‘V [CANCER RESEARCI-154.5106-5110, October 1, 19941
Correlations of Chronic Hepatitis B Virus Infection and Cigarette Smoking with
Elevated Expression of neu Oncoprotein in the Development of
Hepatocellular Carcinoma1
Ming-Whei
Yu, Chien-Jen Chen,2 Jiin-Chyuan
Luo, Paul W. Brandt-Rauf,
Walter P. Carney, and Regina M. Santella
Department of Public Health [M-W. 1'.] and institute of Epidemiology [M-W. 1'., C-i. C.], College of Public Health, National Taiwan University, Taipei lfXfl8, Taiwan; Cancer
Center and Division of Environmental Sciences, School of Public Health, Columbia University, New York, New York 10032 [i-C. L, R. M. S., P. W. B-RI; and Oncogene
Sciences, Cambridge. Massachusetts 02142 [W. P. C.J
ABSTRACT
To investigate the potential role of neu oncogene expression in hepato
carcinogenesis, a nested case-control study was conducted within a cohort
of 9691 male adults in Taiwan. Blood samples of study subjects were collected during 1984—1986and frozen at —30°Cuntil subsequent anal ysis. The neu oncoprotein level in the stored serum was measured by an enzyme-linked immunosorbent assay for 27 cases of newly developed hepatocellular carcinoma (HCC), 12 liver cirrhosis cases, and 40 healthy
controls. The mean level of neu oncoprotein was significantly higher in HCC and liver cirrhosis cases than in controls. The risk of HCC increased significantly with increasing serum level of neu oncoprotein (trend test, P = 0.02). The proportion of subjects having an elevated serum level of
neu oncoprotein, defined as a level greater than the mean level of all controls, was significantly higher among asymptomatic HBsAg carriers than noncarriers (P 0.05), showing a multivariate-adjusted odds ratio of
4.0. Among HCC cases, a strong association was observed between ciga rette smoking and elevated prediagnostic serum level of neu oncoprotein. The association remained highly significant (P 0.017) even when ad
justment was made for potential confounders. The multivarlate-adjusted
odds ratio of having an elevated serum level of neu oncoprotein, defined
as a level greater than the mean plus 1 SD ofcontrol levels, for HCC cases
who smoked more than 10 cigarettes a day was as high as 386.5 compared with the cases who smoked less than 10 cigarettes a day or nonsmoking cases. The results suggest that both HBsAg carrier status and cigarette
smoking are related to the increased expression of neu oncogene, and cigarette smoking seems to play a significant role in the latter stages of hepatocarcinogenesis. There was no association between alcohol drinking
and serum neu oncoprotein leveL
INTRODUCTION
Liver cancer, largely HCC,3 is one of the most common fatal malignant neoplasms in the world. It ranks as the first leading cause
of death due to cancer for men and the third for women in Taiwan (1). Epidemiological studies have provided strong evidence for the link
between chronic HBV infection and the induction of HCC (2, 3). A
variety of mechanisms by which the virus may contribute to the pathogenesis of HCC have also been proposed on the basis of mo
lecular biological and animal studies (4—14).However, many uncer
tainties about the mechanism of HBV-related hepatocarcinogenesis in humans still exist (15).
The evolution of HCC is a pathogenic process involving multiple
stages associated with exposures to multiple risk factors. In addition to HBV chronic carrier status, many possible etiobogical factors,
Received 4/4/94; accepted 7/25/94.
The costs of publication of this article were defrayed in part by the payment of page
charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
I This work was supported by the Department of Health, Executive Yuan, Republic of China, and NIH Grant ESO 5116.
2 To whom requests for reprints should be addressed, at Institute of Epidemiology,
College of Public Health, National Taiwan University, No. 1 Jen-Ai Rd. Sec. 1, Taipei
10018, Taiwan.
3 The abbreviations used are: HCC, hepatocellular carcinoma; CI, confidence interval; HBV, hepatitis B virus.
including hepatitis C virus (16, 17), aflatoxin exposure (18, 19), alcohol consumption, cigarette smoking (2, 3, 20—23),and elevated serum level of endogenous testosterone (24), have also been impli cated in the etiology of HCC. Among these environmental HCC risk factors other than HBV, cigarette smoking, and alcohol consumption are the most common in the general population. Their relationships to risk of HCC have been documented in Taiwan (2, 3) and other countries (20—23).Alcohol drinking has long been postulated as a
HCCriskfactorbecause of its relationshipto the developmentof liver
cirrhosis. Cigarette smoke contains many chemical carcinogens asso ciated with tumor initiation and promotion. A wide range of human malignancies have been associated with cigarette smoking. However, the apparent carcinogenic effect of cigarette smoking differs substan tialby for various cancer sites. There is a strong association between cigarette smoking and lung cancer with a relative risk as high as 10(25), while the relative HCC risk for cigarette smoking is only
2—3-fold(2, 3, 21—23).The robe of cigarette smoking in hepatocarci nogenesis continues to challenge epidemiologists interested in the etiology of HCC.
