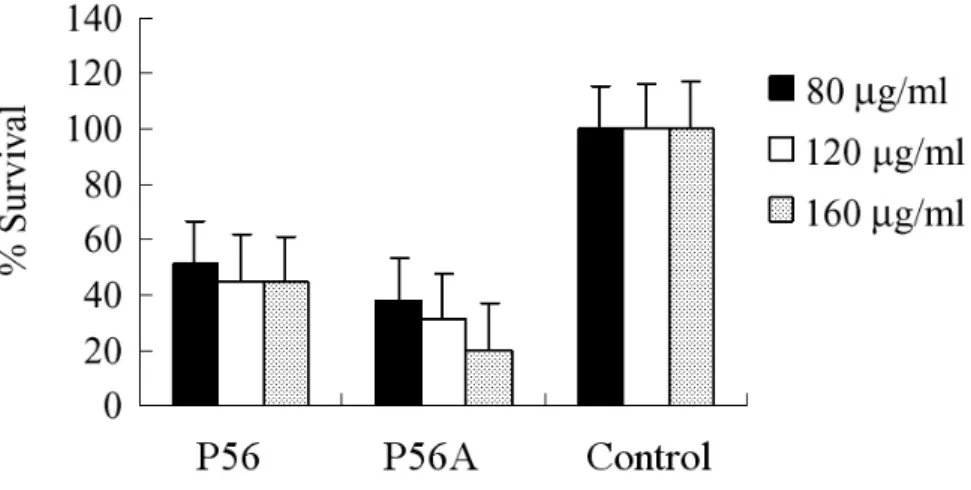
The cytotoxicity of P56 and P56A were tested on mouse macrophage cell line Raw264.7 by the MTT method. The macrophage cells were cultured in RPMI 1640 medium supplemented with 10 % fetal calf serum at 37℃ for 24 hr, and then different amounts of P56 or P56A were added. Figure 4 showed that the survival rate decreased significantly in cells treated with P56 and P56A.
Fig. 1. The nucleotide sequence of flagellar genes in pSC170. Complete nucleotide and deduced amino acid sequences of fliC were shown. ORFs of fliD and fliU were displayed by broken lines. Numbering of the nucleotide sequence commenced with the EcoRI site of insert DNA. Putative –10 and –35 regions of promoters, and Shine-Dalgarno (SD) sequences of each gene were underlined and indicated. The initiation and termination codons were boxed. The sequence of 36 nucleotides in fliC, absent from the same genes of other Salmonella spp. was underlined (nucleotide 1681 to 1716). The nucleotides of fliC in pSC15 different from those in pSC170 were C806→A, T809→C, T812→C, and A1089→T.
Fig. 2. Expression of FliC from pSC15 in E. coli DH5α. Proteins were extracted from cells by a buffer containing SDS, separated on a 12% SDS-PAGE and then stained with Coomassie blue. Lane M, protein size markers (kDa). Lane 1, proteins (50 μg) from pGEX-6P-1 transformants. Lane 2, proteins (50 μg) from pSC15 transformants. Lane 3, fusion protein of GST and P56A purified from pSC15 transformants by a glutathione sepharose 4B column, (20 μg). Lane 4, P56A, purified fusion protein of GST and P56A cleaved by PreScission protease (20 μg). Expression of proteins was induced by 1mM IPTG for 2 hr.
Fig. 3. Western blot analysis of expressed proteins. Lane M, protein size markers (kDa). Lane 1, cell lysate of E. coli DH5α. Lane 2, cell lysate of E. coli DH5α harboring pGEX-6P-1. Lane 3, cell lysate of E. coli harboring pSC15. Expression of proteins was induced by 1mM IPTG for 2 hr. The antibody was prepared against purified P56 from culture supernatant of S. Choleraesuis.
Fig. 4. Cytotoxic effect of P56 and P56A on mouse macrophage Raw264.7 cell line. The Raw264.7 cells were cultured in RPMI 1640 medium containing different amounts of P56 and P56A. The effect of protein on the cell viability was determined by the MTT method. P56 protein was isolated from culture supernatant. P56A was isolated from the purified fusion proteins (GST-P56A) cleaved by PreScission protease. The experiments were performed three times in triplicate.
