Inhibitory effects of 16-hydroxycleroda-3,13(14)E-dien-15-oic acid on
superoxide anion and elastase release in human neutrophils
through multiple mechanisms
Han-Lin Chang
a, Fang-Rong Chang
b, Jin-Shan Chen
c, Hui-Po Wang
d, Yi-Hsiu Wu
a,
Chien-Chiao Wang
a, Yang-Chang Wu
b, Tsong-Long Hwang
a,⁎
aGraduate Institute of Natural Products, Chang Gung University, Taoyuan 333, Taiwan bGraduate Institute of Natural Products, Kaohsiung Medical University, Kaohsiung 807, Taiwan
c
Department of Anatomy, College of Medicine, Taipei Medical University, Taipei, Taiwan
d
Department of Pharmacy, College of Pharmacy, Taipei Medical University, Taipei, Taiwan Received 3 October 2007; received in revised form 29 January 2008; accepted 14 February 2008
Available online 4 March 2008
Abstract
Reactive oxygen species and granule proteases produced by neutrophils contribute to the pathogenesis of inflammatory diseases. In this study, a cellular model in isolated human neutrophils was established to elucidate the anti-inflammatory functions of 16-hydroxycleroda-3,13(14)E-dien-15-oic acid (PL3S), a clerodane diterpenoid from Formosan Polyalthia longifolia var. pendula. PL3S significantly inhibited the generation of superoxide anion and the release of elastase in formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP)-activated human neutrophils in a
concentration-dependent fashion with IC50values of 3.06 ± 0.20 and 3.30 ± 0.48μM, respectively. PL3S did not affect cAMP-dependent pathway,
and the inhibitory effect of PL3S was not reversed by protein kinase A inhibitor. PL3S did not display antioxidant or superoxide anion-scavenging ability, and it failed to alter the subcellular NADPH oxidase activity. PL3S concentration-dependently inhibited calcium mobilization caused by FMLP but not thapsigargin. Furthermore, PL3S attenuated the FMLP-induced protein kinase B (AKT) and p38 mitogen-activated protein kinase phosphorylation. However, PL3S had no effect on FMLP-induced phosphorylation of extracellular regulated kinase and c-Jun N-terminal kinase. In summary, these results indicate that the suppressive effects of PL3S on human neutrophil respiratory burst and degranulation are at least partly mediated by inhibition of calcium, AKT, and p38 signaling pathways.
© 2008 Elsevier B.V. All rights reserved.
Keywords: Clerodane diterpenoid; Elastase; Neutrophil; Polyalthia longifolia; Superoxide anion
1. Introduction
Polyalthia longifolia var. pendula (Annonaceae) is native to the drier regions of Sri Lanka and is locally known as ‘Ulta Ashok’ and is commonly cultivated in Pakistan and India. This plant is used as an antipyretic agent in indigenous systems of medicine (Saleem et al., 2005). Today, P. longifolia var.
pendula is in large-scale cultivation in southern Taiwan as a landscape plant. Pharmacological studies on the bark and leaves of this plant display effective antimicrobial activity (Faizi et al., 2003a,b), cytotoxic function (Chang et al., 2006; Chen et al., 2000), and hypotensive effect (Saleem et al., 2005). In spite of this, the anti-inflammatory effect of P. longifolia var. pendula remains to be established.
Neutrophils are active phagocytes that act as a crucial component of innate immunity. Although antimicrobial func-tions of neutrophils are essential to host defense, their extensive or inappropriate activation often causes unwanted tissue damage, such as rheumatoid arthritis, ischemia-reperfusion
European Journal of Pharmacology 586 (2008) 332–339
www.elsevier.com/locate/ejphar
⁎ Corresponding author. Graduate Institute of Natural Products, College of Medicine, Chang Gung University, 259 Wen-Hwa 1st Road, Kweishan 333, Taoyuan, Taiwan. Tel./fax: +886 3 2118506.
E-mail address:htl@mail.cgu.edu.tw(T.-L. Hwang).
