行政院國家科學委員會專題研究計畫 成果報告
比較異位性皮膚炎病人在使用局部類固醇、tacrolimus、
抗生素前後,疾病的臨床嚴重度與皮膚上金黃色葡萄球菌
的增生率及菌落密度
研究成果報告(精簡版)
計 畫 類 別 : 個別型
計 畫 編 號 : NSC 95-2314-B-002-183-
執 行 期 間 : 95 年 08 月 01 日至 96 年 07 月 31 日
執 行 單 位 : 國立臺灣大學醫學院小兒科
計 畫 主 持 人 : 林于粲
共 同 主 持 人 : 江伯倫
計畫參與人員: 碩士級-專任助理:李秀霞
處 理 方 式 : 本計畫可公開查詢
中 華 民 國 96 年 10 月 03 日
Staphylococcus colonization in atopic dermatitis
treated with fluticasone or tacrolimus with or
without antibiotics
Shuo-Hsun Hung, MD*; Yu-Tsan Lin, MD, PhD*; Chia-Yu Chu, MD†; Chien-Chang Lee, MD*‡;
Tien-Chi Liang, MD*; Yao-Hsu Yang, MD, PhD*; Li-Chieh Wang, MD*; and
Bor-Luen Chiang, MD, PhD*
Background: The skin of patients with atopic dermatitis (AD) exhibits a striking susceptibility to colonization and infection
by Staphylococcus aureus. Treatment with topical anti-inflammatory drugs alone can reduce S aureus colonization.
Objectives: To compare the clinical severity of AD and the S aureus colonization rate between AD patients treated with topical
glucocorticoids and those treated with tacrolimus and to evaluate the effects of complementary topical antistaphylococcal antibiotic therapy and the development of fusidic acid–resistant S aureus.
Methods: Sixty AD patients were enrolled in a prospective, parallel, randomized study of an 8-week treatment with topical
0.05% fluticasone propionate or 0.03% tacrolimus, with or without complementary fusidic acid. Disease severity scoring of AD based on SCORing of Atopic Dermatitis (SCORAD), colonization rate and density of S aureus on the skin, and antibiotic susceptibility of S aureus isolates were evaluated.
Results: The reduction in SCORAD scores correlated with the reduction of S aureus numbers. Treatment with topical
tacrolimus resulted in a comparable reduction in SCORAD scores to fluticasone but a slower eradication of S aureus. Complementary fusidic acid had no additional benefit compared with fluticasone or tacrolimus alone. Two patients developed fusidic acid–resistant S aureus after 8 weeks of fusidic acid treatment.
Conclusion: Tacrolimus is an appropriate alternative treatment for chronic AD. Topical anti-inflammatory therapy alone to
improve the allergic skin inflammation of AD can reduce S aureus colonization of the skin. Topical antibiotics should be reserved for short-term use in obvious secondary bacterial infection.
Ann Allergy Asthma Immunol. 2007;98:51–56.
INTRODUCTION
Atopic dermatitis (AD) is a chronically relapsing pruritic inflammatory skin disorder accompanied by elevated total serum IgE levels and associated with other atopic diseases, such as asthma or allergic rhinitis.1The prevalence of AD is
continuously increasing in developed countries. The skin of AD patients exhibits a striking susceptibility to colonization and infection by Staphylococcus aureus, which can secrete various exotoxins, including staphylococcal enterotoxin A and B (SEA and SEB).2– 4These exotoxins are superantigens.
Staphylococcal superantigens (SsAgs) may penetrate the skin barrier and contribute to the persistence and exacerbation of allergic skin inflammation in AD.1,2,4,5 In addition, SsAgs
may act as allergens and thus induce the production of func-tionally relevant SsAg-specific IgE antibodies in AD pa-tients.3,4
Although topical glucocorticoids are still considered the standard treatment for AD, adverse effects such as skin atrophy, striae, and telangiectasia are not negligible. Glu-cocorticoids are potent immunomodulatory hormones that
adversely affect most cells of the immune system.1 SsAgs
were recently shown to induce glucocorticoid insensitivity of immune cells.6Topical tacrolimus, a calcineurin inhibitor, is
more specific in immunosuppressive action than glucocorti-coids and may be highly effective in suppressing
SsAg-induced inflammation.7 Therefore, tacrolimus provides an
alternative treatment for patients with AD resistant to glu-cocorticoids. Previous studies demonstrated that treatment of AD exacerbations with topical anti-inflammatory drugs alone, whether glucocorticoids8 –14or tacrolimus,15,16caused a
significant reduction of S aureus skin colonization. However, a comparison of the effectiveness of glucocorticoids or ta-crolimus in reducing S aureus colonization has not been reported. The aim of this study was to compare the clinical severity of AD and the S aureus colonization rate in AD patients treated with either topical glucocorticoids or tacroli-mus.