Genome alterations which affect the expression or function of genes controlling cell growth and differentiation have been considered to be the main cause of cancer (26). Molecular research focusing on the identification of genes that are altered in various tumor types and the elucidation of their roles in tumorigenesis have contributed sub
stantially to our understanding of the carcinogenic process. Among
oncogenes known to be important in the development of human malignancies, the neu oncogene (also known as HER-2 or c-erbB-2) has received much aUention for its robe in mammary carcinogenesis (27, 28). The neu gene encodes a M@185,000 transmembrane protein which is structurally and functionally similar to the epidermal growth factor receptor (29). Amplification and/or overexpression of the neu oncogene have been implicated in cell transformation and animal tumorigenesis (30, 31) and in a variety of human cancers, including carcinomas of the breast, ovary, stomach, colon, lung, and salivary gland (27, 28, 32—35).Clinical studies on breast cancer have sug gested a robe of neu oncogene in the neoplastic process (27, 28). Recently, overexpression of this gene in a significant proportion of
HCC and liver cirrhosispatientshas also been reportedfrom a study
of immunohistochemicab staining of a limited number of liver speci
mens (36). This prompted us to further study the expression of neu oncogene during HCC development.
Recent studies have demonstrated that the extracellular domain of the neu oncoprotein is released and is measurable in the serum of patients with various types of cancers using enzyme-linked immu nosorbent assay (37—40).It was also indicated that elevated serum levels of neu oncoprotein are related to the increased expression of this oncoprotein in tumor tissues (39, 41). This suggests that the neu oncoprotein level in the serum can be used as an indicator for
assessing the expression of neu oncogene. We have previously re
ported an increased level of neu oncoprotein in the serum from the individuals who subsequently developed HCC before the clinical diagnosis of the disease was made (38). In our current investigation,
Serum neu Control Liver cancer Liver cirrhosisoncoproteinlevels (HNU―/ml) (n) n OR (95% CI) n OR (95% Cl)985.0― 20 7 lAf 2 1.Oc>985.0 and 16 11 2.0(0.5-7.3) 4 2.5 (0.3—23.0)1501.7>1501.7 4 9 6.4(1.2—36.8) 6 15.0(1.7—174.6)a
HNU, human neu unit; OR, odds ratio.
b The serum levels of neu oncoprotein were trichotomized according to the mean andthe
mean plus 1 SD of the levels of all controls.
C Mantel's @2test for a trend was significant. we applied the same approach to quantitate the neu oncoprotein in the
serum samples from the individuals who subsequently developed
HCC as well as from liver cirrhosispatientsand healthy controls in a
large scale cohort study. We also extended our study to the identifi cation of putative HCC risk factors associated with the overexpressionof neu oncogene. The role of neu oncogene overexpression in human hepatocarcinogenesis will be discussed in this paper.
SUBJECTS AND METHODS
This nested case-control study was conducted within a cohort of 9691 male adults whose blood samples were collected in 1984—1986and stored at —30°C for subsequent assays (24). At the time of blood collection, each study subject was also personally interviewed according to a structured questionnaire, col lecting information of demographic characteristics, long term habits of ciga rette smoking and alcohol drinking, as well as personal and family history of various liver diseases and cancers diagnosed by physicians. A habit of cigarette
smoking was defined as having smoked cigarettes more than 4 days a week for at least 6 months. Alcohol drinking was defined as having drunk alcohol more
than 3 days a week for at least 6 months.
The participantsof this studycohortwere followed by telephoneinterviews
and home visits annually until March 1990. The causes of death of study subjects were investigated through data linkage and matching with a comput erized file of national death certification system in Taiwan. After an average follow-up period of 4.6 years, a total of 36 newly diagnosed liver cancer cases were identified. As the stored serum samples of 9 eligible liver cancer cases were not available for analysis of neu oncoprotein, a total of 27 liver cancer
cases sewed as the case group in the present study. The 27 liver cancer cases
included in this study and the other 9 eligible cases not included were
comparable with respect to all the demographic characteristics and distribu
tions of potential HCC risk factors. It seems reasonable to assume that the
cases included in this study were representative of the eligible cases. The most accepteddiagnosticcriteriafor HCC in Taiwanare eitherpathologicalexam
inations or elevated a-fetoprotein level (400 nglml or greater) combined with
at least one positive image on angiography, sonography, liver scan, and/or
computerized tomography scans. Among the 27 liver cancer cases with avail able sera in this study, 19 (70%) were double-checked with either hospital records or data files of the National Cancer Registry in Taiwan (about one-half
were diagnosedpathologicallyandtheotherhalfby elevateda-fetoproteinand
liver images), whereas 8 were identified only by death certificates.
The subjectsof this studywere selected froma previousnestedcase-control
study on the role of elevated serum testosterone in the development of HCC
(24). All the controls who were matched to the cases in the previous study and
had sufficient serum samples for testing were included as controls in this study.