0014-2999/$ - see front matter © 2008 Elsevier B.V. All rights reserved. doi:10.1016/j.ejphar.2008.02.041
injury, chronic obstructive pulmonary disease, and asthma (Louis et al., 2000; Mohr et al., 1984; Nathan, 2006; Noguera et al., 2001; Vinten-Johansen, 2004). In response to diverse stimuli, activated neutrophils secrete a series of cytotoxins, such as superoxide anion (O2·−), a precursor of other reactive oxygen
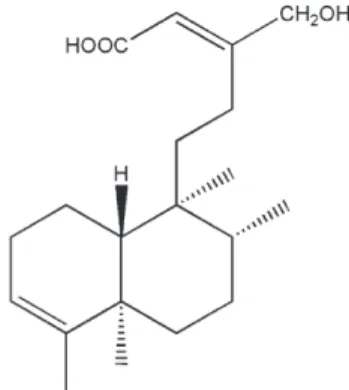
species, and granule proteases (Borregaard, 1988; Klebanoff, 2005). Therefore, it is crucial to restrain respiratory burst and degranulation in physiological conditions while potentiating these functions in infected tissues and organs. However, there are only a few currently available agents that directly modulate neutrophil proinflammatory responses in clinical practice. In a search for new anti-inflammatory agents, two clerodane diterpenoids, 16-hydroxycleroda-3,13(14)E-dien-15-oic acid methyl ester and 16-hydroxycleroda-3,13(14)E-dien-15-oic acid (PL3S) (Fig. 1), from Formosan P. longifolia var. pendula were found to inhibit the generation of O2·−in formyl-L
-methionyl-L-leucyl-L-phenylalanine (FMLP)-activated human neutrophils (Chang et al., 2006). The clerodane diterpenoids constitute a large class of natural products. The number of natural clerodane diterpenoids has grown rapidly in recent years, and many of them exhibit a diverse range of biological activities (Ahmad et al., 2005; Cavin et al., 2006; Huang et al., 2004; Lee et al., 2005; Shen et al., 2005). However, there is little research elucidating the anti-inflammatory function of the clerodane diterpenoids. Since ester structure may undergo hydrolysis, the aims of this study were to investigate the effect of PL3S on O2·− generation and elastase release in human
neutrophils and to elucidate the signaling pathways responsible for the PL3S-caused inhibition of the neutrophil responses. In the present study, our data suggest that the suppressive effects of PL3S on O2·−generation and elastase release in FMLP-induced
human neutrophils are at least partly mediated by the regulation of calcium mobilization and inhibition of p38 mitogen-activated protein kinase (MAPK) and protein kinase B (AKT) activation. 2. Materials and methods
2.1. Materials
PL3S was isolated as pure compound from Formosan P. longifolia var. pendula as described previously (Chen et al., 2000), and was dissolved in dimethyl sulfoxide (DMSO) to
make stock solutions. Aprotinin, leupeptin, phenylmethylsulfo-nyl fluoride (PMSF), and rolipram were obtained from Calbiochem (La Jolla, CA, USA). Fluo-3 AM was purchased from Molecular Probes (Eugene, OR, USA). 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium mono-sodium salt (WST-1) was purchased from Dojindo Laboratories (Kumamoto, Japan). All other chemicals were obtained from Sigma (St. Louis, MO, USA). When drugs were dissolved in DMSO, the final concentration of DMSO in cell experiments did not exceed 0.4% and did not affect the parameters measured. 2.2. Preparation of human neutrophils
Blood was taken from healthy human donors (20–32 years old) by venipuncture, using a protocol approved by the institutional review board at Chang Gung Memorial Hospital. Neutrophils were isolated with a standard method of dextran sedimentation prior to centrifugation in a Ficoll Hypaque gradient and hypotonic lysis of erythrocytes (Boyum et al., 1991). Purified neutrophils that containedN 98% viable cells, as determined by the trypan blue exclusion method, were resuspended in a calcium (Ca2+)-free HBSS buffer at pH 7.4, and were maintained at 4 °C before use.
2.3. Neutrophil fractionation
Neutrophils were pretreated with 1 mM PMSF for 30 min at 4 °C, disrupted in relaxation buffer (100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 1 mM ATP, 1 mM EGTA, and 10 mM PIPES;
pH 7.3) by sonication. Unbroken cells were removed by centrifugation at 300 g for 5 min, and the supernatant was then centrifuged at 100,000 g for 20 min at 4 °C to produce cytosolic and plasma membrane fractions.
2.4. Measurement of O2·−generation
The assay of the generation of O2·−was based on the
SOD-inhibitable reduction of ferricytochrome c (Babior et al., 1973). In brief, after supplementation with 0.5 mg/ml ferricytochrome c and 1 mM Ca2+, neutrophils (1 × 106 cells/ml) were equilibrated at 37 °C for 2 min and incubated with drugs for 5 min. Cells were activated with 100 nM FMLP or 5 nM phorbol myristate acetate (PMA). When FMLP was used as a stimulant, 1μg/ml cytochalasin B (FMLP/cytochalasin B) was incubated for 3 min before peptide activation. O2·−generation by
isolated neutrophil fractionation was measured after the addition of 160 μM NADPH to 800 μl of relaxation buffer containing 4 × 106 cell equivalents of membrane extract, 1.2 × 107cell equivalents of cytosol, 2μM GTP-γ-S, 0.5 mg/ml ferricytochrome c, and 100 μM sodium dodecyl sulfate. To facilitate the assembly of NADPH oxidase components, all constituents (excluding NADPH) were incubated at 37 °C for 3 min before the addition of NADPH. Drugs were incubated for 2 min before NADPH oxidase assembly. Changes in absorbance with the reduction of ferricytochrome c at 550 nm were continuously monitored in a double-beam, six-cell positioner spectrophotometer with constant stirring (Hitachi U-3010,
Fig. 1. Chemical structure of 16-hydroxycleroda-3,13(14)E-dien-15-oic acid (PL3S).
Tokyo, Japan). Calculations were based on differences in the reactions with and without SOD (100 U/ml) divided by the extinction coefficient for the reduction of ferricytochrome c (ε=21.1/mM/10 mm).
2.5. Lactate dehydrogenase (LDH) release
LDH release was determined by a commercially available method (Promega, Madison, WI, USA). Cytotoxicity was represented by LDH release in the cell-free medium as a percentage of the total LDH release. The total LDH release was determined by lysing cells with 0.1% Triton X-100 for 30 min at 37 °C.