Because S aureus plays an important role in the pathogen-esis of AD, antistaphylococcal antibiotic therapy may be important for the disease control of AD.2,17The topical
anti-biotic most commonly used for the reduction of S aureus on
* Department of Pediatrics, National Taiwan University Hospital and Na-tional Taiwan University College of Medicine, Taipei, Taiwan.
† Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan. ‡ Department of Emergency Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan. Received for publication April 20, 2006.
AD skin is fusidic acid, which is effective in the inhibition of methicillin-resistant S aureus.1,17As a secondary objective of
the study, we evaluated the effects of complementary topical fusidic acid therapy on the clinical severity of AD and the S
aureus colonization rate and the development of fusidic acid–
resistant S aureus after treatment.
PATIENTS AND METHODS Study Populations
Sixty AD patients who visited the Department of Dermatol-ogy of the National Taiwan University Hospital as outpatients from February 2004 to February 2005 were recruited for the study. The local ethical committee approved the study, and written informed consent was obtained from each patient or a parent. Criteria for entry to the study were as follows: (1) AD diagnosed according to the criteria of Hanifin and Rajka18; (2)
neither systemic nor topical antibiotics and neither systemic nor topical corticosteroids were used within 4 weeks before entry; (3) no clinical signs of overt secondary infection that obviously needed oral antibiotic therapy; and (4) the AD severity grading was moderate to severe at the time of entry into the study according to the criteria of Rajka and
Lange-land.19 Twenty-six of them were male and 34 were female.
Their ages ranged from 9 months to 33 years, with a mean age of 15.6 years.
Treatment Protocol
The study design was parallel, randomized, and open labeled. Patients were randomly allocated to treatment with 1 of the 4 regimens: a topical application of 0.05% fluticasone propi-onate cream (Cutivate; Glaxo Operations Ltd, Durham, En-gland) twice daily (in the morning and night) for 8 weeks, with or without complementary 2% fusidic acid cream (Fu-cidin; LEO Laboratories, Ltd, Dublin, Ireland); or a topical application of 0.03% tacrolimus ointment (0.03% Protopic; Astellas Pharma, Inc, New York, NY) twice daily (in the morning and night) for 8 weeks, with or without complemen-tary 2% fusidic acid cream. Fluticasone propionate is a mod-erate-potency glucocorticoid, rated in class V in a 7-class arrangement of glucocorticoid potency. The AD patients were instructed to apply the treatment regimen to all affected areas, without occlusive dressings. In the patients with com-plementary fusidic acid cream use, fusidic acid was applied first to all affected areas and followed by application of fluticasone propionate or tacrolimus 20 minutes later. In addition, the use of medicated soaps or detergents was not allowed throughout the study period. The only other topical preparations allowed were the patients’ usual moisturizers, which were instructed to be applied immediately after bathing throughout the study. Oral antihistamine (cetirizine; UCB Pharma, Pianezza, Italy) was given to all patients.
Clinical and Laboratory Evaluations
At the time of enrollment (day 0), both nostrils were exam-ined for S aureus colonization, and the levels of total serum IgE and serum SEA- or SEB-specific IgE were measured by
the Pharmacia CAP assay (Pharmacia and Upjohn, Uppsala, Sweden) according to the manufacturer’s instructions. Clin-ical and laboratory evaluations were performed before treat-ment (at day 0) and after 2 and 8 weeks of treattreat-ment. At these 3 time points, swabs (BBL, Becton, Dickinson and Company, Sparks, MD) for S aureus cultures were taken from the same designated skin lesion, which was the most severe local lesion at the time of enrollment (day 0). Overall clinical severity of AD was evaluated using the SCORing of Atopic Dermatitis (SCORAD) index (range, 0 –103) by 2 separate clinicians (S.-H.H. and C.-Y.C.) at each visit.20Local clinical
severity of AD was evaluated using the modified local SCO-RAD index with 6 intensity items: (1) erythema/darkening; (2) edema/papulation; (3) oozing/crusts; (4) excoriation; (5) lichenification/prurigo; and (6) local dryness. Each item was
graded on a 4-point scale (0 ⫽ absent; 1 ⫽ mild; 2 ⫽
moderate; 3⫽ severe). Scores ranged from 0 to 18.
