Increased Lung Cancer Risk among Patients with Pulmonary
Tuberculosis -A Population Cohort Study
Running title: Tuberculosis and lung cancer
RE: JTO-D-10-00398 revision 1 of“Revised Version"
Yang-Hao Yu, MD1, Chien-Chang Liao, PhD, MS2,3, Wu-Huei Hsu, MD1, Hung-Jen Chen, MD1, Wei-Chih Liao, MD1, Chih-Hsin Muo, MS2,3, Fung-Chang Sung, PhD, MPH2,3, Chih-Yi Chen, MD4
1
Divisions of Pulmonary and Critical Care Medicine, China Medical University and Hospital, Taichung 404, Taiwan
2
Department of Public Health, China Medical University and Hospital, Taichung 404, Taiwan
3
Management Office for Health Data, China Medical University and Hospital, Taichung 404, Taiwan
4
Division of Thoracic Surgery, China Medical University and Hospital, Taichung 404, Taiwan
Words counts: 225 in the abstract; 2925 in the text (including acknowledgement); 4
tables, 1 figure and 46 references in 26 pages.
Correspondence :
Chih-Yi Chen, M.D. Professor
China Medical University and Hospital Division of Thoracic Surgery 2 Yu Der Road, Taichung 404, Taiwan Tel: 886-4-22052121 ext 1921 Fax: 886-4-22070298 e-mail: micc@www.cmuh.org.tw Yu Yang-Hao : yuchest71@gmail.com
Liao Chien-Chang: jacky48863027@yahoo.com.tw Hsu Wu-Huei: hsuwh@mail.cmuh.org.tw
Chen Hung-Jen: redman1025@gmail.com Liao Wei-Chih:weichih.liao@gmail.com Muo Chih-Hsin: b8507006@hotmail.com Sung Fung-Chang: fcsung@mail.cmu.edu.tw Chen Chih-Yi: micc@www.cmuh.org.tw
This work was performed at China Medical University, Taichung, Taiwan.
This work was supported by the Taiwan Department of Health [Grant DOH99-TD-B-111-004], the National Science Council, Executive Yuan, Taiwan (NSC
ABSTRACT
INTRODUCTION: Given one-third of the human population have been infected with
tuberculosis, it is important to delineate the relationship between tuberculosis and lung
cancer. This study explored whether contracting pulmonary tuberculosis is associated
with an increased risk of developing lung cancers. METHODS: In a cohort of 716,872 insured subjects, free from cancers, 20 years of age and above, 4480 patients with
newly diagnosed tuberculosis were identified from the universal insurance claims in
1998-2000, and tracked until 2007 with the remaining insured without tuberculosis. We
compared the incidence of lung cancers between the two cohorts and measured the
associated hazard of developing lung cancer. RESULTS: The incidence of lung cancers was approximately 11-fold higher in the cohort of tuberculosis patients than
non-tuberculosis subjects (26.3 vs. 2.41 per 10,000 person-years). Cox proportional
hazard regression analysis showed a hazard ratio of 4.37 (95% confidence interval (CI)
3.56-5.36) for the tuberculosis cohort after adjustment for the sociodemographic
variables, or 3.32 (95% CI 2.70-4.09) after further adjustment for chronic obstructive
pulmonary disease (COPD), smoking-related cancers (other than lung cancer), etc. The
hazard ratio increased to 6.22 with the combined effect with COPD, or to 15.5 with the
combined effect with other smoking-related cancers. CONCLUSIONS: This study provides a compelling evidence of increased lung cancer risk among individuals with
tuberculosis. The risk may increase further with coexisting COPD or other
smoking-related cancers.
KEYWORD: lung cancer, tuberculosis, chronic obstructive pulmonary disease,
Abbreviations
COPD: Chronic obstructive pulmonary disease
CI: Confidence interval
DOH: Department of health
ICD-9-CM: International Codes of Diseases 9th Edition Clinical Modification
NHI: National Health Insurance
HR: Hazard ratio
SCC: Squamous cell carcinoma
INTRODUCTION
Lung cancers are among the neoplastic diseases with the worst prognosis. The
etiology of the disease has been associated with smoking, occupational exposure toarsenates, nitrosamines, asbestos, and aromatics, and indoor exposures to radon, and
to fumes from fires or cooking stoves.1-4 Outdoor air pollutions also substantially
contribute to the burden of lung cancers in urban dwellers. Inflammation processes
have long been linked to cancer development.5,6 Among intrinsic lung diseases with
inflammatory components, chronic obstructive pulmonary disease (COPD),7 asthma,8
and pulmonary fibrosis9 have been linked to lung cancers. Tuberculosis with more
than 80% of the cases primarily affecting the lungs entails a chronic inflammatory
process. Coexistence of tuberculosis and lung cancers is not uncommon clinically.10,11
However, a clear association of tuberculosis with lung cancers remains to be
established.
