Plasma antioxidant vitamins, chronic hepatitis B virus infection
and urinary aflatoxin B
1
–DNA adducts in healthy males
Ming-Whei Yu
1, Yi-Ching Chiang
1, Ju-Ping Lien
1and
factors for the development of hepatocellular carcinoma (HCC)
Chien-Jen Chen
1,2in Taiwan and other parts of southeastern Asia and Africa
(1–5). AFB
1is carcinogenic in many animal species and its
School of Public Health, College of Public Health, National Taiwancovalent binding to hepatic DNA has been shown to be a
University, Taipei 100, Taiwan and1Graduate Institute of Epidemiology,critical step in hepatocarcinogenesis (6).
College of Public Health, National Taiwan University, No. 1 Jen-Ai Rd.Sec. 1, Taipei 100, Taiwan
The major AFB
1–DNA adduct formed in vivo is AFB
1-N
7-guanine (7). Treatment of AFB
1to rats resulted in the urinary
2To whom correspondence should be addressed
excretion of AFB
1-N
7-guanine adducts. The adduct excretion
Epidemiological evidence indicates that aflatoxin B
1(AFB
1)
following AFB
1administration was shown to be proportional
intake is associated with an increased risk of hepatocellular
to the total amount of AFB
1-N
7-guanine initially formed in
carcinoma (HCC). The hepatocarcinogenesis is initiated by
the hepatic DNA (8). Although the presence of AFB
1–DNA
covalent binding of AFB
1to cellular DNA. To determine
adducts in liver specimens is readily detected by
immunohisto-whether nutritional factors and hormonal status may
influ-chemical methods (3), liver tissues are usually difficult to
ence the binding of AFB
1to hepatic DNA, a cross-sectional
obtain. Measurement of the adduct levels in urine provides a
study was performed on a total of 42 male asymptomatic
non-invasive means of estimating the degree of AFB
1binding
hepatitis B surface antigen (HBsAg) carriers and 43 male
to hepatic DNA. It has been used as an intermediate endpoint
non-carriers in a cohort study on the multistage
develop-in cancer prevention trials for assessdevelop-ing the efficacy of
chemo-ment of HCC in Taiwan. The major AFB
1–DNA adduct
preventive agents that may have an inhibitory effect at early
in vivo, AFB
1-N
7-guanine, was measured by
high-perform-stages of AFB
1-induced hepatocarcinogenesis (6,9). The
rela-ance liquid chromatography in urine. Urinary AFB
1-N
7-tionship between urinary AFB
1-N
7-guanine adducts and HCC
guanine was detectable in 40% of the subjects. HBsAg
risk was recently investigated in a prospective study and the
carriers had a higher detection rate of urinary AFB
1–
presence of the DNA adduct in urine has been shown to be a
DNA adducts than non-carriers and the difference was
significant predictor for the cancer risk (4).
statistically significant after multivariate adjustment. After
In addition to chronic HBV infection and aflatoxin exposure,
taking into account the total AFB
1urinary metabolite
our previous epidemiological studies have linked many risk
level, chronic HBsAg carrier status, and other potential
factors, including hepatitis C virus (HCV), alcohol drinking,
confounders, plasma levels of cholesterol,
α-tocopherol,
cigarette smoking, elevated serum level of endogenous
and
α- and β-carotene were positively associated with
testosterone, low serum retinol level and/or vegetable
consump-the detection rate of consump-the AFB
1–DNA adducts in a
dose-tion, as well as genetic susceptibility, to the development of
dependent manner, whereas plasma lycopene level was
HCC (10–15). There may be a complex interaction among
inversely related to the presence of the adducts in urine.
multiple HCC risk factors at various stages of
hepatocarcino-The association of urinary AFB1–DNA adducts with the
genesis. We previously examined the effect of chronic HBV
plasma levels of cholesterol,
α-tocopherol, lycopene, and
infection, sociodemographic characteristics, and habits of
cigar-α- and β-carotene was observed at both low and high
ette smoking and alcohol drinking on AFB
1–DNA adduct
exposure levels of AFB
1. There was a synergistic interaction
of plasma
α-tocopherol with α- and β-carotene on the
formation (16). Age and habits of cigarette smoking and
adduct levels. No association with the adducts was found
alcohol drinking were found to be positively associated with
for plasma levels of retinol and testosterone. This study
the adduct levels. Whether other factors may influence the
demonstrated different associations of antioxidant vitamins
binding of AFB
1to hepatic DNA have yet to be identified.
with AFB
1–DNA adduct formation. The data consistent
Synthetic antioxidants have been reported to inhibit AFB
1–
with our previous finding in cultured woodchuck hepato-
DNA adduct formation and thus the tumorigenicity of AFB
1cytes that
α-tocopherol and β-carotene enhanced AFB
1–
in rats (17,18). Experimental studies have also suggested that
DNA adduct formation suggest that prospective investi-
dietary antioxidants such as vitamin C, vitamin E and several
gation of the relationship between plasma micronutrients
carotenoids may suppress chemically induced carcinogenesis
and risk of AFB
1-related HCC is warranted.