Bacteriological Protocol
All specimens for bacteriology were coded and processed blindly by the same bacteriologist. Cultures from both ante-rior nostrils were taken using one 360° clockwise rotation of a swab. The most severe local lesion was sampled using a modification of the scrub technique developed by Williamson
and Kligman.21 Bacteria were collected using an electric
rotating blade in 1 mL of tryptic soy broth (pH 7.3) wash solution. Samples of 0.1 mL of the wash solution were inoculated on trypticase soy agar with 5% sheep blood (TSA II, BBL) culture plates using a standard streak method. Plates were incubated at 35°C for 2 days, and a quantitative and qualitative analysis was then conducted. Coagulase-positive S
aureus was identified by testing typical colonies for
coagu-lase activity (BactiStaph Latex, Remel, Lenexa, KS). The colonization density was calculated by counting the number of colony-forming units per 1 cm2 of the investigated skin
surface. S aureus isolates with more than 101 CFU/cm2 but
less than 106CFU/cm2were considered colonized.2Antibiotic
sensitivity testing of S aureus strains was performed on Mueller-Hinton agar (BBL), using the disk diffusion method, and was interpreted according to the Clinical Laboratory Standard Institute comparative method standard.
Statistical Analyses
The data are expressed as mean ⫾ SEM. The data were
analyzed by the principle of intention-to-treat analysis. The clinical scores were compared by nonparametric methods, including the Mann-Whitney U test between 2 treatment groups and the Wilcoxon signed-rank test between different time points in each treatment group. The numbers of patients who had S aureus skin colonization were compared by2test
among different groups. Correlation between skin lesion se-verity and colonization density was established using the Spearman rank correlation. SPSS statistical software, version 12.0 for Windows (SPSS Inc, Chicago, IL), was used for statistical analyses. All tests were 2-tailed, and P⬍ .05 was considered statistically significant.
RESULTS
Patient Characteristics Before Treatment
The initial overall clinical severity of AD by SCORAD was
55.3 ⫾ 1.9. The initial local clinical severity of AD by
modified SCORAD was 10.8⫾ 0.4. Forty-nine (82%) of the
60 patients were colonized with S aureus. In 35 patients (58%), both lesional skin and anterior nostrils were colo-nized. In 8 patients (13%), S aureus colonized only the skin, and in 6 patients (10%), only the anterior nostrils. The density of S aureus isolated from the skin lesions varied between 101.0
and 104.7CFU/cm2. The mean density was 103.4CFU/cm2. A
significant but low correlation was found between the colo-nization density of S aureus on the skin lesions and the local clinical severity of AD at the time of enrollment in the study
(R⫽ ⫹0.320, P ⫽ .01).
Before treatment, no significant difference was found in the rate or density of S aureus colonization and in the overall or local clinical severity of AD among the 4 treatment groups (Table 1). Serum specific IgE antibodies to SEA or SEB were detected in 33 patients (57%). Fifty-four of the 60 patients completed the study. Two patients receiving tacrolimus with complementary fusidic acid dropped out of the treatment protocol because of intolerance to a burning sensation. Two patients receiving tacrolimus only and another 2 receiving fluticasone only dropped out of the treatment protocol be-cause of poor compliance.
Comparison Between Patients Treated With Fluticasone and Tacrolimus
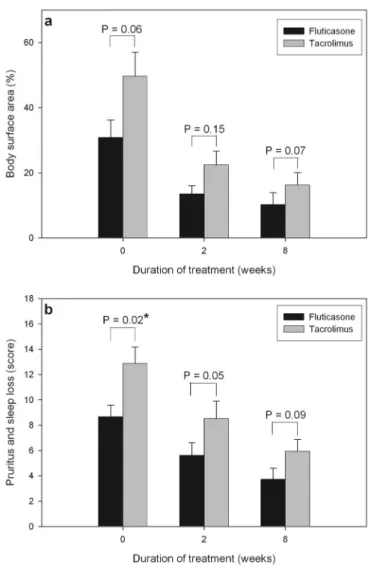
Before and after treatment, no significant difference was found in body surface area involved by AD between patients treated with fluticasone and tacrolimus (Figure 1a). The pa-tients treated with tacrolimus had higher initial subjective scores of pruritus and sleep loss than those treated with fluticasone, but no significant difference was found between the 2 treatment groups after treatment (Figure 1b).
