Atrial Fibrillation linked to Vascular Access Thrombosis in Chronic Hemodialysis Patients
全文
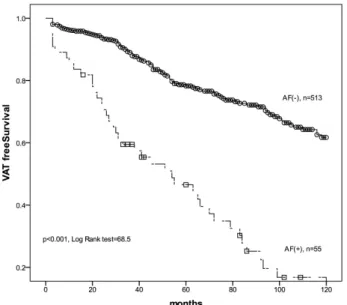
(2) AF and VAT in Chronic HD Patients. 449. patients with AF are more at risk for the development of VAT than those without AF. We conducted this study to determine if AF is linked to the development of vascular access thrombosis events in chronic HD patients with primary functional vascular access.. nary vascular diseases (CVD), self-reported or included in the medical records, included myocardial infarction, coronary artery bypass graft, percutaneous transluminal angioplasty, coronary stenosis greater than 50%, and ischemic stroke at the beginning of HD 12).. Subjects. Statistical analysis. All chronic HD patients (dialyzed for more than 3 months, thrice weekly, 4 hours) were dialyzed via the primary functional native ateriovenous fistula between 1997 and 2006 in China Medical University Hospital.. All data were analyzed using a standard statistical package (SPSS for Windows, version 12; SPSS Inc., Chicago, IL, USA).The clinical and demographic data are reported as the mean±SD or percent frequency, as appropriate. Student’s t-test or the Mann-Whitney U test was used for continuous variables and the chisquare test for categorical variables. The VAT-free survival curve of patients with and without AF was analyzed using Kaplan-Meier estimates. Possible VAT risk factors were analyzed using univariate Cox regression and factors with p < 0.05 were further analyzed using multivariate Cox regression. A p value < 0.05 was considered significant.. Methods The duration of functional primary vascular access was recorded from the initiation of HD treatment via functional vascular access to the date of the first VAT episode or December 2006, censored by death, shifting to peritoneal dialysis, undergoing kidney transplantation, and transfer to other HD center. A VAT event was defined as the sudden cessation of function of the vascular access, rendering HD impossible and requiring thrombectomy, thrombolysis, or acute placement of another HD access 2). Blood pressure (systolic blood pressure, SBP; diastolic blood pressure, DBP) at the beginning (PreHD) and end of HD (PostHD) in the supine position, measured by an automated oscillometric blood pressure device, was recorded every month on the blood sampling day. Changes in SBP (ΔSBP) during HD were calculated by PostHD SBP minus PreHD SBP and changes in DBP (ΔDBP) were calculated by PostHD DBP minus PreHD DBP. Biomarkers, including hematoglobin, serum albumin, creatinine, potassium, total serum calcium, phosphate, cholesterol and triglyceride, were recorded every month. CRP was recorded at the initiation of HD and every year. For patients with more than two biomarker values available, the average value was used for analysis. Atrial fibrillation (AF) was defined as paroxysmal (PAF) for spontaneous resolution of the arrhythmia, and persistent and permanent (CAF) when it could not be interrupted either spontaneously, by using drugs, or by cardioversion 9). Hypertension (HTN) was defined as a history of hypertension (blood pressure > 140/90 mm Hg) for > 2 years that required the initiation of antihypertensive therapy by the primary physician 10). Diabetes mellitus (DM) was defined as a fasting glucose concentration of 126 mg/dL (7 mmol/L) or higher, self-reported diagnosis of DM, or self-reported use of antidiabetic medication 11). Coro-. Results A total of 568 chronic HD patients, including 260 men and 308 women with a mean age of 56.7± 14 years old, were reviewed. Fifty-five (9.7%) patients had AF at the beginning of HD and 154 (27.1%) patients had at least one episode of VAT over an average of 58 months. The demographic characteristics of the entire study population are shown in Table 1. Patients with AF were older ( p < 0.001), had a higher percentage of hypertension ( p = 0.007) and a CVD history ( p = 0.001) than patients without AF. The serum albumin levels were significantly lower among patients with AF ( p = 0.005). Of 89.3% patients without AF, had a primary native-arteriovenous fistula (nAVF), significantly higher than 74.5% of patients with AF ( p = 0.001). In biomarkers, CRP was significantly higher in AF patients than in patients without AF ( p = 0.002). As shown in Table 2, patients who developed VAT events were older ( p = 0.04), and had a higher percentage of AF ( p < 0.001), DM ( p = 0.005) and HTN ( p = 0.038). Of patients who developed VAT events, 34% had an arteriovenous graft (AVG) as primary vascular access, significantly higher than that (7.7%) of patients without VAT events. The cumulative VAT-free survival is shown in Fig. 1. AF patients had a worse VAT-free survival than patients without AF by Kaplan-Meier analysis ( p < 0.001, log rank test). Possible VAT risk factors, including age, AF, DM, HTN, CVD history, PreHD SBP, PreHD DBP,.