through a variety of mechanisms, including reduction of
oxidative DNA damage, modulation of
carcinogen-metaboliz-ing enzymes, and/or trappcarcinogen-metaboliz-ing of electrophilic forms of
carcino-Introduction
gens (19–21). However, vitamin E and
β
-carotene were
observed to enhance AFB
1–DNA adduct formation in our
Ingestion of aflatoxin B
1(AFB
1*) and chronic infection with
previous in vitro study on cultured woodchuck hepatocytes
hepatitis B virus (HBV) have been identified as major risk
(22). In addition to the environmental agents, hormonal status
may also influence the AFB
1binding to DNA. The pathway
*Abbreviations: HCC, hepatocellular carcinoma; HBV, hepatitis B virus;
leading to metabolic activation of AFB
1
was reported to be
AFB1, aflatoxin B1; AFB1-N7-guanine, 8,9-dihydro-8-(N7-guanyl)-9-hydroxy-more active in male than in female rats (23). The role of
aflatoxin B1; HBsAg, hepatitis B surface antigen; HPLC, high-performanceremains to be explored. This study was carried out to elucidate
two HBsAg non-carriers were positive for anti-HCV. AFM
1the potential roles of endogenous testosterone and nutritional
was observed as the most abundant AFB
1metabolite excreted.
factors including common antioxidants at the early stage
The mean level of total AFB
1urinary metabolites (including
of AFB
1-induced hepatocarcinogenesis by investigating the
AFB
1, AFM
1, AFP
1, and AFB
1-N
7-guanine) was 9.47 ng/ml
covalent binding of AFB
1to hepatic DNA. Urinary AFB
1-N
7-
(
616.89 SD). Forty percent (34/85) of the urine samples
guanine was used as a biomarker for assessing AFB
1–DNA
contained a detectable level of AFB
1-N
7-guanine adducts. The
binding in the liver.
mean level of AFB
1
-N
7-guanine adduct in the positive urine
samples was 0.52 ng/ml (
61.02 SD). More than 90% of the
Materials and methods
study subjects had a urinary AFB
1-N
7-guanine adduct level
Study subjects,10% of the total AFB1
metabolites excreted in the urine.
The subjects of the present study have been described in a previous publicationIn univariate analysis, the odds ratios associated with the
that examined associations of multiple factors with urinary levels of AFB1–positivity of urinary AFB
1-N
7-guanine were elevated with
DNA adducts (16). Initially, a total of 43 male asymptomatic HBsAg carriersand 43 male HBsAg non-carriers were included in the study. HBsAg non-
increasing plasma levels of
α
-tocopherol (P for trend
5 0.005)
carriers were matched with the carriers on age (65 years) and time ofand
β
-carotene (P for trend
5 0.018). No significant association
biospecimen collection (63 months). They were randomly selected from thewith the AFB
1–DNA adducts in urine was observed for chronic
cohort members without HCC in a prospective study on the multifactorialetiology of multistage hepatocarcinogenesis in Taiwan. The cohort character-
HBsAg carrier status and plasma levels of testosterone and
istics and method of follow-up have been described in a previous study (15).other nutrients. Since there were correlations among various
The urine and blood samples from study subjects were consecutively collectedplasma nutrients, logistic regression analysis was adopted
between August 1988 and June 1992. Urine samples were kept frozen atto estimate multivariate-adjusted ORs associated with the
–30°C and blood samples were kept at –70°C until they were used in thepresent analyses. At the time of urine and blood collection, each study subject
positivity of urinary AFB
1–DNA adducts. After taking into
was also personally interviewed to obtain information related to habits ofaccount other potential confounders including the total AFB
1 cigarette smoking and alcohol drinking, dietary pattern and health history. In
urinary metabolites, plasma
α
-tocopherol level retained its
the present analysis, study subjects were 42 HBsAg carriers and 43 HBsAgnon-carriers whose plasma samples were sufficient for analysis of micronutri-
significance. Chronic HBsAg carrier status and plasma
choles-ents and testosterone. All the chronic HBsAg carriers included in this studyterol level were also significant factors associated with the
remained asymptomatic throughout a 5-year follow-up period, except twopositivity of urinary AFB
1
–DNA adducts. Increase in the
who were subsequently affected with liver cirrhosis. The mean duration ofdetection rate of the adducts was related to the plasma
α
-biospecimen storage prior to testing is 4.4 years (range: 2.4–6.3 years). Therewas no significant difference in seasons of biospecimen collection between
carotene at a borderline significant level (high versus low
HBsAg carriers and non-carriers.tertile, P
5 0.0548). Although a positive association was also
Laboratory analysesobserved between plasma
β
-carotene levels and urinary AFB
1
–
Serum HBsAg was assayed using a radioimmunoassay (Abbott Laboratories,DNA adducts, this association was not statistically significant.