The clinical severity scores (SCORAD) significantly de-creased after 2 and 8 weeks of treatment in both the
flutica-Figure 1. Comparison of patients treated with fluticasone and tacrolimus. a, Body surface area involved by atopic dermatitis before and after treatment. b, Subjective scores of pruritus and sleep loss before and after treatment. * P⬍ .05.
Table 1. Characteristics of Patients Before Treatment
Characteristics Fluticasone (nⴝ 15) Tacrolimus (nⴝ 15) Fluticasone and fusidic acid (nⴝ 15) Tacrolimus and fusidic acid (nⴝ 15)
Age, mean⫾ SEM, y 17.4⫾ 2.6 15.4⫾ 2.3 12.9⫾ 2.6 16.9⫾ 2.3
Sex, M:F 4:11 8:7 8:7 6:9
Overall AD severity (SCORAD), mean⫾ SEM 54.7⫾ 4.3 56.7⫾ 3.7 50.0⫾ 3.2 59.9⫾ 4.2 Local lesions severity (modified SCORAD), mean⫾ SEM 10.6⫾ 0.8 10.6⫾ 0.6 11.0⫾ 0.7 10.9⫾ 0.9 Colonization rate of Staphylococcus aureus, No. (%) 11 (73) 13 (87) 8 (53%) 13 (87) Colonization density of S aureus, mean⫾ SEM, log10CFU/
cm2
2.5⫾ 2.3 3.7⫾ 3.5 3.4⫾ 3.1 3.1⫾ 2.7 Total IgE, mean⫾ SEM, kU/L 4,552⫾ 1,765 3,299⫾ 1,109 4,283⫾ 1,440 3,316⫾ 936 SEA-specific IgE, No. (%) 6 (40) 8 (53) 6 (40) 7 (47) SEB-specific IgE, No. (%) 8 (53) 9 (60) 8 (53) 7 (47) Abbreviations: AD, atopic dermatitis; SCORAD, SCORing of Atopic Dermatitis; SEA, staphylococcal enterotoxin A; SEB, staphylococcal entero-toxin B.
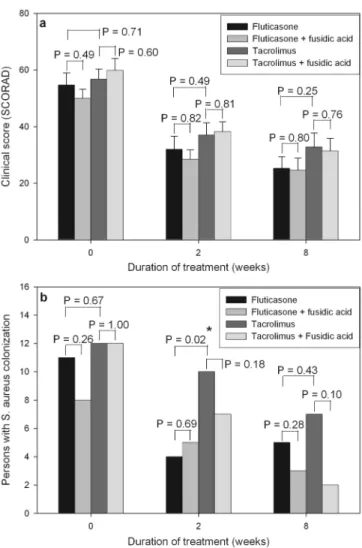
sone group (P⫽ .001) and the tacrolimus group (P ⫽ .002) (Figure 2a). Before and after treatment, there was no signif-icant difference in clinical severity scores between patients treated with fluticasone and tacrolimus. Furthermore, there was no significant difference in the decrease of clinical se-verity scores between the 2 treatment groups.
During treatment, the reduction of S aureus colonization moderately paralleled the improvement of eczema (r⫽ .436,
P ⬍ .001). After 2 weeks of treatment, the patients treated
with fluticasone had a lower colonization rate of S aureus than those treated with tacrolimus (Figure 2b). However, there was no significant difference in the colonization rate of
S aureus between the 2 treatment groups after 8 weeks of
treatment.
Comparison Between Patients Treated With and Without Complementary Fusidic Acid
The clinical severity scores significantly decreased after 2 and 8 weeks of treatment in the 4 treatment groups (all P⬍ .05) (Figure 2a). In both the fluticasone group and the tacroli-mus group, before and after treatment, no significant differ-ence was found in clinical severity scores between patients treated with or without complementary fusidic acid (Figure 2a). Furthermore, no significant difference was found in the decrease of clinical severity scores between patients treated with or without complementary fusidic acid.