Several studies have examined the association between tuberculosis and lung
cancer using hospital/community-based populations.12-21 Results of these studies were
inconclusive. Two studies were conducted in Montreal, Canada in 2 different periods
(1979-1986, and 1996-2001) to evaluate the association between previous lung
diseases and lung cancers.15 For tuberculosis, the evidence is inconsistent between
with respiratory diseases for a median follow-up of 9.1 years, and found 1028 cases of
lung cancers.16 Chronic obstructive pulmonary disease, but not tuberculosis, was
associated with higher risk of lung cancers in this study. Among non-smoker women
in Hong Kong16 and USA,17 pre-existing pulmonary tuberculosis, asthma, pneumonia,
and chronic bronchitis were more frequently noted in patients with lung cancers than
without. However, in these 2 studies only asthma, but not tuberculosis, bore a
significant impact. In a hospital based case-control study in Taiwan, Lee et. al. found
that history of pulmonary tuberculosis was an independent risk factor for lung cancers,
outweighing chronic bronchitis.18
To characterize the relationship between pulmonary tuberculosis and lung
cancers, a cohort study with population-based large representative sample is highly
desirable but has rarely been conducted. The only published cohort study on this topic
to date was conducted among farmers in a remote countryside in China using
retrospective analysis based on self-reported questionnaire data.22 The risk of lung
cancer mortality was 8-fold (25 vs. 3.1 per 1000 person-years) higher for those with
tuberculosis than those without in a population of 42,422. However, this study did not
include review of medical records making it possible for recall biases.
A recent systematic review of 41 studies was performed to determine whether
tuberculosis with lung adenocarcinoma group was noted particularly in
non-westernized countries.23 The impact of tuberculosis on lung cancers varied
among different ethnic groups and in different regions. The inconclusive results led
the authors of this systematic review to call for more cohort studies with larger sample
sizes to confirm the association between tuberculosis and lung cancers.
To gain better knowledge on tuberculosis in relation to lung cancers, we
conducted a population-based cohort study using patient care data compiled into a
large cohort of 1 million patients under the universal National Health Insurance (NHI)
program in Taiwan with a follow-up period of 7 to 9 years.
METHODS AND MATERIALS
Study Design and Sample
The NHI in Taiwan has registered all medical claims since 1996 with insured
identification numbers scrambled for protecting patients’ privacy. Sets of information
available for this study include gender, birthdates, disease codes, health care rendered,
medications prescribed, admissions, discharges, medical institutions and physicians
providing the services and others. In this longitudinal cohort study in a randomly
selected population of 1 million insured subjects, we identified all patients aged 20
cohort and all people without tuberculosis history as the non-exposed cohort also
identified in 1998-2000. We also excluded patients with any cancer diagnosis to make
sure participants were cancer-free at the start of both cohorts. Overall, 716,872
insured adult population were eligible for the prospective analysis. This follow-up
design would last until the date of censored or the end of 2007 for a period of 7 to 9
years to explore whether individuals with tuberculosis were associated with increased
risk of developing lung cancers.
Criteria and Definition
The International Codes of Diseases 9th Edition Clinical Modification
(ICD-9-CM) was used to identify the individual health status. The exposure group
consisted of patients with diagnosis of tuberculosis (ICD-9-CM of 011 and A-code of
A020), and the non-exposure group consisted of all insured without tuberculosis. Both
groups were treated as fixed cohorts. Even if a person develops tuberculosis 2001 or
later, the person remains classified as no tuberculosis by the end of 2007. We then
identified new lung cancer cases (ICD-9-CM of 162 and A-code of A101) from
outpatient and inpatient medical records. To ensure the accuracy of reimbursement
claims, the NHI system required experts review conducted for every 50-100 claims.
The institutions with false diagnosis are subject to penalties.24
inflammatory process, has been linked to lung cancers.7,25 Metabolic syndromes have
also been linked to several types of cancers.26,27 Diabetes was found to be associated
with breast cancer.28 However, its relationship with lung cancers remains
controversial. Two articles addressed its “protective” effect29,30 and another was
negative.31 Impaired glucose intolerance, elevated blood pressure, and dyslipidemia
comprise the major components of metabolic syndromes, which were therefore
included in the comorbidity analysis.
Data analysis
We first compared the distribution of sociodemographic factors between the
cohorts with and without tuberculosis. The proportions of comorbidities were also
compared between the 2 cohorts. The incidence rates of lung cancers were calculated
in the follow-up period until the end of 2007 adjusted by the sociodemographic
factors and comorbidities. The duration of observation for each person was calculated
until lung cancer diagnosed or censored for death, migration or discontinued
enrolment in the insurance system.