North Chicago, IL). All but six study subjects were also tested for antibodiesPlasma lycopene level was inversely associated with the
against HCV (anti-HCV). Anti-HCV was examined by a second-generationenzyme immunoassay (Abbott Laboratories, North Chicago, IL). The urine
positivity of the adducts. No significant association with
samples were assayed for levels of AFB1, two of its main metabolites (AFM1urinary AFB
1
–DNA adducts was found for plasma retinol and
and AFP1) and the AFB1-N7-guanine adducts. Urinary AFB1metabolites weretestosterone. Because plasma levels of
α
-carotene and
β
-quantified by high-performance liquid chromatography (HPLC) as describedpreviously (16). In short, aflatoxin metabolites were extracted from a 5-ml
carotene were highly correlated (r
5 0.64) and the two
aliquot of urine. Analysis of various aflatoxin metabolites was done on ancarotenoids associated with urinary AFB
1
–DNA adducts in a
HPLC gradient liquid chromatograph with a Waters model 470 fluorescencesimilar manner, the levels of the two carotenoids were therefore
detector (Waters Associates, Milford, MA). The HPLC column was a C18combined in the analysis. This made almost no change in the
10-µm (3.9 mm inside diameter3 30 cm length)µBondpak column (Waters).For analysis of AFM1, AFB1-N7-guanine, and AFB1, chromatographic separa-
ORs of the positivity of urinary AFB
1–DNA adducts with
tion was achieved by elution for 12 min with 15% acetonitrile followed byother variables. There was a significant positive association
22% acetonitrile in water. The flow rates were 1.5, 0.8, 0.3 and 1.0 min/mlbetween the adducts and plasma levels of
α
- and
β
-carotene
at 0–12, 13–21, 22–35 and 36–41 min, respectively. Elutes were measured by(Table I).
fluorescence detection with 365 nm excitation and 430 nm emission wavelengthfor AFM1, and 500 nm emission for AFB1and AFB1-N7-guanine. The HPLC
Table II shows the detection rate of AFB
1-N
7-guanine in
analysis for AFP1 was done with a 30-min elution with 12% acetonitrile
urine by levels of total AFB
1
urinary metabolites and plasma
followed by a 12–22% acetonitrile linear gradient generated over 12 min,nutrients. Due to the sample size not being large, study subjects
then elution at 22% acetonitrile. The flow rates were 1.5, 0.3, 1.5, 0.3, 1.0and 0.3 ml/min at 0–17, 18–19, 20–29, 30–41, 42–44 and 45–52 min,
were categorized into only two groups according to the median
respectively. AFP1was measured by monitoring the fluorescence emission atof their distribution. The association of urinary AFB
1
–DNA
500 nm with the excitation wavelength at 365 nm. Authentic aflatoxinadducts with the plasma levels of cholesterol,
α
-tocopherol,
standards were used to determine chromatographic retention times. Allaqueous mobile phases before use were adjusted by orthophosphoric acid and
and
α
- and
β
-carotene was observed at both low and high
triethylammonium formate buffer to pH 3.0. Plasma micronutrients were alsoexposure levels of AFB
1
. The multivariate-adjusted ORs
associ-measured by HPLC using a modification of the method by Miller and Yangated with the positivity of the AFB
1
–DNA adducts in urine
(24). Plasma testosterone levels were determined by a radioimmunoassay kitsuggested a strong synergistic interaction of AFB
1exposure
(Biomerieux, Marcy l’Etoile, France).with plasma levels of these nutrients. In contrast, lycopene
Statistical methodsOdds ratios (ORs) and their 95% confidence intervals (CIs) were computed
appeared to have an inhibitory effect on AFB
1–DNA adduct