A total of 40 controls were thus selected for the 27 liver cancer cases. The controls were individually matched to the cases on age (within 5 years), date
of questionnaire interview and blood collection (within 3 months), and resi
dential townships. They were originally selected from all the cohort members
who were alive and free of cancer on the dates at the diagnosis of liver cancer
cases to whom they were matched. Two controls were matched to each of the
13 liver cancer cases, and one to each of the other 14 cases. As 2 HBsAg
positive and 2 HBsAg-negative controls were selected for each liver cancer case in the previous study, approximately equal numbers of HBsAg-positive
and HBsAg-negative controls were included in this study. This will make the
comparison of serum levels of neu oncoprotein between these 2 groups more efficient. Since only one HBsAg-positive control had a past history of liver
disease at the blood collection, most of the HBsAg-positive controls in this study were asymptomatic chronic HBV carriers.
HCC usually occurs in cirrhotic liver, and liver cirrhosis has long been regarded as the premalignant lesion of HCC. Evaluation of the neu oncogene expression in liver cirrhosis cases will help understand the chronological changes of this gene expression in the pathogenesis of HCC. A total of 12 study subjects who died from liver cirrhosis during the follow-up period were also included in the analyses. They were all HBsAg-positive. As these cirrhotic patients died within 5 years after recruitment, with only 3 report ing a history of physician-diagnosed liver cirrhosis in the baseline inter view, these patients probably already had subclinical liver cirrhosis at the time of blood collection.
All participants in the study cohort were tested for their HBsAg carrier status by reverse passive hemagglutination assay at the initial recruitment examination. Liver cancer cases and matched controls who were HBsAg negative in the initial reverse passive hemagglutination assay were retested by radioimmunoassay using commercial kits (Abbott Laboratories, North Chi cago, IL). Serum levels of the neu oncoprotein were assayed in a manner blinded with respect to disease status by a sandwich enzyme-linked immu
nosorbent assay (Oncogene Science, Inc., Uniondale, NY) using 2 different
mouse monoclonal antibodies that react with independent epitopes on the extracellular domain of the p185 neu oncopeptide, as described in detail
previously (38, 39). All serum samples were measured for the neu oncoprotein
levels on the same day.
Since the distribution of serum neu oncoprotein levels showed no substan tial deviation from normal distributionjudging from histogram and coefficients of skewedness and kurtosis, a t test was applied to compare the mean serum levels of neu oncoprotein between groups. Categorical data analysis was also
performed to assess the dose-response relationship between the serum level of
neu oncoprotein and risk of HCC, and the association between elevated serum level of this protein and HCC risk factors. In the stratified data analysis, the serum level of neu oncoprotein was dichotomized or trichotomized based on the distribution for controls. Mantel's x@test for a trend was used to examine the dose-response relationship. Correlation of the quantity of cigarettes smoked per day with serum neu oncoprotein levels was further examined by Spear man's rank correlation coefficient. Multivariate-adjusted odds ratios and their 95% CI were estimated by modeling the data through unconditional logistic regression. All statistical tests were based on 2-tailed probability.
RESULTS
There were 16 HBsAg-positive and 11 HBsAg-negative liver can cer cases. The age of liver cancer patients at initial recruitment examination ranged from 40 to 74 years. The mean ages ±SD at
recruitment were 58.6 ± 9.7 and 58.2 ± 9.0 years for liver cancer
cases and matched controls, respectively. The age of patients who died from liver cirrhosis ranged from 38 to 66 years, with a mean ±SD of 52.8 ±10.6 years. They were younger than the liver
cancer cases and controls.
Elevated Serum Level of neu Oncoprotein and Risk of HCC. The means ± SD of neu oncoprotein levels in serum were 1324.4 ±590.8, 1482.8 ±656.5, and 985.0 ±516.7 human neu
units/mb, respectively, for liver cancer cases, liver cirrhosis cases, and controls. The differences in mean levels of neu oncoprotein were statistically significant between liver cancer cases and con
trobs (P = 0.02), and between liver cirrhosis cases and controls
(P = 0.008). There was no significant difference in mean levels of neu
oncoprotein between liver cancer and liver cirrhosis cases.
Table 1 shows a clear dose-response relationship between the
serum levels of neu oncoprotein and risk of HCC (trend test,
P = 0.02). As compared with the individuals who had a neu oncop
rotein level below the mean bevel of all controls, the odds ratio ofdeveloping HCC was 2.0 (95% CI = 0.5—7.3)for those who had a neu oncoprotein level greater than the mean but less than the mean plus 1 SD of control levels. The odds ratio increased up to 6.4 (95%
Table 1 Comparisons of the serwn levels ofneu oncoprotein among 27 liver cancer cases. 12 liver cirrhosis cases. and 40 controls
Table 3 Associations between putative HCC riskfactors and elevated prediagnostic serum level of neu oncoprotein (human neu unit/mI) in 27 liver cancercases%
with elevated
Total neu
Variable Group no. oncoprotein―
@ value―HB5Ag ratio P Negative 11 18.2 1.0 Positive 16 43.8 3.5 0.17Alcohol drinking No 20 30.0 1.0 habit Yes 7 42.9 1.8 0.43Cigarette smoking @10 15 6.7 1.0 (cigarettes/day) >10 12 66.7 28.0 0.001a
Elevated serum level of neu oncoprotein was defined as a level greater than the mean
plus 1 SD of control levels.