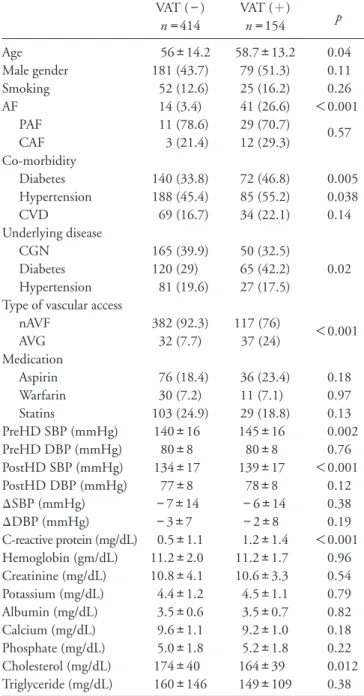
(3) Chou et al .. 450. Table 1. Demographic data of entire study population AF (−) n = 513 Age (years) 55.6±13.9 Male gender 238 (46.4) Co-morbidity Hypertension 237 (46.2) Diabetes 188 (36.6) CVD history 86 (16.8) Underlying disease CGN 199 (38.8) Diabetes 165 (32.2) Hypertension 29 (25.9) Type of vascular access nAVF 458 (89.3) AVG 55 (10.7) PreHD SBP (mmHg) 142±16 PreHD DBP (mmHg) 81±8 PostHD SBP (mmHg) 135±17 PostHD DBP (mmHg) 78±8 ΔSBP (mmHg) −7±14 ΔDBP (mmHg) −3±8 C-reactive protein (mg/dL) 0.6±1.1 Hemoglobin (g/dL) 11.2±1.9 Albumin (g/dL) 3.5±0.6 Creatinine (mg/dL) 10.8±3.3 Potassium (mEq/L) 4.4±1.2 Calcium (mg/dL) 9.5±3.7 Phosphate (mg/dL) 5.0±1.8 Cholesterol (mg/dL) 172±39 Triglyceride (mg/dL) 156±135. AF (+) n = 55 69.5±11.2 22 (40). Table 2. Patients’ clinical characteristics according to the development of vascular access thrombosis (VAT) events p. < 0.001. 0.37. 36 (65.5) 24 (43.6) 17 (30.9). 0.007 0.31 0.01. 16 (29.1) 20 (36.4) 2 (11.1). 0.095. 41 (74.5) 14 (25.5) 138±14 77±8 133±16 75±9 −5±13 −2±9 1.4±1.8 10.8±2.1 3.2±0.7 10.3±7.5 4.4±1.3 9.2±1.0 5.0±1.7 164±47 167±150. 0.001 0.065 0.001 0.26 0.002 0.45 0.79 0.002 0.11 0.005 0.38 0.95 0.48 0.88 0.16 0.56. AF: atrial fibrillation, CVD: cardiovascular disease, CGN: chronic glomerulonephritis, nAVF: native arteriovenous fistula, AVG: arterio-venous graft, PreHD: before hemodialysis sessions, SBP: systolic blood pressure, DBP: diastolic blood pressure, PostHD: end of hemodialysis sessions, Δ: changes of blood pressure during hemodialysis sessions.. PostHD SBP, Post HD DBP, ΔSBP, ΔDBP, types of vascular access, albumin and CRP were analyzed using univariate Cox regression. Parameters including AF, type of vascular access, DM, HTN, age and CRP had p < 0.05 and were further analyzed using multivariate Cox regression. The results are shown in Table 3. AF ( p < 0.001), AVG ( p < 0.001), DM ( p = 0.001), HTN ( p = 0.028), age ( p = 0.049) and CRP ( p = 0.045) were independently linked to the development of VAT events in chronic HD patients with a HR of 2.47 (95% CI: 1.66 to 3.69), 2.1 (95% CI: 1.43 to 3.09), 1.72 (95% CI: 1.25 to 2.39), 1.45 (95% CI: 1.04 to 2.02), 1.01 (95% CI:1.00 to 1.03 for every 1 year older) and. VAT (−) n = 414 Age 56±14.2 Male gender 181 (43.7) Smoking 52 (12.6) AF 14 (3.4) PAF 11 (78.6) CAF 3 (21.4) Co-morbidity Diabetes 140 (33.8) Hypertension 188 (45.4) CVD 69 (16.7) Underlying disease CGN 165 (39.9) Diabetes 120 (29) Hypertension 81 (19.6) Type of vascular access nAVF 382 (92.3) AVG 32 (7.7) Medication Aspirin 76 (18.4) Warfarin 30 (7.2) Statins 103 (24.9) PreHD SBP (mmHg) 140±16 PreHD DBP (mmHg) 80±8 PostHD SBP (mmHg) 134±17 PostHD DBP (mmHg) 77±8 ΔSBP (mmHg) −7±14 ΔDBP (mmHg) −3±7 C-reactive protein (mg/dL) 0.5±1.1 Hemoglobin (gm/dL) 11.2±2.0 Creatinine (mg/dL) 10.8±4.1 Potassium (mg/dL) 4.4±1.2 Albumin (mg/dL) 3.5±0.6 Calcium (mg/dL) 9.6±1.1 Phosphate (mg/dL) 5.0±1.8 Cholesterol (mg/dL) 174±40 Triglyceride (mg/dL) 160±146. VAT ( + ) n = 154 58.7±13.2 79 (51.3) 25 (16.2) 41 (26.6) 29 (70.7) 12 (29.3). p 0.04 0.11 0.26 < 0.001 0.57. 72 (46.8) 85 (55.2) 34 (22.1). 0.005 0.038 0.14. 50 (32.5) 65 (42.2) 27 (17.5). 0.02. 117 (76) 37 (24). < 0.001. 36 (23.4) 11 (7.1) 29 (18.8) 145±16 80±8 139±17 78±8 −6±14 −2±8 1.2±1.4 11.2±1.7 10.6±3.3 4.5±1.1 3.5±0.7 9.2±1.0 5.2±1.8 164±39 149±109. 0.18 0.97 0.13 0.002 0.76 < 0.001 0.12 0.38 0.19 < 0.001 0.96 0.54 0.79 0.82 0.18 0.22 0.012 0.38. PAF: paroxysmal atrial fibrillation, CAF: chronic atrial fibrillation, including permanent and persistent atrial fibrillation, CGN: chronic glomerulonephritis, CVD: cardiovascular disease, nAVF: native arteriovenous fistula, AVG: arteriovenous graft, statins include levostatin, fluvastatin and simvastatin, PreHD: before hemodialysis sessions, SBP: systolic blood pressure, DBP: diastolic blood pressure, PostHD: end of hemodialysis sessions, ∆: changes of blood pressure during hemodialysis sessions.. 1.09 (95% CI: 1.00 to 1.18 for every 1 mg/dL increase of CRP), respectively..