to examine the associations of the positivity of urinary AFB1-N7-guanine withformation at low and high AFB
1exposure. Interactive effect
various variables. Mantel’s chi-square test for a trend was performed to
between plasma
α
-tocopherol and
α
- and
β
-carotene on the
examine the dose–response relationship for the odds ratios. Logistic regressionadduct levels is depicted in Table III. All the ORs associated
was used to estimate ORs when adjusting covariates.with the positivity of urinary AFB
1–DNA adducts in relation
Results
to various variables in this study were not materially changed
when adjustment was also made for storage time of
biospeci-The age range of the subjects was 33 to 66 years, with a mean
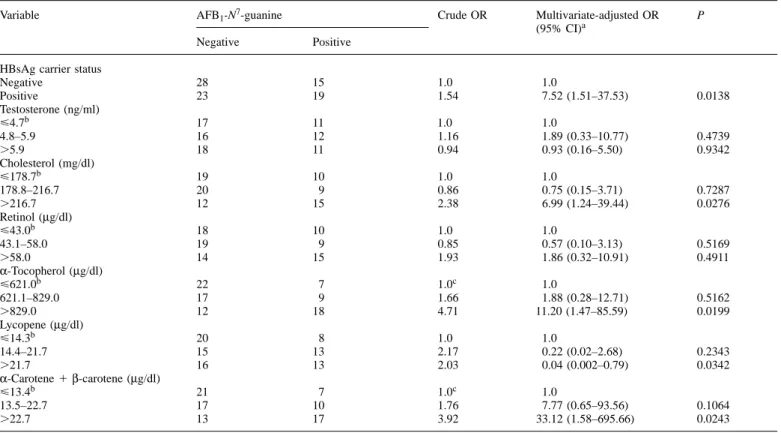
Table I. Associations of the positivity of urinary AFB1-N7-guanine adducts with HBsAg carrier status and plasma levels of testosterone and various nutrients
Variable AFB1-N7-guanine Crude OR Multivariate-adjusted OR P
(95% CI)a Negative Positive
HBsAg carrier status
Negative 28 15 1.0 1.0 Positive 23 19 1.54 7.52 (1.51–37.53) 0.0138 Testosterone (ng/ml) ø4.7b 17 11 1.0 1.0 4.8–5.9 16 12 1.16 1.89 (0.33–10.77) 0.4739 .5.9 18 11 0.94 0.93 (0.16–5.50) 0.9342 Cholesterol (mg/dl) ø178.7b 19 10 1.0 1.0 178.8–216.7 20 9 0.86 0.75 (0.15–3.71) 0.7287 .216.7 12 15 2.38 6.99 (1.24–39.44) 0.0276 Retinol (µg/dl) ø43.0b 18 10 1.0 1.0 43.1–58.0 19 9 0.85 0.57 (0.10–3.13) 0.5169 .58.0 14 15 1.93 1.86 (0.32–10.91) 0.4911 α-Tocopherol (µg/dl) ø621.0b 22 7 1.0c 1.0 621.1–829.0 17 9 1.66 1.88 (0.28–12.71) 0.5162 .829.0 12 18 4.71 11.20 (1.47–85.59) 0.0199 Lycopene (µg/dl) ø14.3b 20 8 1.0 1.0 14.4–21.7 15 13 2.17 0.22 (0.02–2.68) 0.2343 .21.7 16 13 2.03 0.04 (0.002–0.79) 0.0342 α-Carotene1β-carotene (µg/dl) ø13.4b 21 7 1.0c 1.0 13.5–22.7 17 10 1.76 7.77 (0.65–93.56) 0.1064 .22.7 13 17 3.92 33.12 (1.58–695.66) 0.0243
aAll the variables shown in the table, total AFB
1urinary metabolites, age and habits of cigarette smoking and alcohol drinking were also included in the
multiple logistic regression model.
bCategorized according to the tertile distribution of subjects. cTest for trend was statistically significant.
Table II. Detection rate of urinary AFB1-N7-guanine adducts by total AFB1metabolites in urine and plasma levels of selected nutrients
Variable Total no. Detection rate of Crude OR Multivariate-adjusted OR P
AFB1-N7-guanine (%) (95% CI)a
Total urinary AFB1/α-tocopherol
Low/lowb 22 4.5 1.0c 1.0
Low/high 20 45.0 17.18 22.56 (1.64–309.74) 0.0197
High/low 21 47.6 19.09 41.83 (2.99–584.57) 0.0055
High/high 22 63.6 36.75 73.19 (4.56–1173.76) 0.0024
Total urinary AFB1/α-carotene1β-carotene
Low/low 24 12.5 1.0c 1.0
Low/high 18 38.9 4.45 8.63 (1.03–72.34) 0.0470
High/low 19 47.4 6.30 8.10 (1.25–52.29) 0.0280
High/high 24 62.5 11.67 27.68 (3.33–230.41) 0.0021
Total urinary AFB1/lycopene
Low/high 21 28.6 1.0c 1.0
Low/low 21 19.0 0.59 3.02 (0.27–34.35) 0.3725
High/high 21 57.1 3.33 3.45 (0.62–19.02) 0.1556
High/low 22 54.5 3.00 32.55 (3.22–329.40) 0.0032
Total urinary AFB1/cholesterol
Low/low 23 21.7 1.0c 1.0
Low/high 19 26.3 1.29 1.54 (0.21–11.31) 0.6701
High/low 21 47.6 3.27 4.04 (0.69–23.52) 0.1204
High/high 22 63.6 6.30 11.03 (1.78–68.16) 0.0098
aAge, HBsAg carrier status, habits of cigarette smoking and alcohol drinking and plasma levels of testosterone and other nutrients were also included in the
multiple logistic regression model.
bHigh and low level was categorized according to the median of study subjects. cTest for trend was statistically significant.