2.6. O2·−-scavenging activity
The O2
·−-scavenging ability of PL3S was determined using
xanthine/xanthine oxidase in a cell-free system, based on a previously described method (Tan and Berridge, 2000). After 0.1 mM xanthine was added to the assay buffer (50 mM Tris (pH 7.4), 0.3 mM WST-1, and 0.02 U/ml xanthine oxidase) for 15 min at 30 °C, the absorbance associated with the O2·−
-induced WST-1 reduction was measured at 450 nm.
2.7. 1,1-Diphenyl-2-picrylhydrazyl (DPPH)-scavenging activity
An ethanol solution of the stable nitrogen-centered free radical, DPPH (100 μM), was incubated with PL3S or α-tocopherol for 16 min at 25 °C, and the absorbance was measured at 517 nM.
2.8. Measurement of elastase release
Degranulation of azurophilic granules was determined by elastase release as described previously (Coles et al., 2002; Sklar et al., 1982) with some modifications. Experiments were performed using MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide as the elastase substrate. Briefly, after supplementation with MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide (100 μM), neutrophils (6 × 105/ml) were equilibrated at 37 °C for 2 min and incubated with drugs for 5 min. Cells were activated by 100 nM FMLP and 0.5 μg/ml cytochalasin B, and changes in absorbance at 405 nm were continuously monitored to assay elastase release. The results were expressed as the percent of the initial rate of elastase release in the FMLP/cytochalasin B-activated, drug-free control system.
2.9. Determination of cAMP concentrations
cAMP levels were assayed using enzyme immunoassay kits (Amersham Biosciences, Buckinghamshire, England). Human neutrophils were incubated with drugs for 5 min before stimulation with FMLP for another 5 min, and the reaction was terminated by adding 0.5% dodecytrimethylammonium bromide. Samples were then centrifuged at 3000 g for 5 min at 4 °C. The supernatants were used as a source for the cAMP
samples. The assay was performed according to the manufac-turer's instructions.
2.10. Assay of adenylyl cyclase (AC) and phosphodiestase (PDE) activities
Neutrophils (5 × 107 cells/ml) were sonicated in ice-cold buffer, containing 25 mM Tris–HCl (pH 7.5), 0.25 M sucrose, 2 mM EDTA, 5 mM MgCl2, 10μM leupeptin, 100 μM PMSF,
and 10 μM pepstatin. Unbroken cells were removed by centrifugation at 300 g for 5 min, and then supernatant was centrifuged at 100,000 g for 40 min at 4 °C. The pellet and cytosol fraction were respectively used as sources for the AC and PDE enzymes. The reaction mixture (25 mM Tris–HCl (pH 7.5), 15 mM MgCl2, 1 mM 3-isobutyl-1-methylxanthine
(IBMX), 7.5 mM creatine phosphate, and 3 units creatine phosphokinase) contained 0.5 mM dithiothreitol, 1 mM ATP, and the pellet fraction for assessing AC activity. The reaction was carried out for 20 min at 30 °C and was terminated by boiling for 3 min. cAMP contents were assayed using enzyme immunoassay kits.
PDE activity was analyzed using a tritium scintillation proximity assay (SPA) system, and the assay was performed according to the manufacturer's instructions (Amersham Biosciences). Briefly, assays were performed at 30 °C for 10 min in the presence of 50 mM Tris–HCl (pH 7.5) containing 8.3 mM MgCl2, 1.7 mM EGTA, and 0.3 mg/ml bovine serum
albumin. Each assay was performed in a 100-µl reaction volume containing the above buffer, the neutrophil supernatant fraction, and around 0.05μCi [3H]cAMP. The reaction was terminated by the addition of 50 µl PDE SPA beads (1 mg) suspended in 18 mM zinc sulfate. Assays were performed in 96-well microtiter plates. The reaction mix was allowed to settle for 1 h before counting in a microtiter plate counter.
2.11. Measurement of [Ca2+]i
Neutrophils were loaded with 2μM fluo-3 AM at 37 °C for 45 min. After being washed, cells were resuspended in Ca2+ -free HBSS to 3 × 106cells/ml. The change in fluorescence was monitored using a Hitachi F-4500 spectrofluorometer (Tokyo, Japan) in a quartz cuvette with a thermostat (37 °C), while being continuously stirred. The excitation wavelength was 488 nm, and the emission wavelength was 520 nm. FMLP and thapsigargin were used to increase the intracellular con-centration of calcium, [Ca2+]i, in the presence of 1 mM Ca2+.
[Ca2+]i was calibrated by the fluorescence intensity, as
fol-lows: [Ca2+]i= Kd× [(F−Fmin) / (Fmax−F)]; where F is the
observed fluorescence intensity, Fmax and Fmin were
respec-tively obtained by the addition of 0.05% Triton X-100 and 10 mM EGTA, and Kdwas taken to be 400 nM.