The fusion protein of P56A and glutathione S-transferase (GST) had the same toxicity of P56A (data not shown).
To further characterize the mechanism of cell death resulting from P56, the DNA isolated from P56-treated and untreated Raw 264.7 cells were electrophoresed on a 1% agarose gel. DNA fragmentation in the ladder pattern was noted in P56-treated cells but not clearly identified in untreated ones as shown in Fig. 5. The result suggested that the cell death caused by P56 was involved apoptosis.
DISCUSSION
The production of toxin is one of major virulence factors involved in the pathogenesis of Salmonella spp. The enterotoxins are reported to be associated with some S. Typhimurium strains and the symptoms of typhoid fever probably result from the endotoxin, lipopolysaccharide (LPS) of the outer membrane- mediated release of cytokines. However, considerable tissue destruction observed during
Salmonella infection cannot be explained by damage
due to endotoxin or enterotoxin (Salyers and Whitt, 1994; O’Brien and Holmes, 1996). In fact,
Salmonella cytotoxins have long been suspected. In
1983, Koo and Peterson reported a heat-labile cytotoxin that inhibited protein synthesis in cell-free extract of Salmonella. A membrane-bound cytotoxin of S. Typhimurium was reported by Reitmeyer et al. (1986). The production of cytotoxins could account for the tissue damage (Reitmeyer et al., 1986). Libby
et al. (1990) cloned a gene encoding cytotoxin with haemolysin activity from S. Typhimurium. Kita et al. (1993) reported a cytotoxic protein of 32 kDa isolated from L-form S. Typhimurium, with an isoelectric point (pI) of 6.4. This toxin was thermolabile and sensitive to trypsin. It could stimulate macrophages to produce a significant amount of tumor necrosis factor alpha (TNF-alpha) and had a cytolytic effect at concentrations higher than 0.7 μg/mL. Rumeu et al. (1997) characterized another outer membrane-bound, heat-stable cytotoxin produced by S. enterica serovar Eenteritidis (S. Eenteritidis) and S. Typhimurium, that inhibited protein synthesis in culture cells as well as in the intestinal mucosa.
Fig. 5. DNA fragmentation of Raw 264.7 cells. DNA was isolated from Raw 264.7 cells treated (lane 1) or untreated (lane 2) with P56 at a concentration of 80 μg/mL for 72 hr, and then electrophoresed on a 1% agarose gel.
Previously, we have isolated a cytotoxin, P56, with a molecular weight of 56 kDa and a pI of 6.2 from the cell-free supernatant of the S. Choleraesuis CH12440 culture. This cytotoxin caused hepatomegaly, splenomegaly and interstitial pneumonia in mice. It also caused degeneration and necrosis of mouse macrophage cell line P388-D1 in
vitro. A negative Shwartzman’s reaction indicated
that it was not an LPS endotoxin (Chang and Tsai, 1996). P56 can be detected as early as 3 hr in the supernatant of the culture. The secretion of this protein increased by a factor of 3.2 when the cells were grown in BHI supplemented with 10 mM CaCl2, compared to that in the medium without CaCl2
(Ho et al., 2003).
In this communication, the gene encoding P56 was cloned and characterized. The partial amino acid sequence of P56 indicated that it was the protein flagellin of S. Choleraesuis CH12440. A fluorescence confocal microphotograph showed that the periplasm and flagella of S. Choleraesuis were stained with the antibody raised against P56 (data not shown). This result agreed with that flagellin is secreted into the periplasm where it is exported to assemble the flagellum.
Flagella are reported not only necessary for motility but also important for the virulence and invasion of Vibrio cholerae (Freter and Jones, 1976; Attridge and Rowley, 1983) and Vibrio vulnificus (Lee et al., 2004). Drake and Montie (1988) demonstrated that Fla- variants of Pseudomonas
aeruginosa had a diminished virulence and immotile
Fla+ variants lost virulence in animal models. In C.