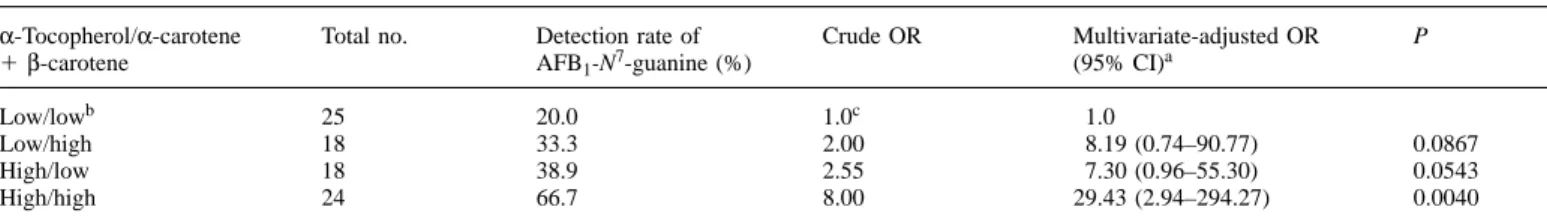
Table III. Detection rate of urinary AFB1-N7-guanine adducts by plasma levels ofα-tocopherol andα-carotene plusβ-carotene
α-Tocopherol/α-carotene Total no. Detection rate of Crude OR Multivariate-adjusted OR P
1β-carotene AFB1-N7-guanine (%) (95% CI)a
Low/lowb 25 20.0 1.0c 1.0
Low/high 18 33.3 2.00 8.19 (0.74–90.77) 0.0867
High/low 18 38.9 2.55 7.30 (0.96–55.30) 0.0543
High/high 24 66.7 8.00 29.43 (2.94–294.27) 0.0040
aAge, total AFB
1urinary metabolites, habits of cigarette smoking and alcohol drinking, HBsAg carrier status and plasma levels of testosterone and other
nutrients were also included in the multiple logistic regression model.
bHigh and low level was categorized according to the median of study subjects. cTest for trend was statistically significant.
Discussion
adducts with DNA, than female rats (23). However, variation
in plasma testosterone levels was not correlated with AFB
1–
The data on urinary aflatoxins were based on spot urine
DNA adducts in this study.
samples. Although no adjustment was made for urine
concen-Low serum retinol level has been associated with the
tration in the calculation of AFB
1-N
7-guanine excretion in this
development of various human cancers, including HCC (14).
study, this may not pose significant problems for investigation
Liver fractions from rats with vitamin A-deficiency formed a
of the associations between various variables and the positivity
higher level of DNA adducts by AFB1
(32). Our previous
of urinary AFB
1–DNA adducts, because total quantity of AFB
1in vitro study with cultured woodchuck hepatocytes
demon-metabolites excreted in urine was included in the multivariate
strated a potent inhibitory effect of retinol on AFB
1–DNA
analyses as a covariate for adjusting its effect.
adduct formation (22). However, we failed to find a significant
Epidemiological studies suggest that HBV and AFB
1may
association of AFB
1–DNA adducts with plasma retinol levels
exert a synergistic effect on the development of HCC (4). The
in this cross-sectional study. The reason for the discrepancy
mechanisms responsible for this interaction remain to be
between our in vitro and human study is unclear but may be
elucidated. In woodchucks, chronic infection with woodchuck
explained by the difference in the effect of retinol between
hepatitis virus, a virus similar in characteristics to HBV,
species. On the other hand, since this study was conducted in
produced an enhanced metabolic activation of chemical
carci-a well-nourished populcarci-ation with low prevcarci-alence of vitcarci-amin
nogen, including AFB
1(25). However, epidemiological studies
A-deficiency, whether a more striking association between
of the relationship between HBsAg carrier status and the
urinary AFB
1–DNA adducts and plasma retinol level may
formation of AFB
1–DNA adducts, using AFB
1-albumin adduct
be shown up in malnourished populations requires further
or urinary AFB
1-N
7-guanine as the surrogate dosimeter for
investigations.
estimating hepatic DNA binding by AFB
1, have been
inconsist-Lycopene,
α
-carotene and
β
-carotene are carotenoids with
ent (16,26–28). We have previously demonstrated that the
a similar chemical structure. They are antioxidant contents of
detection rate of urinary AFB
1-N
7-guanine adducts was higher
vegetables and fruits (21). Experimental and epidemiological
in HBsAg carriers than in non-carriers, but this association
studies on the potential role of
α
-carotene and lycopene
was not statistically significant in univariate analysis or in a
in carcinogenesis are limited (33–37). The influence of
β
-multivariate analysis including the total AFB
1urinary
metabol-carotenoids on susceptibility to various forms of cancers have
ites, age, and habits of cigarette smoking and alcohol drinking,
been evaluated in a number of epidemiological studies (38–
as covariates (16). In the present study, HBsAg carrier status
40). Although the majority of results have shown that high
was strongly associated with urinary AFB
1–DNA adduct levels
intake and/or high serum level of
β
-carotene were associated
after adjustment was made for plasma nutrients and other
with a reduced risk of cancer at several sites (38,39), some
potential confounders. The discrepancy between our two
stud-data are conflicting and the significance of
β
-carotene in
ies may be due to the difference in the control of plasma
carcinogenesis remains unclear (40). The most marked finding
nutrients that were significantly associated with the AFB
1–
of this study was the strong and extremely diverse associations
DNA adduct levels. The possible biological mechanisms for
of various carotenoids with AFB
1-N
7-guanine adducts in urine.