Crude and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for
factors associated with lung cancer risk were calculated using both the univariate and
multivariate Cox proportional hazard analyses with the variables categorized. Two
sex, and occupation. Model 2 further adjusted for diabetes, hypertension, dyslipidemia,
and COPD. We also included in the Model 2 the smoking-related cancers other than
lung cancer (ICD-9-CM of 140-150, 157, 160–161, 189, and A08, A090, A096, A100,
A109 and A123). We further applied a multivariate Cox proportional hazard model to
investigate the combined effect of tuberculosis and COPD or other smoking-related
cancers on lung cancer risk. The Kaplan-Meier model was used to compare the
probabilities of being free from lung cancer between the 2 cohorts. SAS software
version 9.1 (SAS Institute Inc., Carey, NC) was used for data analyses with two-sided
probability values less than 0.05 considered statistically significant.
RESULTS
The eligible study subjects included 4480 persons in the tuberculosis cohort and
712,392 persons in the non-tuberculosis cohort (Table 1). Compared with individuals
without tuberculosis, those with tuberculosis were dominated by males (57.9 vs. 49.2,
p < 0.0001), the elderly with age of 60 or greater (52.4% vs. 19.0%, p <0.0001) and
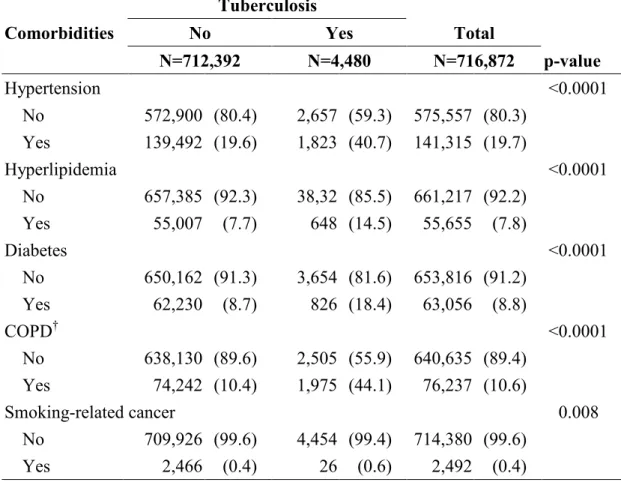
blue collars (18.5% vs. 13.9%, p <0.0001). Table 2 shows that the tuberculosis
patients were more prevalent than the non-tuberculosis group with hypertension,
dyslipidemia, diabetes mellitus and COPD (p <0.0001 for all the listed parameters).
tuberculosis cohort than in the non-tuberculosis cohort (p = 0.008).
The follow-up results showed that tuberculosis patients were 10.9 times more
likely than non-tuberculosis patients to develop lung cancer (26.3 vs. 2.41 per 10,000
person-years) (Table 3). A separate analysis calling deaths as failures showed that the
mortality was also much higher in the tuberculosis patients than in the
non-tuberculosis patients (51.1 vs. 8.2 per 10,000 person-years, data not shown). The
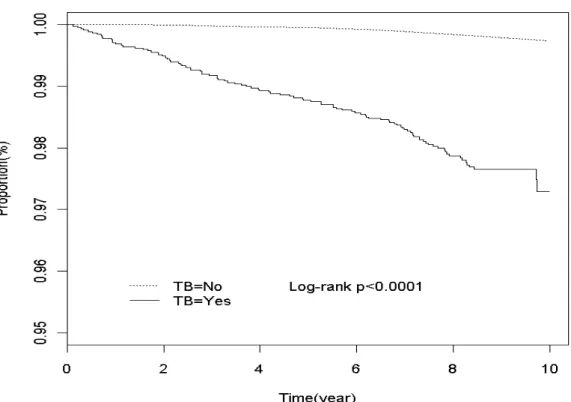
Kaplan-Meier analysis showed that the tuberculosis patients had less subjects
remained in the study than non-tuberculosis patients during a follow-up period of 7-9
years (97.2% vs. 99.8%, Log-rank p <0.0001) (Figure 1).