jejuni, the flagella played an important role in
attachment and invasion (Wassenaar et al., 1991). It was reported that two flagellar genes, fliA and flgM involved in regulation of the flagellar operon might be involved in regulating the expression of virulence genes in S. Ttyphimurium (Carsiotis et al., 1989; Gillen and Hughes, 1991; Schmitt et al., 1994) and that flagella could help S. Typhimurium to survive within murine macrophages (Weinstein et al., 1984). Analysis of nonflagellated Salmonella strains revealed a correlation between the ability to induce TNF-alpha and the expression of the phase-1 filament subunit protein FliC. Furthermore,
Salmonella FliC could restore the TNF-alpha
inducing phenotype in E. coli, which otherwise lacked the activity (Ciacci-Woolwine et al., 1998). Flagellar formation was also suggested to contribute to the virulence of Erwinia carotovora subsp.
carotovora significantly in plants (Matsumoto et al.,
2003).
The overlap between motility and the secretion of virulence proteins has been noted in many organisms. There are significant homologies between several flagellar proteins and proteins involved in the expression of virulence genes, the export of virulence proteins, or both, in plant and animal pathogens. The type III secretion mechanism of bacteria exports many virulence effector proteins involved in subversion of eukaryotic cells. The system requires more than 20 proteins, and its core components are homologous to proteins essential for the assembly of flagella (Harshey and Toguchi, 1996; Bennett and Hughes, 2000).
Although motility and flagella were suggested to play role in bacterial virulence, the role of the flagellum in pathogenesis has not yet been proved directly. Our data provide the evidence that flagellum is directly involved in the pathogenesis of bacteria.
Note: The whole genome of a local S. Choleraesuis isolate with the insert of 36 nucleotides in fliC was sequenced and published after our work was completed (Chiu et al., 2005).
ACKNOWLEDGMENTS
We thank Dr. M. F. Tam, Researcher of the Institute of Molecular Biology, Academia Sinica, Taiwan for determining the N-terminal sequences of the purified P56. We also thank Dr. Te-Chang Lee, Researcher of the Institute of Biomedical Science, Academia Sinica, Taiwan for doing DNA autosequencing. Finally, thanks should go to M. Conrad of University of North Carolina at Chapel Hill for his critical reading and suggestions. This work was partially supported by grant NSC 92-2621-B-002-010 from National Science Council of Taiwan to K.-C. Ho.
LITERATURE CITED
Attridge, S. R. and D. Rowley. 1983. The role of the flagellum in the adherence of Vibrio cholerae. J. Infect. Dis. 147: 864-872.
Bartlett, A. V., D. Prado III, T. G. Cleary and L. K. Pickering. 1986. Production of Shiga toxin and other cytotoxins by serogroups of Shigella. J. Infect. Dis. 154: 996-1002.
Bennett, J. C. and C. Hughes. 2000. From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol. 8: 202-204.
Caprioli, A., G. D'Agnolo, V. Falbo, L. G. Roda and M. Tomasi. 1982. Isolation of Salmonella wien heat-labile enterotoxin. Microbologica 5: 1-10. Carsiotis, M., B. A. Stocker, D. L. Weinstein and A.
D. O'Brien. 1989. A Salmonella typhimurium virulence gene linked to flg. Infect. Immun. 57: 3276-3280.
Chang, G.-N. and S.-S. Tsai. 1996. Characterization of a cytotoxin "P65" produced by Salmonella
choleraesuis: (I) Histopathological study on the
toxicity of P65 in ICR mice. J. Chin. Soc. Vet. Sci. 22: 331-339. (in Chinese, with English abstract)
Chiu, C.-H., P. Tang, C. Chu, S. Hu, Q. Bao, J. Yu, Y.-Y. Chou, H.-S. Wang and Y.-S. Lee. 2005. The genome sequence of Salmonella enterica serovar Choleraesuis, a highly invasive and resistant zoonotic pathogen. Nucl. Acids Res. 33: 1690-1698.
Ciacci-Woolwine, F., I. C. Blomfield, S. H. Richardson and S. B. Mizel. 1998. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect. Immun. 66: 1127-1134.
Cleary, T. G., J. J. Mathewson, E. Faris and L. K. Pickering. 1985. Shiga-like cytotoxin production by enteropathogenic Escherichia coli serogroups. Infect. Immun. 47: 335-337.