(4) AF and VAT in Chronic HD Patients. 451. sociation between AF and VAT events is independent of serum CRP levels. Consistent with our previous findings 2), patients with VAT had a higher CRP (1.2 ±1.4) than patients without VAT (0.5±1.1, p < 0.001, t-test). The independent association between VAT and serum CRP levels can be explained by the limitation of CRP 18) in HD patients. It is possible that AF is linked to VAT through different mechanisms, such as worsening of the local blood flow of vascular access 19-20) or increase of the D-dimer 21), therefore increasing the risk for VAT. The type of vascular access is one of the most important prognostic factors in vascular access patency: an nAVF is better than an AVG and forearm is better than upper-arm vascular access 22-24). The percentage of AVG was significantly higher among AF patients (Table 1), suggesting a poor vascular reserve for vascular access surgery. In our HD program, 87.9% (499/568) of patients had an nAVF as the primary vascular access. Of 41 AF patients with nAVF, 32 (78%) patients had a forearm nAVF and 9 patients had an upper-arm nAVF as the primary vascular access. The percentage of forearm nAVF was significantly lower than that (90.6%) of patients without AF ( p = 0.012, χ2 test). Seventeen (30.9%) of AF patients took aspirin and 10 (7.6%) patients took statins regularly. Five (9.1%) patients took warfarin 67% of the time within the therapeutic range. The use of aspirin, warfarin or statins was not significantly associated with a decrease of VAT risk in univariate Cox regression. Because this is a retrospective observational study, more studies are needed to determine if prophylactic medications reduce VAT risks in chronic HD patients. The CHADS2 score (congestive heart failure, hypertension, age older than 75 years, diabetes, and previous stroke or transient ischemic stroke) 25-26) is a classification scheme for patients with AF to evaluated the need for antithrombotic therapy based on the patientspecific risk of stroke. We found that the CHADS2 score was significantly associated with thromboembol-. Discussion We found that atrial fibrillation is an important risk factor for vascular access thrombosis in chronic HD patients with 9.7% AF prevalence rate 6, 13). This finding was supported by a worse vascular access thrombosis event-free survival curve (Fig. 1) and a two times higher VAT risk in AF patients (Table 3). The association between AF and VAT was independent of the types of vascular access and traditional VAT risk factors, such as DM, HTN and age. The association between AF and VAT may be explained by the common underlying chronic inflammation in AF patients 14-15) and patients who developed VAT 2-3, 5). Mounting evidence links the pathogenesis of AF to chronic inflammation 14-16). In addition, we also found higher serum CRP in AF patients 17); however, the as-. Fig. 1. Vascular access thrombosis event-free survival among patients with and without atrial fibrillation (AF), n = patient number.. Table 3. Hazard ratio of possible risk factors for vascular access thrombosis in multivariate Cox regression HR Atrial fibrillation Vascular access AVG v.s. nAVF Diabetes Hypertension Age CRP (every 1 mg/dL increase). 2.47 2.10 1.72 1.45 1.01 1.09. 95% CI 1.66 1.43 1.25 1.04 1.00 1.00. AVG: arteriovenous graft, nAVF: native arteriovenous fistula, CRP: C-reactive protein. p 3.69 3.09 2.39 2.02 1.03 1.18. < 0.001 < 0.001. 0.001 0.028 0.049 0.045.