2.12. Immunoblotting analysis of whole cell lysates
Neutrophils were incubated with drugs for 5 min at 37 °C before being stimulated by FMLP. After 1 min, reactions were stopped by adding an equal volume of boiling 2× Laemmli
sample buffer (finial concentration 62.5 mM Tris–HCl, pH 6.8, 4% SDS, 5% β-mercaptoethanol, 8.75% glycerol, 2.5 mM orthovanadate, 10 mM paranitrophenylphosphate, 0.00125% bromophenol blue, 1 mM PMSF, and protease inhibitor cocktail (Sigma)) and boiled for 10 min. The samples were centrifuged at 20,000 g for 30 min at 4 °C to yield the whole cell lysates. Proteins derived from whole cell lysates were separated by SDS-PAGE using 12% polyacry-lamide gels and blotted onto polyvinylidene difluoride membranes. Immunoblotting was performed using the indicated antibodies and revealed with the horseradish peroxidase-conjugated secondary anti-rabbit antibodies (1:3000) (Cell Signaling Technology, Beverly, MA, USA). The immunoreactive bands were visualized by the ECL system (Amersham Biosciences).
2.13. Statistical analysis
Results are expressed as the mean ± S.E.M., and comparisons were made using Student's t-test. A probability of 0.05 or less was considered significant.
3. Results
3.1. PL3S inhibits FMLP/cytochalasin B-induced O2 ·−
generation by intact neutrophils but not by reconstituted NADPH oxidase
The O2·−formed in neutrophils can be converted to various
species of oxygen radicals that are strongly antimicrobial but which also directly or indirectly cause damage by destroying the surrounding tissue. To investigate whether PL3S reduces respiratory burst in FMLP/cytochalasin B-treated human neutrophils, the amount of O2·− generated was determined.
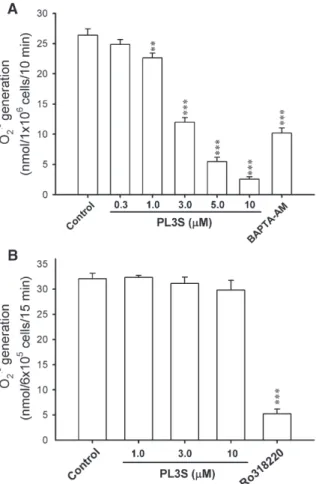
PL3S (0.3–10 μM) failed to alter the basal O2·−generation under
resting conditions, whereas it inhibited O2·−release in FMLP/
cytochalasin B-treated human neutrophils in a concentration-dependent manner with an IC50 value of 3.06 ± 0.20 μM
(Fig. 2A). 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM; 20 μM), a membrane-permeable Ca2+ chelator, was used as a positive control on FMLP/cytochalasin B-caused O2·− generation.
Furthermore, the FMLP-induced O2·−generation in the absence
of cytochalasin B was also inhibited by PL3S with an IC50value
of 2.73 ± 0.09μM. In contrast, PL3S (1, 3, and 10 μM) failed to affect PMA-activated O2·−release. Ro318220 (0.1μM), a
well-documented inhibitor of PKC, was used as a positive control on PMA-caused O2·−generation (Fig. 2B). Culturing with PL3S (up
to 30μM) for 15 min did not affect cell viability, as assayed by LDH release (data not shown). To examine whether NADPH oxidase is involved in the inhibition of PL3S, neutrophil membranes were isolated to assay O2·− production in a
reconstituted system after the addition of NADPH. As shown in Fig. 3, diphenyleneiodonium (10 μM), a NADPH oxidase inhibitor, but not PL3S (3 and 10 μM), suppressed O2
·−
generation. These data indicate that PL3S inhibits FMLP/
Fig. 2. Effects of PL3S on O2·−generation in FMLP/cytochalasin B- or
PMA-activated human neutrophils. O2·− generation was measured using
SOD-inhibitable cytochrome c reduction, as described under Materials and methods. Human neutrophils were incubated with DMSO (control), PL3S (0.3–10 μM), Ro318220 (0.1μM), or BAPTA-AM (20 μM) for 5 min and then activated by FMLP/cytochalasin B (n = 4–9) (A) or PMA (n=4) (B). All data are expressed as mean ± S.E.M. ⁎⁎ Pb0.01; ⁎⁎⁎ Pb0.001 compared with the control.
Fig. 3. Effects of PL3S on O2·−generation by reconstituted NADPH oxidase. O2·−
generation was measured using SOD-inhibitable cytochrome c reduction, as described under Materials and methods. A reactive mixture of the neutrophil cytosolic fraction and membrane fraction was preincubated with DMSO, PL3S (3 and 10μM), or diphenyleneiodonium (DPI, 10 μM) at 37 °C for 2 min before the addition of sodium dodecyl sulfate (100μM). The reaction was initiated by adding 160μM NADPH (n=3–4). All data are expressed as mean±S.E.M. ⁎⁎⁎ Pb0.001 compared with the control.
cytochalasin B-induced O2·−generation by intact neutrophils but
not by reconstituted NADPH oxidase.
3.2. O2·−- and free radical-scavenging activity of PL3S
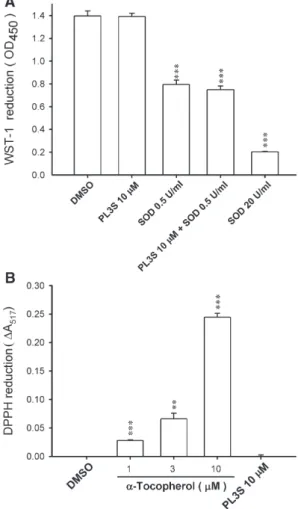
To investigate the ability of PL3S to scavenge O2·−and free
radicals, the effects of PL3S in the cell-free xanthine/xanthine oxidase system and DPPH test were assayed. PL3S, at concentrations of up to 10 μM, failed to alter WST-1 and DPPH reduction. SOD and α-tocopherol were used as the positive controls in the xanthine/xanthine oxidase system and DPPH assay, respectively (Fig. 4). These data rule out the possibility that the inhibitory effect of PL3S on O2·− release
occurs through scavenging of O2·−and free radicals.