HBV involvement in the genesis of HCC have been extensively
α
-Carotene and
β
-carotene were positively associated with the
reviewed (1). This study provides evidence that chronic HBV
DNA adducts, while the adducts appeared to reduce with
infection may interact with AFB
1intake in the initiation
increasing plasma levels of lycopene. Difference in the ability
process of hepatocarcinogenesis and thus result in an increased
of diverse carotenoids to suppress the development of
spontan-risk of AFB
1-related HCC.
eous HCC and chemically-induced neoplastic transformation,
HCC is two to three times more frequent in men than in
enhance gap junctional communication and regulate gene
women (1). The marked sex difference in susceptibility to
expression have been shown in experimental studies (34–
hepatocarcinogenesis was also observed in various animal
37). Although the underlying mechanism for the molecular
models (29–31). A relationship between elevated serum
testo-specificity of each of the carotenoids to influence AFB
1–DNA
sterone level and HCC risk in humans has been documented
adduct formation is unclear, this study provides additional
(12). In experimental studies, the mechanism for the action of
insight into the complexity of the biological function of
testosterone in hepatocarcinogenesis may be through its effects
carotenoids.
on promotion of the growth of tumor and/or modulation of
α
-Tocopherol is among the most potent antioxidants from
the activity of enzymes involved in metabolism of
hepatocarci-natural source (21,36). Several experimental and
epidemiolog-nogens (23,30). For a given level of AFB
1exposure, male rats
Groopman,J.D. (1986) Modulation of aflatoxin metabolism, aflatoxin-N7
-(41). The potential use of
α
-tocopherol in cancer
chemopreven-guanine formation and hepatic tumorigenesis in rats fed ethoxyquin: role
tion has been evaluated in lung cancer, oral leukoplakia and
of induction of glutathione S-transferases. Cancer Res., 46, 3924–3931.
colorectal polyps. However, epidemiologic evidence for its
7. Croy,R.G., Essigmann,J.M., Reinhold,V.N. and Wogan,G.N. (1978)chemopreventive action is inconsistent (40,42,43). In this
Identification of the principal aflatoxin B1–DNA adduct formed in vivo inrat liver. Proc. Natl Acad. Sci. USA, 75, 1745–1749.
study, the detection rate of the AFB
1–DNA adducts in urine
8. Bennett,R.A., Essigmann,J.M. and Wogan,G.N. (1981) Excretion of an
was significantly elevated with increasing plasma levels of
α
-aflatoxin-guanine adduct in the urine of aflatoxin B1-treated rats. Cancer
tocopherol. There was a synergistic interaction between
α
-Res., 41, 650–654.
tocopherol and
α
- and
β
-carotene on the DNA adduct levels.
9. Groopman,J.D., Wogan,G.N., Roebuck,B.D. and Kensler,T.W. (1994)The data demonstrating a positive association between
Molecular biomarkers for aflatoxins and their application to human cancerurinary AFB
1–DNA adducts and the plasma levels of
α
-
prevention. Cancer Res., 54, 1907s–1911s.10. Yu,M.W., You,S.L., Chang,A.S., Lu,S.N., Liaw,Y.F. and Chen,C.J. (1991)
tocopherol and
β
-carotene are in accordance with our previous
Association between hepatitis C virus antibodies and hepatocellular
in vitro study in which
α
-tocopherol and
β
-carotene enhanced
carcinoma in Taiwan. Cancer Res., 51, 5621–5625.
the adduct formation in cultured woodchuck hepatocytes (22).
11. Chen,C.J., Yu, M.W., Wang,C.J., Huang,H.Y. and Lin,W.C. (1993) MultipleUrinary AFB
1–DNA adduct levels can be influenced by carci-
risk factors of hepatocellular carcinoma: a cohort study of 13737 malenogen metabolism and DNA repair. However, it was shown
adults in Taiwan. J. Gastroenterol. Hepatol., 8, 83s–87s.12. Yu,M.W. and Chen,C.J. (1993) Elevated serum testosterone levels and risk
that a supplement with antioxidant vitamins had no significant
of hepatocellular carcinoma. Cancer Res., 53, 790–794.
effect on DNA repair activity (44,45). Thus, our findings cast
13. Yu,M.W., Chen,C.J., Luo,J.C., Brandt-Rauf,P.W., Carney,W.P. and
doubts on the potential role of certain dietary antioxidants,
Santella,R.M. (1994) Correlations of chronic hepatitis B virus infection
such as
α
-tocopherol,
α
-carotene and
β
-carotene, in prevention
and cigarette smoking with elevated expression of neu oncoprotein in theof AFB
1-induced DNA damage. However, it is premature to
development of hepatocellular carcinoma. Cancer Res., 54, 5106–5110.14. Yu,M.W., Hsieh,H.H., Pan,W.H., Yang,C.S. and Chen,C.J. (1995) Vegetable
conclude that these antioxidant vitamins have an adverse effect
consumption, serum retinol level and risk of hepatocellular carcinoma.
on the development of HCC. The pathogenesis of cancer is a
Cancer Res., 55, 1301–1305.
multistage process including DNA alteration and cell
prolifera-15. Yu,M.W., Gladek-Yarborough,A., Chiamprasert,S., Santella,R.M.,
tion. Vitamins may have multiple biological mechanisms to
Liaw,Y.F. and Chen,C.J. (1995) Cytochrome P-450 2E1 and glutathioneinhibit or retard one or more stages of carcinogenesis. The
S-transferase M1 polymorphisms and susceptibility to hepatocellularcarcinoma. Gastroenterology, 109, 1266–1273.
association of HCC risk with plasma levels of various
antioxid-16. Yu,M.W., Lien,J.P., Liaw,Y.F. and Chen,C.J. (1996) Effects of multiple
ant vitamins remains to be elucidated. On the other hand, our
risk factors for hepatocellular carcinoma on formation of aflatoxin B1–
prospective study has demonstrated that an increased risk of
DNA adducts. Cancer Epidemiol. Biomark. Prev., 5, 613–619.