The colonization rate of S aureus was decreased after 2 and 8 weeks of treatment in the 4 treatment groups (Figure 2b). In the tacrolimus group, the patients treated with complemen-tary fusidic acid had a lower colonization rate of S aureus than those treated without complementary fusidic acid, al-though the difference was not statistically significant (Figure 2b). Furthermore, no significant difference was found in the decrease in the colonization rate of S aureus between patients treated with or without complementary fusidic acid.
S aureus was eliminated in 82% of patients treated with
complementary fusidic acid compared with 54% of patients treated without complementary fusidic acid. The colonization density of S aureus decreased from 103.3⫾ 103.6to 102.5⫾
103.3 CFU/cm2 in the patients treated with complementary
fusidic acid and from 103.5⫾ 104.0to 102.9⫾ 103.5CFU/cm2
in those treated without complementary fusidic acid.
Antibiotic-Resistant Strains of S aureus
Ninety-eight isolates of S aureus were cultured from lesional skin during the study period. Among the 61 isolates cultured from patients treated without complementary fusidic acid, 43 (70%) were methicillin sensitive, 16 (26%) were methicillin resistant, and 2 (3%) were fusidic acid resistant. In the pa-tients treated with fluticasone or tacrolimus alone, the anti-biotic sensitivity pattern was not changed. However, in the patients treated with complementary fusidic acid, the percent-age of both methicillin-sensitive and methicillin-resistant strains decreased significantly (P ⫽ .04). Of the 5 patients with persistent S aureus colonization, 2 (40%) developed fusidic acid–resistant strains after 8 weeks of fusidic acid treatment.
DISCUSSION
It is well established that skin colonization with S aureus may contribute to the persistence and exacerbation of AD.1,2,4,5,17S
aureus is not considered a member of the resident skin
microflora in healthy populations whose carry rate is less than
10%.2 Eighty-three percent of our AD patients were
colo-nized with S aureus. The density of S aureus isolated from the skin lesions in our AD patients varied between 101.0and
104.7 CFU/cm2, which is compatible with the findings in
previous studies.12,22 During treatment, the reduction of S
aureus colonization paralleled the improvement in eczema,
which verified earlier investigations that had shown a
signif-Figure 2. Comparison of patients treated with fluticasone and tacrolimus with and without complementary fusidic acid. a, Clinical severity scores (SCORing of Atopic Dermatitis) before and after treatment. b, Numbers of patients with Staphylococcus aureus colonization before and after treatment. * P⬍ .05.
icant correlation between colonization density and clinical severity of AD.11–16,22,23
Reitano et al24found that the efficacy of 0.03% tacrolimus
ointment was higher than that of 1% hydrocortisone acetate (low-potency glucocorticoid) but lower than that of 0.1% hydrocortisone butyrate ointment (medium- to high-potency glucocorticoid). In our study, a comparable clinical improve-ment of AD was noted between topical 0.03% tacrolimus and 0.05% fluticasone propionate treatment, but a more rapid reduction of S aureus was found in patients treated with fluticasone. The slower clearance of S aureus in the tacroli-mus group was possibly attributed to the humid and occlusive environment of the ointment, which favored S aureus colo-nization. However, ointments as vehicles enhance the absorp-tion of the active ingredient of tacrolimus. With relatively large molecular size and high lipophilicity, the absorption of tacrolimus decreases as treatment continues and clinical im-provement occurs. Therefore, tacrolimus may be more suit-able than glucocorticoids for AD patients in intermittent or continuous long-term maintenance therapy.