Addition-ally, PL3S (10μM) did not affect the removal of O2·−by SOD
(0.5 U/ml) (Fig. 4A).
3.3. PL3S inhibits FMLP/cytochalasin B-induced elastase release
Neutrophil degranulation was measured according to the
extent of release of the primary granule-derived protease, elastase. PL3S (0.3–10 μM) inhibited elastase release by human neutrophils in response to FMLP/cytochalasin B in a concen-tration-dependent manner with an IC50value of 3.30 ± 0.48μM.
BAPTA-AM (20μM) was used as a positive control (Fig. 5). On the other hand, PL3S did not alter the basal level of elastase release under resting conditions (data not shown).
3.4. Effect of PL3S on cAMP pathway
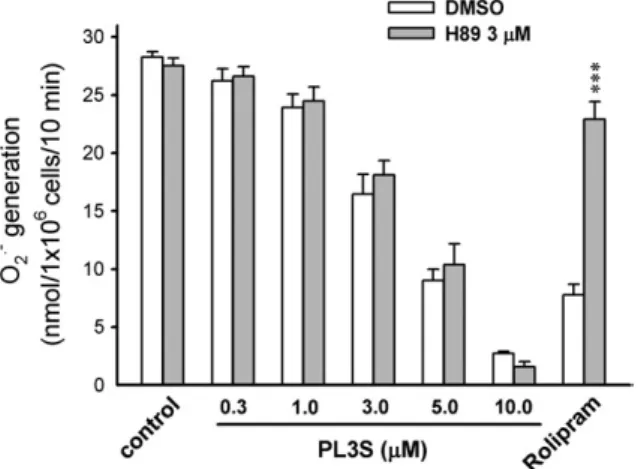
Cellular cAMP concentrations are modulated either by synthesis via AC or by degradation via PDEs. To examine whether cAMP pathways are involved in the inhibitory effect of PL3S, cAMP concentration, AC function, and PDE activity were assayed. Rolipram (a PDE4 inhibitor, 3μM), but not PL3S (10 μM), increased cAMP levels in FMLP-activated human neutrophils (Table 1). Furthermore, neither AC nor cAMP PDE activities were altered by PL3S. Forskolin and rolipram were used as positive controls for activating AC and inhibiting cAMP PDE, respectively (Table 1). Moreover, the PKA inhibitor H89
Fig. 4. Antioxidant effects of PL3S in a cell-free xanthine/xanthine oxidase system and DPPH assay. Reduction of WST-1 (n = 4) (A) and DPPH (n = 3) (B) was respectively measured spectrophotometrically at 450 and 517 nm, as described under Materials and methods. All data are expressed as mean ± S.E.M. ⁎⁎ Pb0.01; ⁎⁎⁎ Pb0.001 compared with the control.
Fig. 5. Inhibition of elastase release in human neutrophils by PL3S. Human neutrophils were incubated with DMSO (control), PL3S (0.3–10 μM), or BAPTA-AM (20μM) for 5 min and then activated by FMLP/cytochalasin B. Elastase release was induced by FMLP/cytochalasin B and measured spectro-photometrically at 405 nm, as described under Materials and methods. All data are expressed as mean ± S.E.M. (n = 4). ⁎⁎ Pb0.01; ⁎⁎⁎ Pb0.001 compared with the control.
Table 1
Effects of PL3S on cAMP level, AC function, and cAMP PDE activity in human neutrophils
Drugs cAMP Level AC activity cAMP PDE activity (pmol/2.5 × 106 cells/10 min) (pmol/1.5 × 106 cells/20 min) (% of inhibition) Control 1.46 ± 0.19 3.40 ± 0.83 0.00 ± 0.00 PL3S 10μM 1.49 ± 0.26 3.39 ± 0.26 10.53 ± 15.47 Forskolin 30μM – 10.47 ± 1.86a – Rolipram 3μM 8.03 ± 0.49b – 59.69 ± 7.00b All data are expressed as mean ± S.E.M. (n = 3–5).
a Pb0.05. b
did not restore the PL3S-induced inhibition of O2·− release
(Fig. 6).
3.5. Effect of PL3S on [Ca2+]i
Many cellular functions of neutrophils, such as respiratory burst and degranulation, are regulated by the Ca2+signals. Peak [Ca2+]ivalues and the time it took for [Ca2+]ito return to half of
the peak values (t1/2) were significantly inhibited by PL3S (1, 3,
and 10 μM) in FMLP-induced human neutrophils in a
concentration-dependent fashion (Fig. 7A and Table 2). In contrast, PL3S failed to change the [Ca2+]i mobilization of
human neutrophils caused by thapsigargin, an endoplasmic reticular Ca2+-ATPase inhibitor (Fig. 7B).