(5) Chou et al .. 452. ic events in end-stage renal disease patients in the previous study 17); however, no correlation was found between the CHADS2 score and the development of VAT. The average CHADS2 score was 1.6±1 for AF patients who developed VAT and 2.0±1.2 for AF patients without VAT events ( p = 0.28). In addition, the CHADS2 score was not correlated to serum CRP levels in our study ( p = 0.23, Pearson’s correlation analysis), which can be explained by the different nature of thromboembolic events and VAT 26-28). Neointimal hyperplasia may play a key role in the development of VAT, but not in thromboembolic events among AF patients. In conclusion, atrial fibrillation is associated with the development of vascular access thrombosis among chronic HD patients with primary functional vascular access. The association between atrial fibrillation and VAT is independent of the vascular access type, chronic inflammation markers and traditional VAT risk factors. Competing Interests There are no conflicts of interest in this study. References 1) Roy-Chaudhury P, Kelly BS, Zhang J, Narayana A, Desai P, Melham M, Duncan H, Heffelfinger SC: Hemodialysis vascular access dysfunction: from pathophysiology to novel therapies. Blood Purif, 2003; 21: 99-110 2) Chou CY, Kuo HL, Yung YF, Liu YL, Huang CC: C-reactive protein predicts vascular access thrombosis in hemodialysis patients. Blood Purif, 2006; 24: 342-346 3) Sirrs S, Duncan L, Djurdjev O, Nussbaumer G, Ganz G, Frohlich J, Levin A: Homocyst(e)ine and vascular access complications in haemodialysis patients: insights into a complex metabolic relationship. Nephrol Dial Transplant, 1999; 14: 738-743 4) Chen TC, Wang IK, Lee CH, Chang HW, Chiou TT, Lee CT, Fang JT, Wu MS, Hsu KT, Yang CC, Wang PH, Chuang FR: Hyperhomocysteinaemia and vascular access thrombosis among chronic hemodialysis patients in Taiwan: a retrospective study. Int J Clin Pract, 2006; 60: 1596-1599 5) Chang CJ, Ko YS, Ko PJ, Hsu LA, Chen CF, Yang CW, Hsu TS, Pang JH: Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int, 2005; 68: 1312-1319 6) Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A: Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis, 2005; 46: 897-902 7) Chung MK, Martin DO, Sprecher D, Wazni O, Kanderi-. an A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR: C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation, 2001; 104: 2886-2891 8) Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, Burzotta F, Iacoviello L, Donati MB, Schiavello R, Maseri A, Possati G: The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation, 2003; 108 Suppl 1: Ⅱ195-Ⅱ199 9) Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, Valsecchi MG: Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis, 2008; 51: 255-262 10) Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. The American Journal of Medicine, 2003; 115: 291-297 11) Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care, 2003; 26: 3160-3167 12) Schwaiger JP, Lamina C, Neyer U, Konig P, Kathrein H, Sturm W, Lhotta K, Grochenig E, Dieplinger H, Kronenberg F: Carotid plaques and their predictive value for cardiovascular disease and all-cause mortality in hemodialysis patients considering renal transplantation: a decade follow-up. Am J Kidney Dis, 2006; 47: 888-897 13) Zebe H: Atrial fibrillation in dialysis patients. Nephrol Dial Transplant, 2000; 15: 765-768 14) Korantzopoulos P, Kolettis TM, Kountouris E: Inflammation and anti-inflammatory interventions in atrial fibrillation. Int J Cardiol, 2005; 104: 361-362 15) Korantzopoulos P, Kolettis TM, Siogas K, Goudevenos JA: The emerging role of inflammation in atrial