Sts@tiod analyseswere performedby Fisher's exact test.
Table 2 Associations between putative HCC riskfactors and elevated serum level (human neu unit/mI) ofneu controls%oncoprotein in 40
with elevated Total neu Variable Group no. oncoproteina
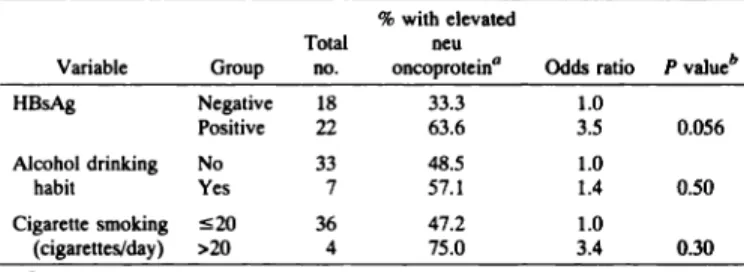
@ valuebHBsAg ratio P Negative 18 33.3 1.0 Positive 22 63.6 3.5 0.056Alcohol drinking No 33 48.5 1.0 habit Yes 7 57.1 1.4 0.50Cigarette smoking 20 36 47.2 1.0 (cigarettes/day) >20 4 75.0 3.4 0.30a
Elevated
serum
level
ofneu
oncoprotein
was
defined
asalevel
greater
than
themean
level of all controls.
b Statistical analyses were performed by Fisher's exact test.
INCREASED EXPRESSION OF neu ONCOPROTEIN AND HEPATOCELLULARCARCINOMA CI = 1.2—36.8)for those who had a neu oncoprotein level greater than
the mean plus 1 SD of control levels. A strong association was also observed between liver cirrhosis and elevated serum level of neu oncoprotein. The percentage of liver cirrhosis cases who had serum neu oncoprotein levels greater than the mean but less than the mean plus 1 SD of control levels was 33.3% (4 of 12) compared with 40% (16 of 40) for the controls, the percentage of the liver cirrhosis cases who had serum neu oncoprotein levels greater than the mean plus 1 SD of control levels was 50% (6 of 12) compared with 10% (4 of 40) for the controls. When an analysis was performed for the association between liver cirrhosis and serum neu oncoprotein level, a highly significant trend for the odds ratios was noted (P = 0.006).
There were 13 liver cancer cases diagnosed within 2 years and 14 cases diagnosed more than 2 years after blood collection. The distri
bution of serum neu oncoprotein level in the early onset cases was
similar to that of the late onset cases.
Serum Level of neu Oncoprotein and HCC Risk Factors, In this study, the serum samples of the liver cancer cases were collected up to 5 years prior to the occurrence of clinical disease. It seems reason able to speculate that the liver cancer cases had preneoplastic lesions or small tumors at the time of blood collection, but they were asymp tomatic. Since chronic HBV infection, cigarette smoking, and alcohol drinking may have a different effect on the expression of neu onco gene at different stages of neoplastic process, the associations of these factors with serum neu oncoprotein levels were evaluated in controls and liver cancer cases, separately.
Table 2 shows the association with elevated serum level of neu oncoprotein for HBsAg carrier status, alcohol drinking, and cigarette smoking among controls. More HBsAg-positive controls (63.6%) than HBsAg-negative controls (33.3%) had an elevated serum level of neu oncoprotein, defined as a level greater than the mean bevel of all controls, giving an odds ratio of 3.5 (95% CI = 0.9—13.0,P = 0.056). The association between HBsAg carrier status and elevated serum level of neu oncoprotein was statistically significant after adjustment
for age, cigarette smoking, and alcohol drinking (P = 0.05). The
multivariate-adjusted odds ratio of having an elevated serum level of neu oncoprotein for HBsAg carrier status was 4.0 (95% CI 1.0— 16.0). Alcohol drinking and cigarette smoking were not significantly associated with the elevated neu oncoprotein level among controls.
The associations of elevated prediagnostic serum level of neu oncoprotein with HBsAg carrier status, alcohol drinking, and cigarette smoking among liver cancer cases are depicted in Table 3. The percentage of liver cancer cases who had a serum neu oncoprotein bevel greater than the mean plus 1 SD of control levels was 43.8% for HBsAg-positive cases and 18.2% for HBsAg-negative cases, showing
an odds ratio of 3.5 (95% CI = 0.6—21.7). But the association
between HBsAg carrier status and elevated serum level of neu onco protein was not statistically significant. No association with the ele vated serum neu oncoprotein level was observed for alcohol drinking.