These findings, combined with the fact that neither fluti-casone nor tacrolimus has a direct antistaphylococcal activity, are consistent with the concept of the inflammatory skin condition in AD being itself a major predisposing factor for colonization with S aureus. Indeed, S aureus colonization is both a cause and a consequence of allergic skin inflammation. Mechanisms by which allergic skin inflammation of AD promotes the increase in S aureus colonization include skin barrier dysfunction, increased synthesis of extracellular ma-trix adhesins for S aureus, and defective innate immune responses due to decreased production of endogenous anti-microbial peptides.1,4,14,25
It has been shown that systemic or topical antibiotic treat-ment without glucocorticoids can temporarily decrease the number of S aureus in skin lesions and lead to some improve-ment.9,21,26 –28However, these effects are not sustained after 4
to 8 weeks.26 –30In AD patients with obvious secondary skin
infection or S aureus colonization densities above 106CFU
per cm2, treatment with a combination of antistaphylococcal
antibiotic and topical glucocorticoid produces superior clini-cal effects to treatment with topiclini-cal glucocorticoids alone.9,31
In clinically noninfected areas of AD, although one can still isolate S aureus, little or no evidence exists for any additional benefit from antistaphylococcal therapy when the usual der-matitis treatment is used.17
The most contentious clinical situation is where there is no overt sign of infection but the eczema is moderate to severe. Wachs and Maibach8reported that the combination of topical
antibiotic and glucocorticoid gave a better clinical response than glucocorticoid alone. However, others have documented no additional benefit of systemic or topical antibiotics over glucocorticoid alone in patients with moderate to severe AD.10,12In our study, no significant difference was found in
clinical improvement and S aureus clearance between AD patients treated with or without complementary topical anti-biotics. A possible reason for persistent S aureus colonization
may be seeding from other reservoirs, such as the nasal cavity, unaffected skin, or family members.30Another
possi-ble reason is the appearance of fusidic acid–resistant strains. In our study, fusidic acid–resistant strains of S aureus ap-peared in 2 of 5 patients with persistent S aureus colonization after 8 weeks of fusidic acid treatment. Antibiotic resistance of S aureus is a serious and growing problem in dermatolog-ical practice, and fusidic acid resistance may threaten the efficacy of systemic fusidic acid for the treatment of serious
S aureus infections.32,33 Therefore, fusidic acid– containing
preparations should be reserved for short-term treatment of obvious secondary skin infections only.
SsAgs contribute to the persistence and exacerbation of allergic skin inflammation in AD.1– 6,17,28,29However, no
cor-relation was found between levels or positive rates of serum SsAg-specific IgE antibodies and the presence of previous staphylococcal skin infection in AD patients.3This suggests
that S aureus induces the production of SsAg-specific IgE antibodies, not by infection but by skin penetration of exo-toxins.3 Colonization of S aureus on the skin is a constant
feature of AD, and therapeutic strategies aimed at the eradi-cation of S aureus may not always be appropriate.
In conclusion, this study showed that topical 0.03% tacroli-mus treatment had clinical efficacy comparable to topical 0.05% fluticasone in AD. Although initial, more rapid clear-ance of S aureus appeared in the patients treated with fluti-casone, no difference was found between the 2 treatment groups after long-term use. These results suggest that tacroli-mus is an appropriate alternative treatment for chronic AD. Complementary topical antibiotic treatment did not provide additional benefit compared with topical fluticasone or ta-crolimus treatment alone, suggesting that topical anti-inflam-matory therapy alone can improve allergic skin inflammation and reduce S aureus colonization in AD and that topical antibiotics should be reserved for short-term use in obvious secondary bacterial infection.
REFERENCES
1. Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361: 151–160.
2. Lu¨bbe J. Secondary infections in patients with atopic dermatitis.
Am J Clin Dermatol. 2003;4:641– 654.
3. Lin YT, Shau WY, Wang LF, et al. Comparison of serum specific IgE antibodies to staphylococcal enterotoxins between atopic children with and without atopic dermatitis. Allergy. 2000;55:641– 646.
4. Lin YT, Wang CT, Chiang BL. Role of bacterial pathogens in atopic dermatitis. Clin Rev Allergy Immunol. In press. 5. Lin YT, Wang CT, Hsu CT, et al. Differential susceptibility to
staphylococcal superantigen (SsAg)-induced apoptosis of CD4⫹ T cells from atopic dermatitis patients and healthy subjects: the inhibitory effect of IL-4 on SsAg-induced apoptosis. J Immunol. 2003;171:1102–1108.
6. Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial su-perantigens. J Allergy Clin Immunol. 2000;105:782–787. 7. Hauk PJ, Leung DY. Tacrolimus (FK506): new treatment
dermati-tis? J Allergy Clin Immunol. 2001;107:391–392.
8. Wachs GN, Maibach HI. Co-operative double-blind trial of an antibiotic/corticoid combination in impetiginized atopic derma-titis. Br J Dermatol. 1976;95:323–328.
9. Leyden JJ, Kligman AM. The case for steroid-antibiotic com-binations. Br J Dermatol. 1977;96:179 –187.
10. Hjorth N, Schmidt H, Thomsen K. Fusidic acid plus betametha-sone in infected or potentially infected eczema.
Pharmathera-peutica. 1985;4:126 –131.