fibrillation and the potential of anti-inflammatory interventions. Eur Heart J, 2005; 26: 2207-2208; author reply 22082209 16) Liu T and Li G: Is atrial fibrillation an inflammatory disease? Med Hypotheses, 2005; 64: 1237-1238 17) Chou CY, Kuo HL, Wang SM, Liu JH, Lin HH, Liu YL, Huang CC: Outcome of atrial fibrillation among patients with end-stage renal disease. Nephrol Dial Transplant, 2010; 25: 1225-1230 18) Kaysen GA, Levin NW, Mitch WE, Chapman AL, Kubala L, Eiserich JP: Evidence that C-reactive protein or IL-6 are not surrogates for all inflammatory cardiovascular risk factors in hemodialysis patients. Blood Purif, 2006; 24: 508-516 19) Gustafsson C, Blomback M, Britton M, Hamsten A, Svensson J: Coagulation factors and the increased risk of stroke in nonvalvular atrial fibrillation. Stroke, 1990; 21: 47-51 20) Comerota AJ, Stewart GJ, Alburger PD, Smalley K, White JV: Operative venodilation: a previously unsus-.
(6) AF and VAT in Chronic HD Patients. pected factor in the cause of postoperative deep vein thrombosis. Surgery, 1989; 106: 301-308: discussion 308309 21) Lip GY, Lowe GD, Rumley A, Dunn FG: Increased markers of thrombogenesis in chronic atrial fibrillation: effects of warfarin treatment. Br Heart J, 1995; 73: 527533 22) Berardinelli L and Vegeto A: Lessons from 494 permanent accesses in 348 haemodialysis patients older than 65 years of age: 29 years of experience. Nephrol Dial Transplant, 1998; 13 Suppl 7: 73-77 23) Dixon BS, Novak L, Fangman J: Hemodialysis vascular access survival: upper-arm native arteriovenous fistula. Am J Kidney Dis, 2002; 39: 92-101 24) Erkut B, Unlu Y, Ceviz M, Becit N, Ates A, Colak A, Kocak H: Primary arteriovenous fistulas in the forearm for hemodialysis: effect of miscellaneous factors in fistula patency. Ren Fail, 2006; 28: 275-281. 453. 25) Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ: Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. Jama, 2001; 285: 28642870 26) Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, Petersen P: Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation, 2004; 110: 2287-2292 27) Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L: Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant, 2009; 24: 2786-2791 28) Castier Y, Lehoux S, Hu Y, Foteinos G, Tedgui A, Xu Q: Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney Int, 2006; 70: 315-320.
(7)
數據
相關文件
The functional fall of the salivary secretion observed at 180 days post irradiation was not only associated with a reduction of gland mass but also to an alteration of the
Diabetic uremic patients undergoing hemodialysis exhibited a higher risk for dental decay and xerostomia Ogunbodede, et al.732005Cross-sectional1 and 265/5425 to
KEYWORDS: acute closed lock, chronic closed lock, disc displacement without reduction, jaw locking, locking duration, temporomandibular joint.. Accepted for publication 29
Accordingly, the present retrospective study analyzed bone loss as an objective clinical parameter for chronic periodontitis as a potential risk factor for the presence of OSCC in
In the present investigation “mean” and “highest” bone loss could be confirmed in univariate and multivariate analysis as independent risk factor for OSCC. As the alveolar
Sjögren’s syndrome is a chronic, systemic autoimmune disorder involves salivary and lacrimal glands resulting in xerostomia(dry mouth), xerophthalimia (dry
Chronic traumatic ulcers are commonly found on the mucosa that is subjected to trauma from dentition such as buccal mucosa, lateral border of the tongue or
Chronic traumatic ulcers are commonly found on the mucosa that is subjected to trauma from dentition such as buccal mucosa, lateral border of the tongue or lips.. Lesions on