HCC is associated with low vegetable intake (14). Vegetables
17. Jhee,E.C., Ho,L.L. and Lotlikar,P.D. (1988) Effect of butylatedcontain a wide variety of phytochemicals with the potential
hydroxyanisole pretreatment on in vitro hepatic aflatoxin B1–DNA binding
to modulate carcinogenesis (21). Whether constituents in
and aflatoxin B1–glutathione conjugation in rats. Cancer Res., 48, 2688–2692.
vegetables other than
α
-carotene and
β
-carotene may be
18. Mandel,H.G., Manson,M.M., Judah,D.J. and Simpson,J.L. (1987)
important protective factors for AFB
1-induced HCC required
Metabolic basis for the protective effect of antioxidant ethoxyquin on
further studies.
aflatoxin B1hepatocarcinogenesis in the rat. Cancer Res., 47, 5218–5223.
To date we have not found any reports on the effect of
19. Duthie,S.J., Ma,A., Ross,M.A. and Collins,A.R. (1996) Antioxidantcholesterol on AFB
1–DNA adduct formation. The finding of
supplementation decreases oxidative DNA damage in human lymphocytes.Cancer Res., 56, 1291–1295.
the positive association of plasma cholesterol with urinary
20. Van Lieshout,E.M.M., Peters,W.H.M. and Jansen,J.B.M.J. (1996) Effect
AFB
1–DNA adducts in this study warrants further
investi-of oltipraz,α-tocopherol,β-carotene and phenethylisothiocyanate on rat
gation.
oesophageal, gastric, colonic and hepatic glutathione, glutathione S-transferase and peroxidase. Carcinogenesis, 17, 1439–1445.
Acknowledgements
21. Frei,B. (1994) Natural Antioxidants in Human Health and Disease.Academic Press, San Diego, California. This study was supported by grants from the National Science Council (NSC
22. Yu,M.W., Zhang,Y.J., Blaner,W.S. and Santella,R.M. (1994) Influence of 84-2331-B-002-183, NSC 85-2331-B-002-264) and National Institute of
vitamins A, C and E andβ-carotene on aflatoxin B1binding to DNA in
Health, Department of Health, Executive Yuan (DOH86-HR-627) of the
woodchuck hepatocytes. Cancer, 73, 596–604. Republic of China.
23. O’Brien,K., Mose,E., Judah,D. and Neal,G. (1983) Metabolic basis of the species difference to aflatoxin B1-induced hepatotoxicity. Biochem.
References
Biophys. Res. Commun., 114, 813–827.24. Miller,K.W. and Yang,C.S. (1985) An isocratic high-performance liquid 1. Yu,M.W. and Chen,C.J. (1994) Hepatitis B and C viruses in the development
chromatography method for the simultaneous analysis of plasma retinol, of hepatocellular carcinoma. Crit. Rev. Oncol. Hematol., 17, 71–91.
α-tocopherol and various carotenoids. Anal Biochem., 145, 21–26. 2. Chen,C.J., Yu,M.W., Liaw,Y.F., Wang,L.W., Chiamprasert,S., Matin,F.,
25. De Flora,S., Hietanen,E., Bartsch,H., Camoirano,A., Izzotti,A., Hirvonen,A., Bell,D.A. and Santella,R.M. (1996) Chronic hepatitis B
Bagnasco,M. and Millman,I. (1989) Enhanced metabolic activation of carriers with null genotypes of glutathione S-transferase M1 and T1
chemical hepatocarcinogens in woodchucks infected with hepatitis B virus. polymorphisms who are exposed to aflatoxin are at increased risk of
Carcinogenesis, 10, 1099–1106. hepatocellular carcinoma. Am. J. Hum. Genet., 59, 128–134.
26. Allen,S.J., Wild,C.P., Wheeler,J.G., Riley,E.M., Montesano,R., Bennett,S., 3. Chen,C.J., Zhang,Y.J., Lu,S.N. and Santella,R.M. (1992) Aflatoxin B1
Whittle,H.C., Hall,A.J. and Greenwood,B.M. (1992) Aflatoxin exposure, DNA adducts in smeared tumor tissue from patients with hepatocellular
malaria and hepatitis B infection in rural Gambian children. Trans. R. Soc. carcinoma. Hepatology, 16, 1150–1155.