A significantly higher proportionof liver cancer cases who smoked
more than 10 cigarettes a day had elevated serum bevels of neu oncoprotein (66.7%) than those who smoked less than 10 cigarettes a day or nonsmokers (6.7%), giving an odds ratio of 28.0 (95%CI = 2.7—295.7,
P = 0.001). The association of cigarette smoking
with elevated serum levels of neu oncoprotein was highly significantafter adjustment for potential confounders including age, onset time of
liver cancer, HB5Ag carrier status, and alcohol drinking (P 0.017). The multivariate-adjusted odds ratio for those who smoked more than 10 cigarettes a day was as high as 386.5 (95% CI = 3.0—50,531.3) compared with those who smoked less than 10 cigarettes a day or nonsmokers. There was no association between elevated serum level of neu oncoprotein and age, onset time of liver cancer, HBsAg carrier status, and alcohol drinking among liver cancer cases.
The correlation between the quantity of cigarettes consumed per day and serum neu oncoprotein level was further examined using Spearman's rank correlation coefficient. This analysis showed a sig nificant correlation between cigarettes smoked per day and precliag nostic serum levels of neu oncoprotein among liver cancer cases
(r 0.58, P = 0.0015). There was essentially no correlation between
cigarettes smoked per day and the serum neu oncoprotein bevels among controls (r = —0.14,P = 0.4).
DISCUSSION
Although various oncogenes in hepatocarcinogenesis have been investigated in rat liver cancers and rat or human liver cancer cell lines
(42—45),only a few studies have been done on oncogenes in human
HCC (36, 46, 47). This nested case-controlstudy suggests an associ
ation between elevated prediagnostic serum levels of neu oncoprotein and the development of HCC. However, the exact role for overex pression of neu oncoprotein in hepatocarcinogenesis is unknown. Because the neu oncoprotein is presumed to be a growth factor receptor, the elevated expression of neu oncoprotein may simply reflect the increased cell proliferation in preneoplastic liver. Abterna tively, the increased expression may result from exposure to carcin ogens in the course of hepatocarcinogenesis. The close association between elevated serum level of neu oncoprotein and some putativeHCC risk factors observed in this study is compatiblewith the latter
hypothesis and suggests a robe for neu oncogene in the induction ofHCC in humans.
HBsAg carrier status has been well-documented as the most im
portant environmental risk factor of HCC, with a relative risk 10 to 20
times higher for HBsAg carriers as compared with noncarriers (2, 3).
Based on the strong epidemiologic association between HBV and
HCC, the role of HBV in hepatocarcinogenesishas been extensively
explored in molecular and animal studies (4—14).However, many uncertainties about the mechanism of HBV-related human hepato carcinogenesis remain (15). In this study, a significantly higher pro 5108portion of asymptomatic HBsAg carriers were found to have ele vated serum levels of neu oncoprotein than noncarriers. Whether the increased neu expression in chronic HBV carriers plays a rela tively early role in HBV-modubated hepatocarcinogenesis needs to be established.
The mechanism by which chronic HBV infection causes an in creased serum level of neu oncoprotein remains to be elucidated. In view of observations from previous experimental work, it is likely that multiple independent or interdependent mechanisms are involved.
HBV has been proposed to act as an insertional mutagen that may
alter cellular gene structures and/or functions (4—9).HBV X-gene product has been demonstrated to be a transcriptional activator abbe to stimulate a variety of cellular promoters which do not share a common cis-regulatory element (10—12).As the X protein has the ability to affect signal transduction (12), it may also perturb the flow of infor mation in the cell and thus affect the expression of many genes. Persistent infection with HBV is also associated with chronic phasic necroinflammation and regenerative hyperplasia of the liver (48). The continuous hepatocyte regeneration caused by prolonged hepatocel lular injury has been shown to initiate a cascade of events associated with the pathogenesis of HCC including transcriptional deregulation and chromosomal aberrations in HBV transgenic mouse model (14). In this study, we also observed a close relationship between liver cirrhosis and elevated serum level of neu oncoprotein. This result provides evidence supporting the theory that prolonged hepatocellular injury caused by chronic HBV infection results in overexpression of neu oncoprotein. Many oncogenes may be expected to be overex pressed in regenerating livers (42). Significantly higher levels ofc-myc gene expression have also been observed in liver cirrhotic
tissues (47). As liver cirrhosis has long been regarded as the prema bignant lesion of HCC, the elucidation of oncogenes associated with liver cirrhosis may help us to understand the multistep hepatocarci nogenesis at the molecular level in humans.