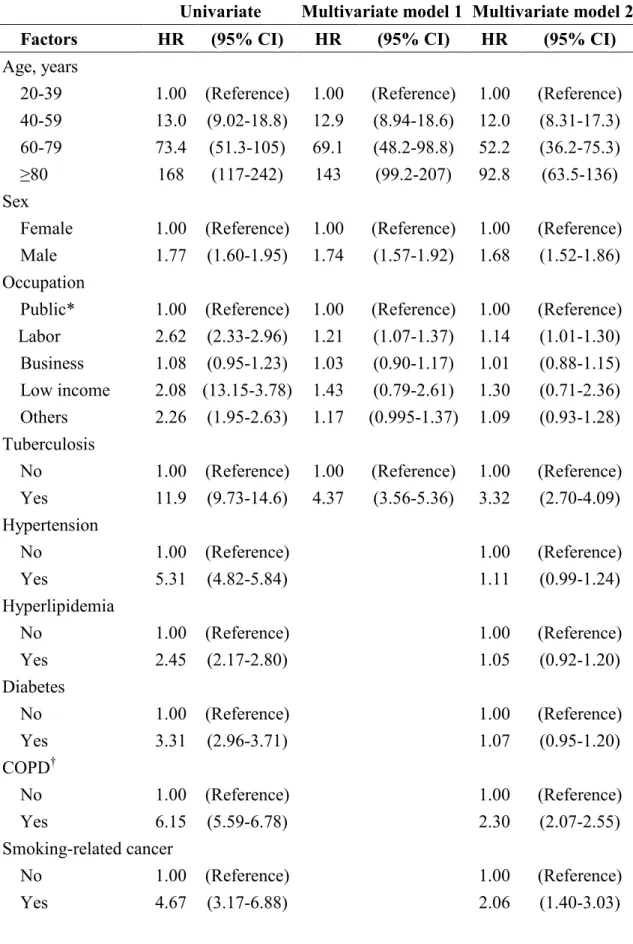
In Table 4, the univariate Cox proportional regression model shows that, in addition to
tuberculosis, age, sex, occupation, hypertension, dyslipidemia, diabetes and COPD
were also significant factors relevant to the development of lung cancers. After
controlling for these variables, the tuberculosis patients had a HR of 4.37 (95% CI
3.56-5.36) for lung cancers (model 1). When all comorbidities were included in model
2 multivariate analyses, the HR decreased to 3.32 (95% CI 2.70-4.09). COPD and other
smoking-related cancers remained as significant independent comorbidities associated
with lung cancer development. This model also shows that the risk of lung cancers
increased as age advanced, was higher in men than in women, and higher in blue collar
effect on the risk of lung cancers with an HR of 6.22 (95% CI = 4.87-7.94) (data not
shown). The HR increased to 15.5 (95% CI = 2.17-110) with the combined effect with
other smoking-related cancers.
DISCUSSION
Previous studies on association between tuberculosis and lung cancer using
hospital/community-based populations gave conflicting conclusions.12-22 The recent
meta-analysis of 41 studies has concluded that tuberculosis link to lung cancers varied
among different ethnic groups and in different regions.23 The inconclusive results
raise the need for cohort studies with larger sample sizes to confirm the association
between tuberculosis and lung cancers. However, a large cohort study conducted in a
remote country side in China, based on self-reported questionnaire data, was without
valid confirmation of diagnosis for both tuberculosis and lung cancer.22
The present cohort study explored the longitudinal association between
tuberculosis and lung cancer risk using a nationwide population-based sample of
patients and complete ascertainment of care that are verified with stringent national
health insurance claim procedures. Our analyses revealed that the incidence of lung
cancer is much greater in tuberculosis patients than in the general population, with an
observe a much higher mortality in the tuberculosis cohort.
Results from the present study are consistent with the report by Gao and Blot et.
al. that lung cancers were more frequently found in recent survivors of tuberculosis
infection.32 The risk is higher for men than for women and much higher for the elderly.
The data also shows tuberculosis is an independent predictor of lung cancer risk,
stronger than COPD. The changing incidence shows a trend of lung cancer shifting
from developed to less-developed countries,33,34 where tuberculosis poses a major
health risk. Our findings point to a potential health burden of lung cancer risk in
developing countries. In these countries, the populations are also aging with
tuberculosis more prevalent in men. These features together with results presented
here heighten the need for the developing countries to contain tuberculosis.
Smoking and air pollutions are the two major risk factors causing airway
diseases by repeatedly irritating respiratory epithelium, resulting in a chronic
inflammatory condition. The link of chronic inflammation to the lung cancer
development has been demonstrated in animal models.35,36 COPD is a known risk for
lung cancer. Cohort studies have shown the association of COPD with lung cancers.37
It has been reported that smokers with COPD had increased risk of developing lung
cancers by 1.3 to 4.5 folds in comparison with smokers without COPD.37-39 Our
hazard ratio of 2.30 (Table 4). The combined effect of tuberculosis and COPD
increased the hazard ratio of lung cancer risk from 3.32 to 6.22, a risk measure
comparable to smoking, the major etiologic factor of lung cancer.40,41
This causal association between chronic inflammatory conditions and lung
cancers has been observed not only clinically but also in a mice model. Using mutated
K-ras restricted to Clara cells of the conducting airway, Moghaddam et. al. reported
that a chronic inflammatory airway, mimicking COPD condition, promoted cancer
progression.35 The infected sites of tuberculosis are under a chronic inflammatory
condition with inflammatory cells and mediators that may facilitate carcinogenesis.