3.6. Effect of PL3S on phosphorylation of MAPKs and AKT To determine whether MAPKs and AKT are involved in the inhibitory effects of PL3S, activation of these kinases was assayed using antibodies specific for the phosphorylated, activate forms of p38, extracellular regulated kinase (ERK), and c-Jun N-terminal kinase (JNK) as well as AKT (Ser 473) determined by Western blotting. As shown inFig. 8, stimulation of human neutrophils with FMLP resulted in a rapid phosphorylation of MAPKs and AKT. FMLP-induced phos-phorylation of p38 MAPK and AKT was diminished by PL3S (1, 3, and 10 μM) in a concentration-dependent manner. However, PL3S did not alter FMLP-induced ERK and JNK activation in human neutrophils.
Fig. 6. Effects of a PKA inhibitor on the inhibition of O·−2 generation by PL3S in
human neutrophils. H89 (3μM) was preincubated for 5 min before the addition of PL3S (0.3–10 μM) or rolipram (1 μM). O2·− generation was induced by
FMLP/cytochalasin B and measured using SOD-inhibitable cytochrome c reduction, as described under Materials and methods. All data are expressed as the mean ± S.E.M. (n = 3–4). ⁎⁎⁎ Pb0.001 compared with the corresponding control.
Fig. 7. Typical traces of the effects of PL3S on Ca2+mobilization in FMLP- and
thapsigargin-activated human neutrophils. Human neutrophils were incubated with DMSO or PL3S (1, 3, and 10μM) for 5 min before stimulation with FMLP (A) or thapsigargin (B). Mobilization of Ca2+was determined in real time in a
spectrofluorometer. Representative traces from one of four–six experiments are shown.
Table 2
Effects of PL3S on the peak [Ca2+]iand the time taken for this concentration to
decline to half of its peak value (t1/2) in FMLP-activated neutrophils
Drug peak [Ca2+]
i t1/2 (nM) (s) Control 457.31 ± 16.37 24.85 ± 1.03 PL3S 1μM 423.83 ± 13.31 24.42 ± 1.58 PL3S 3μM 407.28 ± 11.23a 19.77 ± 1.16b PL3S 10μM 347.53 ± 20.71b 11.05 ± 0.99c
Neutrophils labeled with fluo-3/AM as described under Materials and methods were stimulated with 0.1μM FMLP, and fluorescence was monitored at 37 °C with stirring. All data are expressed as mean ± S.E.M. (n = 5–6).
a
Pb0.05.
b
Pb0.01.
c
Pb0.001 compared with the control.
Fig. 8. Effects of PL3S on phosphorylation of MAPKs and AKT in human neutrophils. Human neutrophils were incubated with DMSO or PL3S (1, 3, and 10 μM) for 5 min before stimulation with FMLP for 1 min at 37 °C. Phosphorylation of p38, p42/44, and JNK (A) as well as AKT (B) was analyzed by immunoblotting analysis using antibody against the phosphorylated form and the total of each protein as described under Materials and methods. Representative images from one of three experiments are shown.
4. Discussion
It is widely accepted that neutrophils contribute to inflam-matory diseases. Reactive oxygen species and granule proteases produced by neutrophils can directly or indirectly cause damage by destroying surrounding tissue. Herein, the effects of PL3S, a clerodane diterpenoid from Formosan P. longifolia var. pendula, on respiratory burst and degranulation in human neutrophils were investigated. Our data showed that PL3S significantly inhibited the release of O2·−and elastase in
FMLP-activated human neutrophils in a concentration-dependent fashion. Investigation of the signal transduction pathways indicates that the inhibitory effects of PL3S are associated with inhibition of calcium mobilization as well as p38 MAPK and AKT activation, but not with regulation of cAMP concentration and ERK and JNK phosphorylation.
Stimulation of neutrophils leads to increases in their oxygen consumption through the activity of NADPH oxidase which generates O2·−, a precursor of other reactive oxygen species. The
formation of O2·−in neutrophils can be inhibited by modulating
the cellular signaling pathways, but also by directly scavenging O2·−. PL3S did not scavenge O2·−or DPPH radicals in cell-free
systems, indicating that the inhibition of PL3S on O2·−release is
mediated via modulating the cellular signaling pathways. Furthermore, the lack of direct inhibition of NADPH oxidase by PL3S shows that this compound exerts its inhibitory influence upstream of NADPH oxidase. In addition to respiratory burst, degranulation also plays a pivotal role in most neutrophil functions. Neutrophil granules contain many antimicrobial and potentially cytotoxic substances. Neutrophil elastase is a major secreted product of stimulated neutrophils and a major contributor to destruction of tissue in chronic inflammatory disease. Therefore, elastase appears to be a target for therapy of chronic inflammatory diseases (Faurschou and Borregaard, 2003; Pham, 2006). PL3S suppressed FMLP-induced human neutrophil elastase release in a concentration-dependent manner. This data also supports our hypothesis that PL3S could act as an anti-inflammatory agent.