A number of case-control studies have investigated the association between cigarette smoking and HCC. Most of the studies reported a relative risk of 2—3for cigarette smoking (3, 21, 23). The association between cigarette smoking and HCC has also been documented in 2 large-scale cohort studies (2, 22). An unsolved issue is the mechanism of action of cigarette smoking. The most marked finding of this study was the strong association between elevated prediagnostic serum levels of neu oncoprotein and cigarette smoking among HCC patients. Although a recent smoking habit may be changed due to intercurrent illness and thus may result in a misclassification of exposure to smoking, the misclassification may be nondifferential. In other words, it may not be associated with the serum levels of neu oncoprotein. Even if cigarette-smoking liver cancer patients with high neu oncop rotein levels may be more likely to quit smoking than smoking patients with low neu oncoprotein levels due to unidentified symp toms, this may bias the effect of smoking toward the null only. This means that the association between cigarettes smoked per day and serum neu oncoprotein in this study was estimated under a conserv ative circumstance. In contrast, there was no significant association between cigarette smoking and elevated serum level of neu oncopro tein among controls. These results suggest that cigarette smoking may play a role in the late promotion and/or progression stages of HCC development. A significant etiological role of cigarette smoking in the development of HCC from liver cirrhosis was recently reported by a prospective study of Japanese patients with chronic liver disease (17). In this study, the relative risk of developing HCC from liver cirrhosis was much higher for current smokers than for exsmokers. This result
is compatible with our hypothesis that cigarette smoking may act as a
strong promoter or progressor in the late stages of hepatocarcinogen
esis. It will be of interest to determine whether liver cirrhosis patients
who smoked cigarettes had a higher serum level of neu oncoprotein than nonsmoking patients. However, we were not able to test the hypothesis in this study because of the small number of liver cirrhosis
cases. Further study on this hypothesis may help clarify the role of
cigarette smoking in the development of HCC from liver cirrhosis. It has been reported that the overexpression of neu oncogene can be caused by gene amplification and gene deregulation through epige netic mechanisms (49). Activation of the transforming potential of neu protooncogene caused by point mutation have also been shown in vitro and in chemically induced rat tumors (31, 50, 51). Cigarette smoke is a mixture of over 3800 chemical substances containing at least 43 known carcinogens. Many substances in cigarette smoke can be metabolized to form reactive intermediate products which can bind to DNA (52). The precise mechanism for cigarette smoking-associ ated overexpression of neu oncogene to induce HCC requires further examination.
There was no significant association between alcohol drinking and elevated serum level of neu oncoprotein. The average quantity of alcohol consumed by people in Taiwan is not large. In this study, habitual alcohol drinking was defmed as having consumed alcohol more than 3 days a week for at least 6 months. Ethanol itself is not carcinogenic. There is good evidence suggesting that alcohol con sumption may be a HCC risk factor only because it is involved in the development of liver cirrhosis (17, 53). Liver cirrhosis was shown to be significantly associated with an increase in serum bevel of neu oncoprotein in this study, but no significant association between alcohol thinking and elevated neu oncoprotein level was observed. This discrepancy may be due to the small quantity of alcohol con sumed by habitual alcohol drinkers in this study, an amount pos sibly insufficient to induce liver cirrhosis. However, only a small fraction of cases and controls were habitual alcohol drinkers in our study. The sample size is too small to draw a definite conclusion on the association between alcohol drinking and serum bevel of neu oncoprotein.
To date, observations on the role of oncogenes in hepatocarcino genesis and their relationship to risk factors of HCC are still sparse. This nested case-control study of HCC provided the first link between increased expression of neu oncogene and human hepatocarcinogen esis. However, since this study was based on a small sample size, further prospective studies on a larger number of cases are needed to validate this fmding. On the other hand, only a fraction of the HCC patients in this study had increased prediagnostic serum bevels of neu oncoprotein. There probably exists a variety of pathways for hepato
carcinogenesis. The use of serum oncoprotein levels as a biomarker
offers a way to search for the genetic alterations involved in the development of HCC. Whether there are other oncogenes critical for the pathogenesis of HCC remains to be determined.
REFERENCES
1. Yu, M. W., Tsai, S. F., Hsu, K. H., You, S. L., et a!. Epidemiologic characteristics of
malignant neoplasms in Taiwan. II. Liver cancer. J. Natl. Public Health Assoc., 8:
125—138,1988.
2. Chen, C. J., Yu, M. W., Wang, C. J., Huang, H. Y., and Un, W. C. Multiple risk
factors of hepatocellular carcinoma: a cohort study of 13737 male adults in Taiwan. 3. Gastroenterol. Hepatol., 8(Suppl): s83-s87, 1993.
3. Chen, C. J., Liang, K. Y., Chang, A. S., Chang, Y. C., et al. Effects of hepatitis B
virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology (Baltimore), 13: 398—406,1991.
4. Rogler, C. E., Sherman, M., Su, C. Y., Shafritz, D. A., et at. Deletion in chromosome 1ip associated with a hepatitis B integration site in hepatocellular carcinoma. Science
(Washington DC), 230: 319—322,1985.
5. Hino, 0., Shows, T. B., and Rogler, C. E. Hepatitis B virus integration site in
hepatocellular carcinoma at chromosome 17:18 translocation. Proc. NatI. Acad. Sci. USA, 83: 8338—8342,1986.
6. Meyer, M., Wiedorn, K. H., Hofschneider, P. H., Koshy, R., et aL A chromosome
17:7 translocation is associated with a hepatitis B virus DNA integration in human
hepatocellular carcinoma DNA. Hepatology (Baltimore), 15: 665—671,1992. 5109
INCREASED EXPRESSION OF neu ONCOPROTEIN AND HEPATOCELLULARCARCINOMA
7. Fourel, G., Trepo, C., Bougueleret, L., Henglein, B., et a!. Frequent activation of
N-myc genes by hepadna virus insertion in woodchuck liver tumors. Nature (Land.), 347: 294—298,1990.