The longitudinal survey applied in the present study is a better approach in
establishing a link of tuberculosis to lung cancers. It avoids the selection and recall
biases in previous cross-sectional and case-control studies.12-14,17,21 The
population-based insurance data allow this study to avoid recall biases inherent to a
previous self-reported questionnaire study, which has been the only cohort study on
association of tuberculosis to lung cancers published to date.22 The larger
representative sample sizes collected in the present study provide a more reliable
statistical power for assessing the increase in lung cancer risk in patients with
tuberculosis as compared to a control cohort without tuberculosis.
of clinical coding could be questioned. Tuberculosis is one of the communicable
diseases under intense national surveillance in Taiwan. Reporting patients with
tuberculosis is mandatory and is enforced by the Department of Health (DOH) in
Taiwan. Cases reported to the Center for Disease Control, DOH, were under the
WHO recommended Directly Observed Therapy Short-course (DOTS) Care.
The diagnosis of cancers, including lung cancer, entitles the patients to qualify
for special healthcare privileges in the class of “major critical diseases” in Taiwan’s
NHI system. Once a patient is claimed to have this disease entity; co-payments for
healthcare are waived. This health benefit program has been under stringent NHI
auditing to avoid abuse or frauds. Thus, results derived from the NHI database for the
diagnosis of tuberculosis and lung cancer are reliable.
In this study, none of the three metabolic syndrome related comorbidities was
significantly associated with the lung cancer risk in the multivariate analysis. There is
a significant collinearity among the components of the metabolic syndrome,
supporting the validity of the data retrieved from the NHI cohorts. The findings that
metabolic syndrome related comorbidities did not bear any weight on lung cancer
development in these cohorts support the contention that a close association of
tuberculosis with lung cancer is not a chance observation.
this study is that the smoking data of the study cohorts were available only for the
smoking cessation history. Some studies have addressed the positive association
between smoking and tuberculosis.40,42 The estimate of smoking is an important issue
in the risk measurement of lung cancers.40 The prevalence of smoking in Taiwan has
been in the range of 50–60% in men and 3-4% in women.43,44 We used COPD and
other smoking-related cancer to substitute smoking as one of covariates in the
adjustment measures. The present study showed patients with other smoking-related
cancers were more prevalent in tuberculosis cohort. A case-control study has
indicated that 40% of patients with tuberculosis in Taiwan were smokers.45 A lower
smoking rate in tuberculosis patients reflects greater efforts for this group to quit
smoking. Thus, the confounding impact of smoking on the risk of lung cancer may be
lower in the tuberculosis group. A higher risk of lung cancer in men than in women
may reflect the smoking impact. Smoking has been associated with metabolic
syndrome.46 The negative associations between metabolic syndromes related
comorbidities and lung cancer risk may also alleviate the potential bias that is not
adjusted without smoking data.
Second, even if tuberculosis is associated with lung cancers, more questions
could be raised. Does tuberculosis affects some types of lung cancer but not others?
coexistence of tuberculosis and lung cancers.11 SCC of lung was also found in mice
subjected to chronic infection of mycobacterial tuberculosis.36 A recent meta-analysis
of epidemiological data, however, revealed the association was only significant with
adenocarcinoma, but not SCC.23 Without information on lung cancer types, whether
tuberculosis is preferentially associated with select types of lung cancer cannot be
addressed based on results derived from the present study. Finally, there is a remote
possibility that a small number of tuberculosis patients may have the disease before
being selected into the cohorts because of receiving no medical care until 1998-2000.
It is likely, however, the bias will affect both groups with and without lung cancer.
Further more, 1,584 lung cancer patients in the non-tuberculosis cohort had received
2,480 person-times of X-ray examinations, while there were 1,973 person-times for
the 100 patients in the tuberculosis cohort necessary for the treatment progress. It is
possible the x-ray examinations increased the risk of lung cancer as well.
In conclusion, this nationwide population-based cohort study provides evidence
supporting the contention that patients with pulmonary tuberculosis carried higher risk
of developing lung cancers. COPD and smoking enhanced the risk of lung cancer
further in patients with tuberculosis.
This work was supported by the National Science Council, Executive Yuan, Taiwan (NSC
97-2625-M-039-003), Department of Health Clinical Trial and Research Center of
Excellence [DOH99-TD-B-111-004]; and China Medical University Hospital [Grant
1MS1]. The authors declare that there are no conflicts of interest.