Intracellular Ca2+ is a key regulator in all cells of the immune system. When chemoattractants bind to the G protein-coupled receptor, inositol 1,4,5-triphosphate (IP3) triggers rapid
Ca2+ release from endoplasmic reticulum by activating IP3
receptors. Such Ca2+ release depletes endoplasmic reticulum Ca2+stores and subsequently activates extracellular Ca2+influx across the plasma membrane (Berridge, 1993). The magnitude and duration of [Ca2+]isignal responses to G protein-coupled
chemoattractants are obviously important. Increases in [Ca2+]i
have profound effects on neutrophils, including the initiation of cytoskeletal changes, degranulation, and respiratory burst (Tintinger et al., 2005). Consistent with this, BAPTA-AM, a cell permeable Ca2+ chelator, inhibited the generation of O2·−
and the release of elastase in FMLP-activated human neutrophils. Significant inhibition on FMLP-induced increase in [Ca2+]i mobilization by PL3S was observed in human
neutrophils. However, PL3S did not change [Ca2+]i
concentra-tion of human neutrophils caused by thapsigargin. These results indicate that PL3S specifically inhibits Ca2+ mobilization
caused by FMLP. PL3S at higher concentrations did not completely inhibit FMLP-induced [Ca2+]i mobilization,
sug-gesting that PL3S may exhibit an additional Ca2+-independent mechanism of action. On the other hand, increases in intracellular cAMP concentrations in neutrophils are associated with a decrease in several neutrophil functions, including respiratory burst and degranulation (Hwang et al., 2006). cAMP is formed from ATP by the action of the enzyme, AC, and is degraded by cAMP PDE, which catalyzes the hydrolysis of cAMP to inactive 5′-AMP. Our results are in line with previous findings that rolipram, a well-documented inhibitor of PDE4, increased cAMP concentration and inhibited O2·− release in
FMLP-induced human neutrophils. In contrast, PL3S failed to elevate the concentrations of cAMP and to alter the activities of AC and cAMP PDE. Moreover, pretreatment of PKA inhibitor did not restore the PL3S-induced inhibition of O2·− release.
These results indicate that cAMP-dependent pathway does not mediate the inhibition of respiratory burst and degranulation by PL3S.
FMLP is known to activate phospholipase C and phospha-tidylinositol-3-kinase (PI3K)/AKT, and it also activates MAPKs. The activation of these signal transduction pathways is known to be responsible for various physiological responses (Selvatici et al., 2006). The MAPK family of signaling cascades consists of ERK, p38 kinase, JNK, ERK3/4, and the big mitogen-activated protein kinase 1. Upon activation of PI3K, AKT is recruited to the plasma membrane, where it undergoes phosphorylation and activation. Activation of p38 and PI3K/ AKT has been shown to contribute to human neutrophils functions, such as respiratory burst and degranulation (Azuma et al., 2007; Chen et al., 2003; Nanamori et al., 2007; Partrick et al., 2000). Consistent with this, FMLP-induced generation of O2
·− and release of elastase were significantly inhibited by
inhibitors of p38 (SB202190) and PI3K (LY294002) (data not shown). The present study shows that the FMLP-induced phosphorylation of p38 MAPK and AKT, but not ERK and JNK, was diminished by PL3S in a concentration-dependent manner, suggesting that p38 and AKT are involved in PL3S-induced inhibition.
In summary, the present study shows that PL3S, a clerodane diterpenoid from Formosan P. longifolia var. pendula, inhibits human neutrophil proinflammatory responses, including respiratory burst and degranulation. These inhibitory effects by PL3S are mediated through the blockade of Ca2+, p38 MAPK, and AKT signaling pathways.
Acknowledgements
This work was supported by grants from the Chang Gung Medical Research Foundation and the National Science Council, Taiwan.
References
Ahmad, V.U., Khan, A., Farooq, U., Kousar, F., Khan, S.S., Nawaz, S.A., Abbasi, M.A., Choudhary, M.I., 2005. Three new cholinesterase-inhibiting cis-clerodane diterpenoids from Otostegia limbata. Chem. Pharm. Bull. (Tokyo). 53, 378–381.
Azuma, Y., Kosaka, K., Kashimata, M., 2007. Phospholipase D-dependent and -independent p38MAPK activation pathways are required for super-oxide production and chemotactic induction, respectively, in rat neutrophils stimulated by FMLP. Eur. J. Pharmacol. 568, 260–268.
Babior, B.M., Kipnes, R.S., Curnutte, J.T., 1973. Biological defense mechan-isms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 52, 741–744.
Berridge, M.J., 1993. Inositol trisphosphate and calcium signalling. Nature 361, 315–325.
Borregaard, N., 1988. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 41, 401–413.
Boyum, A., Lovhaug, D., Tresland, L., Nordlie, E.M., 1991. Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality. Scand. J. Immunol. 34, 697–712.
Cavin, A.L., Hay, A.E., Marston, A., Stoeckli-Evans, H., Scopelliti, R., Diallo, D., Hostettmann, K., 2006. Bioactive diterpenes from the fruits of Detarium microcarpum. J. Nat. Prod. 69, 768–773.
Chang, F.R., Hwang, T.L., Yang, Y.L., Li, C.E., Wu, C.C., Issa, H.H., Hsieh, W.B., Wu, Y.C., 2006. Anti-inflammatory and cytotoxic diterpenes from Formosan Polyalthia longifolia var. pendula. Planta Med. 72, 1344–1347.