8. Benbrook, D., Lernhardt, E., and Pfahl, M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature (Land.), 333: 669—672, 1988.
9. Wang, J., Chenivesse, X., Henglein, B., and Bréchot,C. Hepatitis B virus integration
in a cyclin A gene in a hepatocellular carcinoma. Nature (Land.), 343: 555—557,
1990.
10. Kim, C. M., Koike, K., Saito, I., Miyamura, T., et al. HBx gene of hepatitis B virus
induces liver cancer in transgenic mice. Nature (Land.), 351: 317—320,1991.
11. Wu, J. Y., Thou, Z. Y., Judd, A., Cartwright, C. A., et a!. The hepatitis B virus
encoded transcriptional trans-activator hbx appears to be novel protein serine/threo
nine kinase. Cell, 63: 687—695,1990.
12. Kekule, A. S., Lauer, U., Weiss, L., Luer, B., and Hofschneider, P. H. Hepatitis B
virus transactivator HBx uses a tumor promoter signalling pathway. Nature (Land.),
36!: 742—745,1993.
13. Kekule, A. S., Lauer, U., Meyer, M., Caselmann, W. H., et aL The preS2/S region of
integrated hepatitis B virus DNA encodes a transcriptional transactivator. Nature (Land.), 343: 457—461,1990.
14. Chisari, F. V., Klopchin, K., Moriyama, T., Pasquinelli, C., et al. Molecular patho
genesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell, 59: 1145—1156,1989.
15. Yu, M. W., and Chen, C. J. Hepatitis B and C viruses in the development of
hepatocellular carcinoma. Crit. Rev. Oncol./Hematol., in press, 1994.
16. Yu, M. W., You, S. L, Chang, A. S., Lu, S. N., Liaw, Y. F., and Chen, C. J.
Association between hepatitis C virus antibodies and hepatocellular carcinoma in
Taiwan. Cancer Res., 51: 5621—5625,1991.
17. Tsukuma, H., Hiyama, T., Tanaka, S., Nakao, M., etal. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N. EngI. J. Med., 328: 1797—
1801, 1993.
18. Ross, R. K., Yuan, J. M., Yu, M. C., Wogan, G. N., et al. Urinary aflatoxin
biomarkers and risk of hepatocellular carcinoma. Lancet, 339: 943—946,1992.
19. Hatch, M. C., Chen, C. J., Levin, B., Ji, B. T., et al. Urinary aflatoxin levels, hepatitis
B virus infection and hepatocellular carcinoma in Taiwan. Int. J. Cancer, 54: 931— 934, 1993.
20. International Agency for Research on Cancer. Alcohol drinking. in: International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks
to Humans, Vol. 44, pp. 207—215.Lyon, France: International Agency for Research on Cancer, 1988.
21. Yu, M. C., Tong, M. J., Govindarajan, S., and Henderson, B. E. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles
county, California. J. NatI. Cancer Inst., 83: 1820—1826,1991.
22. Hirayama, 1. A. A large-scale cohort study on risk factors for primary liver cancer,
with special reference for the role of cigarette smoking. Cancer Chemother. Pharma
col., 23(Suppl): si 14-si 17, 1989.
23. Tsukuma, H., Hiyama, T., Oshima, A., Sobue, T., et a! A case-control study of hepatocellular carcinoma in Osaka, Japan. tnt. J. Cancer, 45: 231—236,1990.
24. Yu, M. W., and Chen, C. J. Elevated serum testosterone levels and risk of hepato cellular carcinoma. Cancer Res., 53: 790—794,1993.
25. Doll, R., and Peto, R. The causes of cancer: quantitative estimates of avoidable risks
of cancer in the United States today. J. Nafi. Cancer Inst., 66: 1191—1308,1981. 26. Fearon, E. R., and Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell,
61: 759—767,1990.
27. Slamon, D. J., Clark, G. M., Wong, S. G., Levin, W. J., et a!. Human breast cancer:
correlation of relapse and survival with amplification of the HER-2/neu oncogene.
Science (Washington DC), 235: 177—182,1987.
28. Press, M. F., Pike, M. C., Chazin, V. R., Hung, G., et aL Her-2lneu expression in
node-negative breast cancer: direct tissue quantitation by computerized image anal ysis and association of overexpression with increased risk of recurrent disease. Cancer
Res., 53: 4960—4970, 1993.
29. Coussens, L., Yang-Fen, T. L., Liao, Y. C., et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu onco
gene. Science (Washington DC), 230: 1132—1139, 1985.
30. HUZIak, R. M., Schlessinger, J., and Ullrich, A. Increased expression of the putative
growth factor receptor p18SH@ causes transformation and tumorigenesis of NIH
3T3 cells. Proc. Nati. Acad. Sci. USA, 84: 7159—7163, 1987.
31. Di Fiore, P. P., Pierce, J. H., K.raus, M. H., Segatto, 0., et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science (Washington DC), 237:
178—182,1987.