ACKNOWLEDGEMENTS: The authors are grateful to Prof. Chung Y. Hsu for his
critical review of this manuscript. The authors’ contributions are detailed here: Yu
YH, Sung FC and Chen CY initiated this project; Yu YH, Liao CC, Muo CH and
Sung FC designed the study, analyzed data and drafted the manuscript; Sung FC and
Hsu WH provided conceptual and technical input and assisted in revising the
manuscript; Chen HJ and Liao WC conducted literature review and provided
background information in relation to the novel findings reported in this manuscript.
Sung FC is the co-corresponding author.
REFERENCES
1. Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989;81:745-757. 2. Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest 2003;123: 21S-49S. 3. Ahsan H, Thomas DC. Lung cancer etiology: independent and joint effects of
genetics, tobacco, and arsenic. JAMA 2004;292:3026-3029.
4. Yang GH, Zhong NS. Effect on health from smoking and use of solid fuel in China. Lancet 2008;372:1445-1446.
5. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539-545.
6. Mantovani A. Cancer: inflammation by remote control. Nature 2005;435:752-753.
7. Hopkins RJ, Christmas T, Black PN et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J
2009;34:380-386.
8. Santillan AA, Camargo CA Jr, Colditz GA. A meta-analysis of asthma and risk of lung cancer (United States). Cancer Causes Control 2003;14:327-334.
9. Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med
2000;161:5-8.
10. Ashizawa K, Matsuyama N, Okimoto T et al. Coexistence of lung cancer and tuberculoma in the same lesion: demonstration by high resolution and
contrast-enhanced dynamic CT. Br J Radiol 2004;77:959-962.
11. Cicenas S, Vencevicius V. Lung cancer in patients with tuberculosis. World J Surg Oncol 2007;5:22-26.
12. Wang XR, Yu IT, Chiu YL et al.Previous pulmonary disease and family cancer history increase the risk of lung cancer among Hong Kong women. Cancer Causes Control 2009;20:757-763.
13. Zatloukal P, Kubík A, Pauk N et al. Adenocarcinoma of the lung among women: risk associated with smoking, prior lung disease, diet and menstrual and
pregnancy history. Lung Cancer 2003;41:283-293.
14. Galeone C, Pelucchi C, La Vecchia C et al. Indoor air pollution from solid fuel use, chronic lung diseases and lung cancer in Harbin, Northeast China. Eur J Cancer Prev 2008;17:473-478.
15. Ramanakumar AV, Parent ME, Menzies D et al. Risk of lung cancer following nonmalignant respiratory conditions: evidence from two case-control studies in Montreal, Canada. Lung Cancer 2006;53:5-12.
16. Littman AJ, Thornquist MD, White E et al. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control 2004;15:819-827. 17. Wu AH, Fontham ET, Reynolds P et al. Previous lung disease and risk of lung
cancer among lifetime nonsmoking women in the United States. Am J Epidemiol 1995;141:1023-1032.
18. Lee CH, Ko YC, Cheng LS et al. The heterogeneity in risk factors of lung cancer and the difference of histologic distribution between genders in Taiwan. Cancer Causes Control 2001;12:289-300.
19. Park SK, Cho LY, Yang JJ et al.Lung cancer risk and cigarette smoking, lung tuberculosis according to histologic type and gender in a population based case-control study. Lung Cancer 2009.[Epub ahead of print].
20. Bae J, Gwack J, Park SK et al. Cigarette smoking, alcohol consumption, tuberculosis and risk of lung cancer: the Korean multi-center cancer cohort study. J Prev Med Public Health 2007;40:321-328.
21. Tamura A, Hebisawa A, Iuchi K et al. Lung cancer in patients with chronic pyothorax. Respirology 2008;13:585-589.
22. Engels EA, Shen M, Chapman RS et al. Tuberculosis and subsequent risk of lung cancer in Xuanwei, China. Int J Cancer 2009;124:1183-1187.
23. Liang HY, Li XL, Yu XS et al. Facts and fiction of the relationship between pre-existing tuberculosis and lung cancer risk: A systematic review. Int J Cancer 2009;125:2936-2944.
24. Tseng CH. Mortality and causes of death in a national sample of diabetes patients in Taiwan. Diabetes Care 2004;27:1605-1609.
25. Kiri VA, Soriano J, Visick G et al. Recent trends in lung cancer and its
association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J 2009. [Epub ahead of print]
26. Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer 2008;44:293-297.
27. Lindgren A, Pukkala E, Nissinen A et al. Blood pressure, smoking, and the incidence of lung cancer in hypertensive men in North Karelia, Finland. Am J Epidemiol 2003;158:442-447.