Doll, L. and G Frankel. 1993. fliU and fliV: two flagellar genes essential for biosynthesis of
Salmonella and Escherichia coli flagella. J. Gen.
Microbiol. 139: 2415-2422.
Drake, D. and T. C. Montie. 1988. Flagella motility and invasive virulence of Pseudomonas
aeruginosa. J. Gen. Microbiol. 134: 43-52.
Freter, R. and G. W. Jones. 1976. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surface. Infect. Immun. 14: 246-256.
Giannella, R. A., R. E. Gots, A. N. Charney, W. B. Greenough and S. B. Formal. 1975. Pathogenesis of Salmonella-mediated intestinal fluid secretion: activation of adenylate cyclase and inhibition by indomethacin. Gastroenterology 69: 1238-1245. Gillen, K. and K. T. Hughes. 1991. Molecular
characterization of flgM, a gene encoding a negative regulator of flagellin synthesis in
Salmonella typhimurium. J. Bacteriol. 173:
6453-6459.
Guerrant, R. L., C. A. Wanke and R. A. Pennie. 1987. Production of unique cytotoxin by
Campylobacter jejuni. Infect. Immun. 55:
2526-2530.
Harshey, R. M. and A. Toguchi. 1996. Spinning tails: homologies among bacterial flagellar systems. Trends Microbiol. 4: 226-231.
Hirano, T., T. Minamino, K Namba and R. M. Macnab. 2003. Substrate specificity classes and the recognition signal for Salmonella Type III flagellar export. J. Bacteriol. 185: 2485-2492. Ho, K.-C. and G.-N. Chang. 2000. The fliU and fliV
genes are expressed as a single ORF in
Salmonella choleraesuis. Can. J. Microbiol. 46:
1149-1152.
Ho, K.-C., D.-C. Kuo and G.-N. Chang. 2003. Biphasic modulation of mouse macrophage cell growth by the Salmonella choleraesuis cytotoxin P56. Taiwania 48: 224-231.
Ho, K.-C., V. E. Quarmby, F. S. French and E. M. Wilson. 1992. Molecular cloning of rat prostate transglutaminase complementary DNA: The major androgen-regulated protein DP1 of rat dorsal prostate and coagulating gland. J. Biol. Chem. 267: 12660-1266.
Ho, K.-C., C.-C. Tsai and T.-L. Chung. 2001. Organization of ribosomal genes from a loofah witches' broom phytoplasma. DNA Cell Biol. 20: 112-122.
Josenhans, C. and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291: 605-614.
Joys, T. M. 1985. The covalent structure of the phase-1 flagellar filament protein of Salmonella
typhimurium and its comparison with other
flagellins. J. Biol. Chem. 260: 15758-15761. Kita, E., N. kamikaidou, A. Nakano and S. Kashiba.
1993. Isolation of a cytotoxin from L-form
Salmonella typhimurium. FEMS Microbiol. Lett. 103: 179-184.
Kondoh, H. and H. Hotani. 1974. Flagellin from
Escherichia coli k12: polymerization and
molecular weight in comparison with Salmonella flagellin. Biochim. Biophys. Acta 336: 117-139. Konowalchuk, J., J. I. Speirs and S. Stavric. 1977.
Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18: 775-779.
Koo, F. C. W. and J. W. Peterson. 1983. Cell-free extracts of Salmonella inhibit protein synthesis and cause cytotoxicity in eukaryotic cells. Toxicon 21: 309-320.
Kutsukake, K., Y. Ohya and T. Iino. 1990. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 172: 741-747.
Lee, J.-H., J. B. Rho, K. J. Park, C. B. Kim, Y.-S. Han, S. H. Choi, K.-H. Lee and S. J. Park. 2004. Role of flagellum and motility in pathogenesis of
Vibrio vulnificus. Infect. Immun. 72: 4905-4910.
Libby, S. J., W. Goebel, S. Muir, J. G. Songer and F. Heffron. 1990. Cloning and characterization of a cytotoxin gene from Salmonella typhimurium. Res. Microbiol. 141: 775-783.