11. Nilsson E, Henning C, Hjo¨rleifsson ML. Density of the micro-flora in hand eczema before and after topical treatment with a potent corticosteroid. J Am Acad Dermatol. 1986;15:192–197. 12. Nilsson EJ, Henning CG, Magnusson J. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. J Am Acad
Dermatol. 1992;27:29 –34.
13. Stalder JF, Fleury M, Sourisse M, Rostin M, Pheline F, Litoux P. Local steroid therapy and bacterial skin flora in atopic der-matitis. Br J Dermatol. 1994;131:536 –540.
14. Guzik TJ, Bzowska M, Kasprowicz A, et al. Persistent skin colonization with Staphylococcus aureus in atopic dermatitis: relationship to clinical and immunological parameters. Clin Exp
Allergy. 2005;35:448 – 455.
15. Pournaras CC, Lu¨bbe Jann, Saurat JH. Staphylococcal coloni-zation in atopic dermatitis treatment with topical tacrolimus (Fk506). J Invest Dermatol. 2001;116:480 – 481.
16. Remitz A, Kyllo¨nen H, Granlund H, Reı¨tamo S. Tacrolimus ointment reduces staphylococcal colonization of atopic derma-titis lesions. J Allergy Clin Immunol. 2001;107:196 –197. 17. Williams RE. The antibacterial-corticosteroid combination:
what is its role in atopic dermatitis? Am J Clin Dermatol. 2000;1:211–215.
18. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis.
Acta Dermatol Venereol (Stockh). 1980;92(suppl):44 – 47.
19. Rajka G, Langeland T. Grading of the severity of atopic der-matitis. Acta Derm Venereol Suppl. 1989;144:13–14.
20. Kunz B, Oranje AP, Labre`ze L, Stalder JF, Ring J, Taı¨eb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Der-matitis. Dermatology. 1997;915:10 –19.
21. Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965;45: 498 –503.
22. Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90: 525–530.
23. Gilani SJ, Gonzalez M, Hussain I, Finlay AY, Patel GK.
Staph-ylococcus aureus re-colonization in atopic dermatitis: beyond
the skin. Clin Exp Dermatol. 2004;30:10 –13.
24. Reitamo S, Rustin M, Ruzicka T, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone bu-tyrate ointment in adult patients with atopic dermatitis. J Allergy
Clin Immunol. 2002;109:547–555.
25. Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model.
J Invest Dermatol. 2001;116:658 – 663.
26. Lever R, Hadley K, Downey D, Mackie R. Staphylococcal colonization in atopic dermatitis and the effect of topical mupi-rocin therapy. Br J Dermatol. 1988;119:189 –198.
27. Ewing CI, Ashcroft C, Gibbs AC, Jones GA, Connor PJ, David TJ. Flucloxacillin in the treatment of atopic dermatitis. Br J
Dermatol. 1998;138:1022–1029.
28. Breuer K, Ha¨ussler S, Kapp A, Werfel T. Staphylococcus
aureus: colonizing features and influence of an antibacterial
treatment in adults with atopic dermatitis. Br J Dermatol. 2002; 147:55– 61.
29. Boguniewicz M, Sampson H, Leung SB, Harbeck R, Leung DY. Effects of cefuroxime axetil on Staphylococcus aureus colonization and superantigen production in atopic dermatitis. J
Allergy Clin Immunol. 2001;108:651– 652.
30. Patel GK, Wyatt H, Kubiak EM, Clark SM, Mills CM.
Staph-ylococcus aureus colonization of children with atopic eczema
and their parents. Acta Derm Venereol. 2001;81:366 –367. 31. David TJ, Cambridge GC. Bacterial infection and atopic
ec-zema. Arch Dis Child. 1986;61:20 –23.
32. Shah M, Mohanraj M. High levels of fusidic acid-resistant
Staphylococcus aureus in dermatology patients. Br J Dermatol.
2003;148:1018 –1020.
33. Peeters KA, Mascini EM, Sanders CJ. Resistance of
Staphylo-coccus aureus to fusidic acid. Int J Dermatol. 2004;43:235–236.
Requests for reprints should be addressed to: Bor-Luen Chiang, MD, PhD
Department of Pediatrics
National Taiwan University Hospital No. 7
Chung-Shan South Road Taipei 100, Taiwan.