32. Park, J. B., Rhim, J. S., Park, S. C., Kimm, S. W., et al. Amplification, Overexpres. sion, and rearrangement of the erbB-2 protooncogene in primary human stomach
carcinomas. Cancer Res., 49: 6605—6609,1989.
33. D'Emilia, J., Bulovas, K., D'Ercole, K., Wolf, B., et al. Expression of the c.erbB..2
gene product (p185) at different stages of neoplastic progression in the colon.
Oncogene, 4: 1233—1239,1989.
34. Kern, J. A., Schwartz, D. A., Nordberg, J. E., Weiner, D. B., et al. pl8sneu expression
in human lung adenocarcinomas predicts shortened survival. Cancer Res., 50: 5184— 5191, 1990.
35. Semba, K., Kamata, N., Toyoshima, K., and Yamamoto, T. A v-erbB-related pro. tooncogene, c-erbB-2 is distinct from the c-erbB-1/epidermal growth factor-receptor
gene and is amplified in a human salivary gland adenocarcinoma. Proc. Natl. Acad.
Sci. USA, 82: 6497—6501,1985.
36. Brunt, E. M., and Swanson, P. E. Immunoreactivity for c-erbB-2 oncopeptide in
benign and malignant diseases of the liver. Am. J. Clin. Pathol., 97(Suppl. 1)
s53-s61, 1992.
37. Carney, W. P., Hamer, P. J., Petit, D., Retos, C., et a!. Detection and quantitation of the human neu oncoprotein. Tumor Marker Oncol., 6: 53—72,1991.
38. Lao, J. C., Yu, M. W., Chen, C. J., Santella, R. M., et a!. Serum c-erbll-2 oncopeptide
in hepatocellular carcinogenesis. Med. Sci. Res., 21: 305—307,1993.
39. Brandt-Rauf, P. W., Lao, J. C., Carney, W. P., Smith, S., et a! Detection of increased
amounts of the extracellular domain of the c-erbB-2 oncoprotein in serum during
pulmonary carcinogenesis in humans. tat. J. Cancer, 56: 383—386,1994. 40. Wu, J. T., Astill, M. E., and Zhang, P. Detection of the extracellular domain of
c-erbB-2 oncoprotein in sera from patients with various carcinomas: correlation with tumor markers. J. Chin. Lab. Anal., 7: 31-40, 1993.
41. Breuer, B., Lao, J. C., DeVivo, I., Pincus, M., et aL Detection of elevated c-erbB-2
oncopeptide in the serum and tissue in breast cancer. Med. Sci. Res., 21: 383-384,
1993.
42. Fausto, N., and Shank, P. R. Oncogene expression in liver regeneration and hepato.
carcinogenesis. Hepatology, 3: 1016—1023,1983.
43. Reynolds, S. H., Stowers, S. J., Patterson, R. M., Maronpot, R. R., et a!. Activated
oncogenes in B&3F1 mouse liver tumors: imp'ications for risk assessment. Science
(Washington DC), 237: 1309—1316,1987.
44. Nagy, P., Evans, R. P., Marsden, E., Roach, J., et al. Cellular distribution of c-myc
transcripts during chemical hepatocarcinogenesis in rats. Cancer Res., 48: 5522—
5527,1988.
45. Richards, C. A., Short, S. A., Thorgeirsson, S. S., and Huber, B. E. Characterization of a transforming N-ras gene in the human hepatoma cell line Hep 02: additiOnal evidence for the importance of c-myc and ras cooperation in hepatocarcinogenesis.
Cancer Res., 50: 1521—1527,1990.
46. FarSIsid, M., and Tabor, E. Expression of oncogenes and tumor suppressor genes in human hepatocellular carcinoma and hepatoblastoma cell lines. J. Med. Virol., 38:
235—239,1992.
47. Himeno, Y., Fukuda, Y., Hatanaka, M., and Imura, H. Expression of oncogenes in
human liver disease. Liver, 8: 208—212,1988.
48. Popper, H. Shafritz, D. A., and Hoofnagle, J. H. Relation of the hepatitis B virus
carrier state to hepatocellular carcinoma. Hepatology, 7: 764—772,1987. 49. Kraus, M. H., Popescu, N. C., Amsbaugh, S. C., and King, C. R. Overexpression of
the EGF receptor-related proto-oncogene erbB-2 in human mammary tumor cell lines
by different molecular mechanisms. EMBO J., 6: 605—610,1987.
50. Bargmann, C. I., Hung, M. C., and Weinberg, R. A. Multiple independent activations
of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell, 45: 649—657,1986.
51. Bargmann, C. I., and Weinberg, R. A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J., 7: 2043-2052, 1988. 52. Perera, F. P., Poirier, M. C., Yuspa, S. H., Nakayama, J., et a!. A pilot project in
molecular cancer epidemiology: determination of benzo[ajpyrene.DNA addn@ts in
animal and human tissues by immunoassays. Carcinogenesis (Land.), 3: 1405—1410, 1982.
53. Adami, H-O., Hsing, A. W., McLaughlin, J. K., et al. Alcoholism and liver cirrhosis in the etiology of primary liver cancer. lnt. J. Cancer, 51: 898—902, 1992.