Trop. Med. Hyg., 86, 426–430. 4. Qian,G.S., Ross,R.K., Yu,M.C., Yuan,J.M., Gao,Y.T., Henderson,B.E.,
27. Wild,C.P., Fortuin,M., Donato,F., Whittle,H.C., Hall,A.J., Wolf,C.R. and Wogan,G.N. and Groopman,J.D. (1994) A follow-up study of urinary
Montesano,R. (1993) Aflatoxin, liver enzymes and hepatitis B virus markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s
infection in Gambian children. Cancer Epidemiol. Biomark. Prev., 2, Republic of China. Cancer Epidemiol. Biomark. Prev., 3, 3–10.
555–561. 5. Van Rensburg,S.J., Cook-Mozaffari,P., Van Schalkwyk,D.J., Van Der
28. Groopman,J.D., Hall,A.J., Whittle,H., Hudson,G.J., Wogan,G.N., Watt,J.J., Vincent,T.J. and Purchase,I.F. (1985) Hepatocellular carcinoma
Montesano,R. and Wild,C.P. (1992) Molecular dosimetry of aflatoxin-N7
-and dietary aflatoxin in Mozambique -and Transkei. Br. J. Cancer, 51,
guanine in human urine obtained in the Gambia, west Africa. Cancer 713–726.
29. Sawaki,M., Enomoto,K., Takahashi,H., Nakajima,Y. and Mori,M. (1990) Phenotype of preneoplastic and neoplastic liver lesions during spontaneous liver carcinogenesis of LEC rats. Carcinogenesis, 11, 1857–1861. 30. Moore,M.R., Drinkwater,N.R., Miller,E.C., Miller,J.A. and Pitot,H.C.
(1981) Quantitative analysis of the time-dependent development of glucose-6-phosphatase-deficient foci in the livers of mice treated neonatally with diethylnitrosamine. Cancer Res., 41, 1585–1593.
31. Kim,C.M., Koike,K., Saito,I., Miyamura,T. and Jay,G. (1991) HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature, 351, 317–320.
32. Bhattacharya,R.K., Prabhu,A.L. and Aboobaker,V.S. (1989) In vivo effect of dietary factors on the molecular action of aflatoxin B1: role of vitamin
A on the catalytic activity of liver fractions. Cancer Lett., 44, 83–88. 33. Ziegler,R.G., Colavito,E.A., Hartge,P., McAdams,M.J., Schoenberg,J.B.,
Mason,T.J., Fraumeni,J.F. Jr (1996) Importance ofα-carotene,β-carotene and other phytochemicals in the etiology of lung cancer. J. Natl Cancer Inst., 88, 612–615.
34. Murakoshi,M., Nishino,H., Satomi,Y. et al. (1992) Potent preventive action ofα-carotene against carcinogenesis: spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by α-carotene than by β-carotene. Cancer Res., 52, 6583–6587.
35. Bertram,J.S., Pung,A., Churley,M., Kappock IV,T.J., Wilkins,L.R. and Cooney,R.V. (1991) Diverse carotenoids protect against chemically induced neoplastic transformation. Carcinogenesis, 12, 671–678.
36. Zhang,L.X., Cooney,R.V. and Bertram,J.S. (1991) Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/ 2 cells: relationship to their cancer chemopreventive action. Carcinogenesis, 12, 2109–2114.
37. Zhang,L.X., Cooney,R.V. and Bertram,J.S. (1992) Carotenoids up-regulate Connexin43 gene expression independent of their provitamin A or antioxidant properties. Cancer Res., 52, 5707–5712.
38. Byers,T. and Perry,G. (1992) Dietary carotenes, vitamin C and vitamin E as protective antioxidants in human cancers. Annu. Rev. Nutr., 12, 139–159. 39. Comstock,G.W., Bush,T.L. and Helzlsouer,K. (1992) Serum retinol, beta-carotene, vitamin E and selenium as related to subsequent cancer of specific sites. Am. J. Epidemiol., 135, 115–121.
40. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med., 330, 1029–1035.
41. Bertram,J.S., Kolonel,L.N. and Meyskens,F.L. Jr (1987) Rationale and strategies for chemoprevention of cancer in humans. Cancer Res., 47, 3012–3031.
42. DeCosse,J.J., Miller,H.H. and Lesser,M.L. (1989) Effects of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J. Natl Cancer Inst., 81, 1290–1297.
43. Benner,S.E., Wargovich,M.J., Lippman,S.M., Fisher,R., Velasco,M.,Winn,R.J. and Hong,W.K. (1994) Reduction in oral mucosa micronuclei frequency following α-tocopherol treatment of oral leukoplakia. Cancer Epidemiol. Biomark. Prev., 3, 73–76.
44. Wei,Q., Matanoski,G.M., Farmer,E.R., Hedayati,M.A. and Grossman,L. (1994) DNA repair related to multiple skin cancers and drug use. Cancer Res., 54, 437–440.
45. Hu,J.J., Roush,G.C., Berwick,M., Dubin,N., Mahabir,S., Chandiramani,M. and Boorstein,R. (1996) Effects of dietary supplementation ofα-tocopherol on plasma glutathione and DNA repair activities. Cancer Epidemiol. Biomark. Prev., 5, 263–270.
Received on November 4, 1996; revised on February 7, 1997; accepted on February 20, 1997