Macnab, R. M. 1996. Flagella and motility. In: Neidhardt, F. C., R. Curtiss III, J. L. Ingraham, E.C.C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter and H. E. Umbarger (eds.), Escherichia coli and
Salmonella typhimurium: cellular and molecular
biology, 2nd ed. ASM Press, Washington, D.C., USA. pp. 123-145.
Marques, L. R. M., M. A. Moore, J. G. Wells, I. K. Wachsmuth and A. D. O'Brien. 1986. Production of Shiga-like toxin by Escherichia coli. J. Infect. Dis. 154: 338-341.
Matsumoto, H., H. Muroi, M. Umehara, Y. Yoshitake and S. Tsuyumu. 2003. Peh production, flagellum synthesis, and virulence reduced in Erwinia carotovora subsp. carotovora by mutation in a homologue of cytR. Mol. Plant-Microbe Interact. 16: 389-397.
Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65: 55-63.
Nnalue, N. A. 1990. Mice vaccinated with a non-virulent, aromatic-dependent mutant of
Salmonella choleraesuis die from challenge with
its virulent parent but survive challenge with
Salmonella typhimurium. J. Med. Microbiol. 31:
225-233.
O'Brien, A. D. and R. K. Holmes. 1996. Protein toxins of Escherichia coli and Salmonella. In: Neidhardt, F. C., R. Curtiss III, J. L. Ingraham, E.C.C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter and H. E. Umbarger (eds.), Escherichia coli and
Salmonella typhimurium: cellular and molecular
biology, 2nd ed. ASM Press, Washington, D.C., USA. pp. 2788-2802.
Penn, C. W. and C. J. Luke. 1992. Bacterial flagellar diversity and significance in pathogenesis. FEMS Microbiol. Lett. 79: 331-336.
Reitmeyer, J. C., J. W. Peterson and K. J. Wilson. 1986. Salmonella cytotoxin: a component of the bacterial out membrane. Microbial. Pathog. 1: 503-510.
Roussel, A. F. and Y. A. Chabbert. 1978. Taxonomy and epidemiology of gram- negative bacterial
plasmids studied by DNA-DNA filter hybridization in formamide. J. Gen. Microbiol.
104: 269-276.
Rumeu, M. T., M. A. Suarez, S. Morales and R. Rotger. 1997. Enterotoxin and cytotoxin production by Salmonella enteritidis strains isolated from gastroenteritis outbreaks. J. Appl. Microbiol. 82: 19-31.
Salyers, A. A. and D. D. Whitt. 1994. Bacterial pathogenesis: A molecular approach. ASM Press, Washington, D. C., USA. pp. 229-243.
Sambrook, J., E. F. Fritsch and T. Maniatis. 1989. Molecular cloning: A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY., USA. pp. 125-130.
Schmitt, C. K., S. C. Darnell, V. L. Tesh, V. A. D. Stocker and A. D. O'Brien. 1994. Mutation of
flgM attenuates virulence of Salmonella typhimurium, and mutation of fliA represses the
attenuated phenotype. J. Bacteriol. 176: 368-377. Singh, S. P., V. D. Sharma and I. P. Singh. 1985.
Isolation of an enterotoxic factor from culture supernatant of Salmonella saintpaul. FEMS Microbiol. Lett. 26: 301-304.
Tam, M. F., C.-H. Hsieh, S.-P. Tsai and T.-C. S. Tam. 1998. Amino acid sequence of glutathione S-transferase rGSTM5* from rat testis. Biochem. J. 333: 735-739.
Vanegas, R. A. and T. M. Joys. 1995. Molecular analyses of the phase-2 antigen complex 1.2 of
Salmonella spp. J. Bacteriol. 177: 3863-3864.
Wassenaar, T. M., N. M. C. Bleumink-Pluym and M. A. M. van der Zeijst. 1991. Inactivation of
Campylobacter jejuni flagellin genes by
homologous recombination demonstrates that
flaA but not flaB is required for invasion. The
EMBO J. 10: 2055-2061.
Wei, L.-N. and T. M. Joys. 1985. Covalent structure of three phase-1 flagellar filament proteins of
Salmonella. J. Mol. Biol. 186: 791-803.
Weinstein, D. L., M. Carsiotis, C. R. Lissner and A. D. O'Brien. 1984. Flagella help Salmonella
typhimurium survive within murine