28. Wolf I, Sadetzki S, Catane R et al. Diabetes mellitus and breast cancer. Lancet Oncol 2005;6:103-111.
29. Hanbali A, Al-Khasawneh K, Cole-Johnson C et al. Protective effect of diabetes against metastasis in patients with non-small cell lung cancer. Arch Intern Med 2007;167:513.
30. de Giorgio R, Barbara G, Cecconi A et al. Diabetes is associated with longer survival rates in patients with malignant tumors. Arch Intern Med
2000;160:2217.
31. Satoh H, Ishikawa H, Kurishima K et al. Diabetes is not associated with longer survival in patients with lung cancer. Arch Intern Med 2001;161:485.
32. Zheng W, Blot WJ, Liao ML et al. Lung cancer and prior tuberculosis infection in Shanghai. Br J Cancer 1987;56:501-504.
33. Toh CK. The changing epidemiology of lung cancer. Methods Mol Biol 2009;472:397-411.
34. Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol
2008;3:819-31.
chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol 2009;40:443-453. 36. Nalbandian A, Yan BS, Pichugin A et al. Lung carcinogenesis induced by
chronic tuberculosis infection: the experimental model and genetic control. Oncogene 2009;28:1928-1938.
37. Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med 1986;105:503-507.
38. Purdue MP, Gold L, Järvholm B et al. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 2007;62:51-56. 39. Tockman MS, Anthonisen NR, Wright EC et al. Airways obstruction and the
risk for lung cancer. Ann Intern Med 1987;106:512-518.
40. Lin HH, Murray M, Cohen T et al. Effects of smoking and solid-fuel use on COPD, lung cancer, and tuberculosis in China: a time-based, multiple risk factor, modelling study. Lancet 2008;372:1473-1483.
41. Freedman ND, Leitzmann MF, Hollenbeck AR et al. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol 2008;9:649-656.
42. Leung CC, Li T, Lam TH et al. Smoking and tuberculosis among the elderly in Hong Kong. Am J Respir Crit Care Med 2004;170:1027-1033.
43. Ko YC, Lee CH, Chen MJ et al. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int J Epidemiol 1997;26:24-31.
44. Wen CP, Tsai SP, Chen CJ et al. The mortality risks of smokers in Taiwan: Part I: cause-specific mortality. Prev Med 2004;39:528-535.
45. Wang JY, Hsueh PR, Jan IS et al. The effect of smoking on tuberculosis: different patterns and poorer outcomes. Int J Tuberc Lung Dis 2007; 11:143-149.
46. Liu CS, Lin WY, Wu MT et al. Association among cigarette smoking, metabolic syndrome, and its individual components: the metabolic syndrome study in Taiwan. Metabolism 2008;57:544-548.
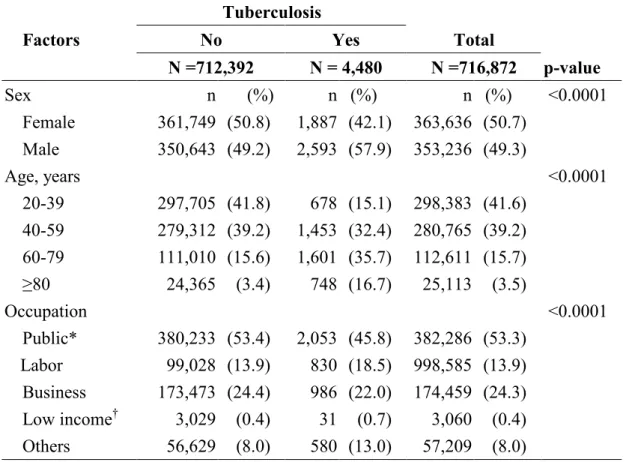
Table 1. Comparison in sociodemographic factors between cohorts with and without
tuberculosis.