Chen, C.Y., Chang, F.R., Shih, Y.C., Hsieh, T.J., Chia, Y.C., Tseng, H.Y., Chen, H.C., Chen, S.J., Hsu, M.C., Wu, Y.C., 2000. Cytotoxic constituents of Polyalthia longifolia var. pendula. J. Nat. Prod. 63, 1475–1478.
Chen, Q., Powell, D.W., Rane, M.J., Singh, S., Butt, W., Klein, J.B., McLeish, K.R., 2003. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J. Immunol. 170, 5302–5308.
Coles, B., Bloodsworth, A., Clark, S.R., Lewis, M.J., Cross, A.R., Freeman, B. A., O'Donnell, V.B., 2002. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ. Res. 91, 375–381.
Faizi, S., Khan, R.A., Azher, S., Khan, S.A., Tauseef, S., Ahmad, A., 2003a. New antimicrobial alkaloids from the roots of Polyalthia longifolia var. pendula. Planta med. 69, 350–355.
Faizi, S., Mughal, N.R., Khan, R.A., Khan, S.A., Ahmad, A., Bibi, N., Ahmed, S.A., 2003b. Evaluation of the antimicrobial property of Polyalthia longifolia var. pendula: isolation of a lactone as the active antibacterial agent from the ethanol extract of the stem. Phytother. Res. 17, 1177–1181. Faurschou, M., Borregaard, N., 2003. Neutrophil granules and secretory vesicles
in inflammation. Microbes Infect. 5, 1317–1327.
Huang, D.M., Shen, Y.C., Wu, C., Huang, Y.T., Kung, F.L., Teng, C.M., Guh, J.H., 2004. Investigation of extrinsic and intrinsic apoptosis pathways of new clerodane diterpenoids in human prostate cancer PC-3 cells. Eur. J. Pharmacol. 503, 17–24.
Hwang, T.L., Yeh, S.H., Leu, Y.L., Chern, C.Y., Hsu, H.C., 2006. Inhibition of superoxide anion and elastase release in human neutrophils by 3 ′-isopropoxychalcone via a cAMP-dependent pathway. Br. J. Pharmacol. 148, 78–87.
Klebanoff, S.J., 2005. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625.
Lee, D.Y., Ma, Z., Liu-Chen, L.Y., Wang, Y., Chen, Y., Carlezon Jr., W.A., Cohen, B., 2005. New neoclerodane diterpenoids isolated from the leaves of Salvia divinorum and their binding affinities for human kappa opioid receptors. Bioorg. Med. Chem. 13, 5635–5639.
Louis, R., Lau, L.C., Bron, A.O., Roldaan, A.C., Radermecker, M., Djukanovic, R., 2000. The relationship between airways inflammation and asthma severity. Am. J. Respir. Crit. Care Med. 161, 9–16.
Mohr, W., Pelster, B., Wessinghage, D., 1984. Polymorphonuclear granulocytes in rheumatic tissue destruction. VI. The occurrence of PMNs in menisci of patients with rheumatoid arthritis. Rheumatol. Int. 5, 39–44.
Nanamori, M., Chen, J., Du, X., Ye, R.D., 2007. Regulation of leukocyte degranulation by cGMP-dependent protein kinase and phosphoinositide 3-kinase: potential roles in phosphorylation of target membrane SNARE complex proteins in rat mast cells. J. Immunol. 178, 416–427.
Nathan, C., 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182.
Noguera, A., Batle, S., Miralles, C., Iglesias, J., Busquets, X., MacNee, W., Agusti, A.G., 2001. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax 56, 432–437.
Partrick, D.A., Moore, E.E., Offner, P.J., Meldrum, D.R., Tamura, D.Y., Johnson, J.L., Silliman, C.C., 2000. Maximal human neutrophil priming for superoxide production and elastase release requires p38 mitogen-activated protein kinase activation. Arch. Surg. 135, 219–225.
Pham, C.T., 2006. Neutrophil serine proteases: specific regulators of inflamma-tion. Nat. Rev. Immunol. 6, 541–550.
Saleem, R., Ahmed, M., Ahmed, S.I., Azeem, M., Khan, R.A., Rasool, N., Saleem, H., Noor, F., Faizi, S., 2005. Hypotensive activity and toxicology of constituents from root bark of Polyalthia longifolia var. pendula. Phytother. Res. 19, 881–884.
Selvatici, R., Falzarano, S., Mollica, A., Spisani, S., 2006. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur. J. Pharmacol. 534, 1–11.
Shen, Y.C., Cheng, Y.B., Ahmed, A.F., Lee, C.L., Chen, S.Y., Chien, C.T., Kuo, Y.H., Tzeng, G.L., 2005. Cytotoxic clerodane diterpenoids from Casearia membranacea. J. Nat. Prod. 68, 1665–1668.
Sklar, L.A., McNeil, V.M., Jesaitis, A.J., Painter, R.G., Cochrane, C.G., 1982. A continuous, spectroscopic analysis of the kinetics of elastase secretion by neutrophils. The dependence of secretion upon receptor occupancy. J. Biol. Chem. 257, 5471–5475.
Tan, A.S., Berridge, M.V., 2000. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J. Immunol. Methods 238, 59–68.
Tintinger, G., Steel, H.C., Anderson, R., 2005. Taming the neutrophil: calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin. Exp. Immunol. 141, 191–200.
Vinten-Johansen, J., 2004. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 61, 481–497.