Tuberculosis
Factors No Yes Total
N =712,392 N = 4,480 N =716,872 p-value Sex n (%) n (%) n (%) <0.0001 Female 361,749 (50.8) 1,887 (42.1) 363,636 (50.7) Male 350,643 (49.2) 2,593 (57.9) 353,236 (49.3) Age, years <0.0001 20-39 297,705 (41.8) 678 (15.1) 298,383 (41.6) 40-59 279,312 (39.2) 1,453 (32.4) 280,765 (39.2) 60-79 111,010 (15.6) 1,601 (35.7) 112,611 (15.7) ≥80 24,365 (3.4) 748 (16.7) 25,113 (3.5) Occupation <0.0001 Public* 380,233 (53.4) 2,053 (45.8) 382,286 (53.3) Labor 99,028 (13.9) 830 (18.5) 998,585 (13.9) Business 173,473 (24.4) 986 (22.0) 174,459 (24.3) Low income† 3,029 (0.4) 31 (0.7) 3,060 (0.4) Others 56,629 (8.0) 580 (13.0) 57,209 (8.0) *Government, education and military
Table 2. Comparison in comorbidity between cohorts with and without tuberculosis. Tuberculosis
Comorbidities No Yes Total
N=712,392 N=4,480 N=716,872 p-value Hypertension <0.0001 No 572,900 (80.4) 2,657 (59.3) 575,557 (80.3) Yes 139,492 (19.6) 1,823 (40.7) 141,315 (19.7) Hyperlipidemia <0.0001 No 657,385 (92.3) 38,32 (85.5) 661,217 (92.2) Yes 55,007 (7.7) 648 (14.5) 55,655 (7.8) Diabetes <0.0001 No 650,162 (91.3) 3,654 (81.6) 653,816 (91.2) Yes 62,230 (8.7) 826 (18.4) 63,056 (8.8) COPD† <0.0001 No 638,130 (89.6) 2,505 (55.9) 640,635 (89.4) Yes 74,242 (10.4) 1,975 (44.1) 76,237 (10.6) Smoking-related cancer 0.008 No 709,926 (99.6) 4,454 (99.4) 714,380 (99.6) Yes 2,466 (0.4) 26 (0.6) 2,492 (0.4) *
Missing value: 11 in urbanization.
†
Chronic obstructive pulmonary disease.
‡
ICD-9-CM and A-codes for hypertension were 401, 402, 403, 404, A260, A269; for hyperlipidemia 272.0, 272.1, 272.2, 272.3, 272.4, A189; for diabetes 250, A181; for COPD 491, 492, 496, A323.01, A323.03, A325; and for Smoking-related cancer 140-150, 157, 160–161, 189, A08, A090, A096, A100, A109, A123.
Table 3. Incidence of lung cancer between cohorts with and without
tuberculosis
Tuberculosis Population Person-years Cancer, n Incidence rate*
No 712,392 6,571,088 1,584 2.41
Yes 4,480 37,951 100 26.3
*
Table 4. Crude and adjusted hazard ratios and 95% confidence intervals of lung cancer
and associated factors
Univariate Multivariate model 1 Multivariate model 2
Factors HR (95% CI) HR (95% CI) HR (95% CI)
Age, years
20-39 1.00 (Reference) 1.00 (Reference) 1.00 (Reference) 40-59 13.0 (9.02-18.8) 12.9 (8.94-18.6) 12.0 (8.31-17.3) 60-79 73.4 (51.3-105) 69.1 (48.2-98.8) 52.2 (36.2-75.3) ≥80 168 (117-242) 143 (99.2-207) 92.8 (63.5-136) Sex
Female 1.00 (Reference) 1.00 (Reference) 1.00 (Reference) Male 1.77 (1.60-1.95) 1.74 (1.57-1.92) 1.68 (1.52-1.86) Occupation
Public* 1.00 (Reference) 1.00 (Reference) 1.00 (Reference) Labor 2.62 (2.33-2.96) 1.21 (1.07-1.37) 1.14 (1.01-1.30) Business 1.08 (0.95-1.23) 1.03 (0.90-1.17) 1.01 (0.88-1.15) Low income 2.08 (13.15-3.78) 1.43 (0.79-2.61) 1.30 (0.71-2.36) Others 2.26 (1.95-2.63) 1.17 (0.995-1.37) 1.09 (0.93-1.28) Tuberculosis
No 1.00 (Reference) 1.00 (Reference) 1.00 (Reference) Yes 11.9 (9.73-14.6) 4.37 (3.56-5.36) 3.32 (2.70-4.09) Hypertension No 1.00 (Reference) 1.00 (Reference) Yes 5.31 (4.82-5.84) 1.11 (0.99-1.24) Hyperlipidemia No 1.00 (Reference) 1.00 (Reference) Yes 2.45 (2.17-2.80) 1.05 (0.92-1.20) Diabetes No 1.00 (Reference) 1.00 (Reference) Yes 3.31 (2.96-3.71) 1.07 (0.95-1.20) COPD† No 1.00 (Reference) 1.00 (Reference) Yes 6.15 (5.59-6.78) 2.30 (2.07-2.55) Smoking-related cancer No 1.00 (Reference) 1.00 (Reference) Yes 4.67 (3.17-6.88) 2.06 (1.40-3.03)
*Government, education and military; † Chronic obstructive pulmonary disease.
Figure 1. Kaplan-Meier curves for probabilities of study subjects remained in the